

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456924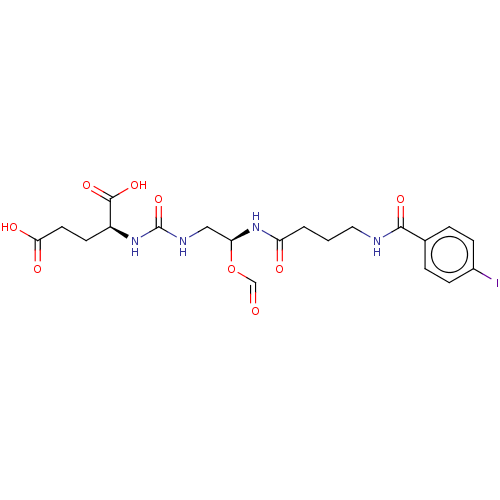 (US10736974, Compound YC-I-27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456928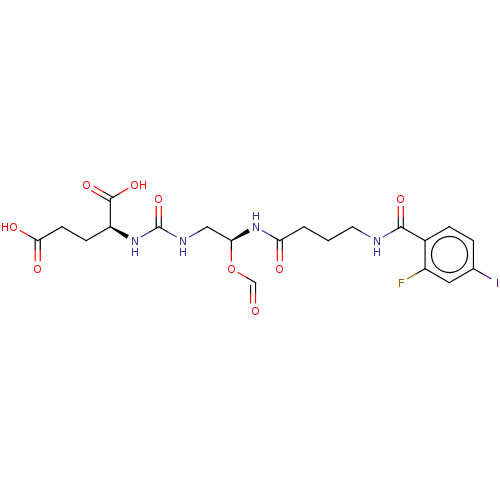 (US10736974, Compound XY-44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456930 (US10736974, Compound XY-59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456923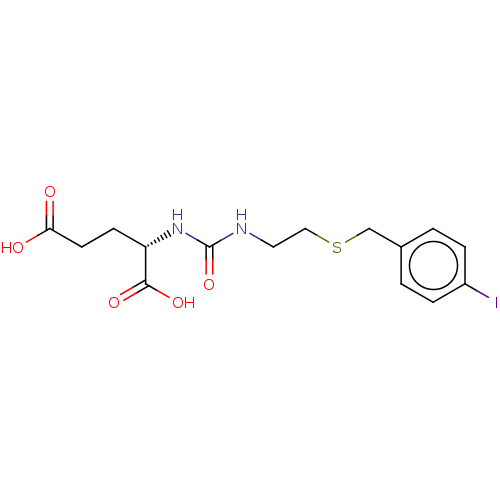 (US10736974, Compound DCIBC) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578399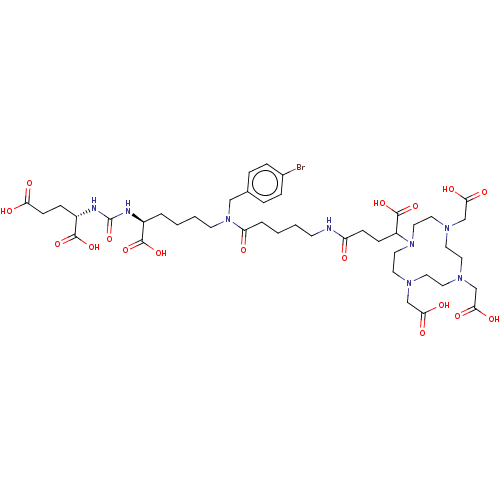 (US11478558, Compound L9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456931 (US10736974, Compound XY-58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456929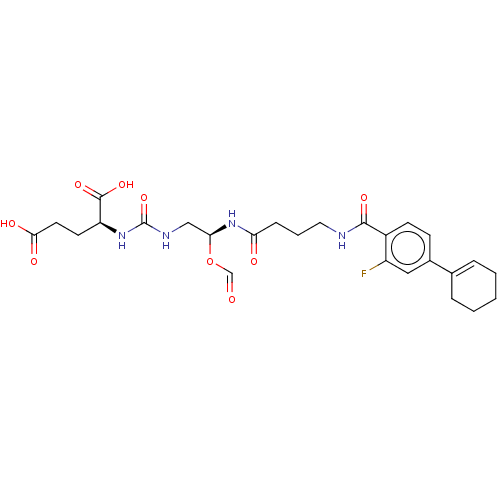 (US10736974, Compound XY-45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578398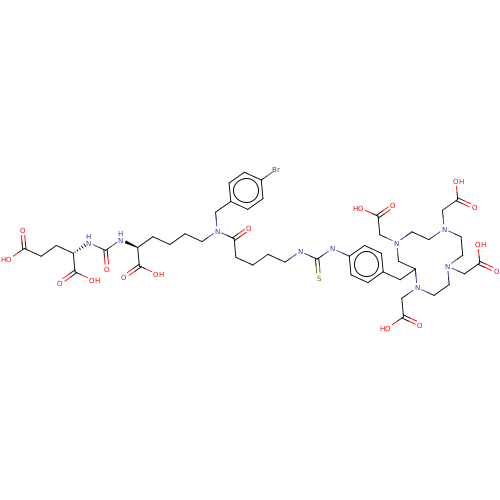 (US11478558, Compound L8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246934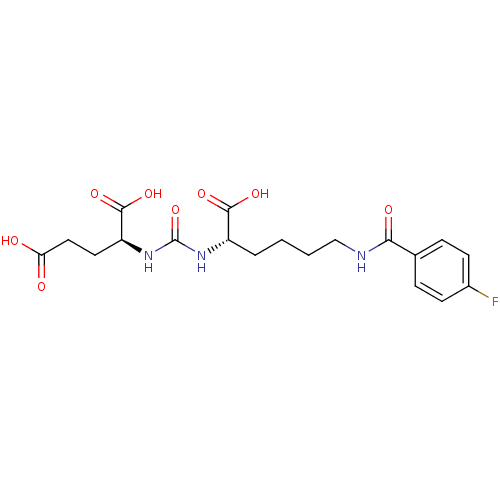 ((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298756 ((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246934 ((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456920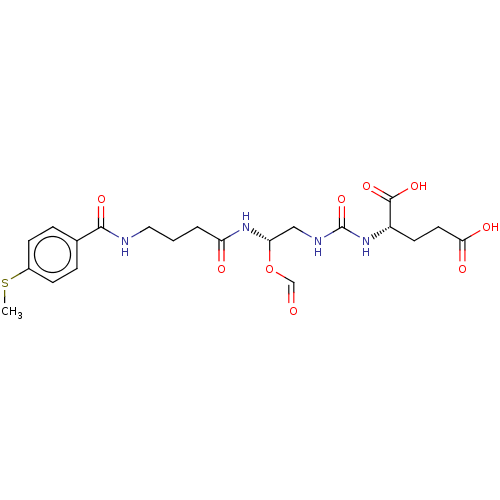 (US10736974, Entry 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578392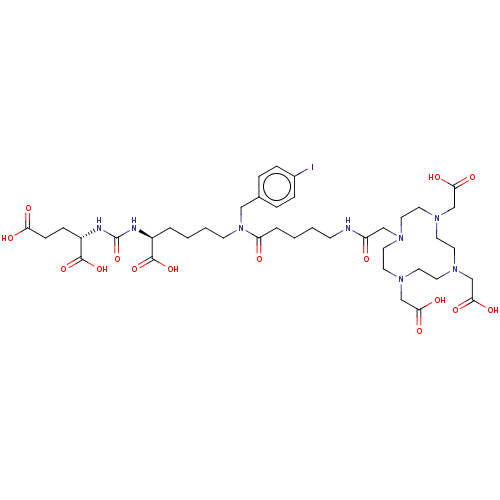 (US11478558, Compound L2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578394 (US11478558, Compound L4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456925 (US10736974, Compound YC-I-26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456914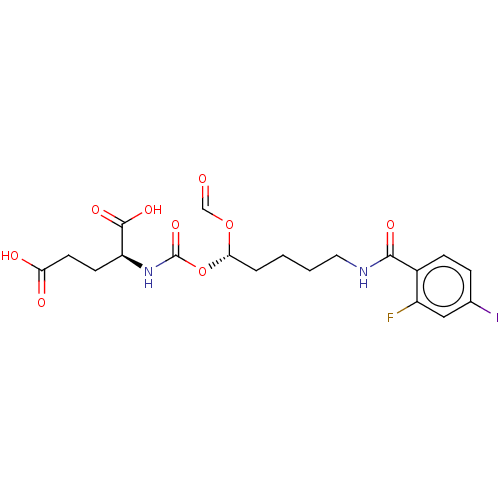 (US10736974, Compound XY-43 | US10736974, Entry 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456914 (US10736974, Compound XY-43 | US10736974, Entry 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456927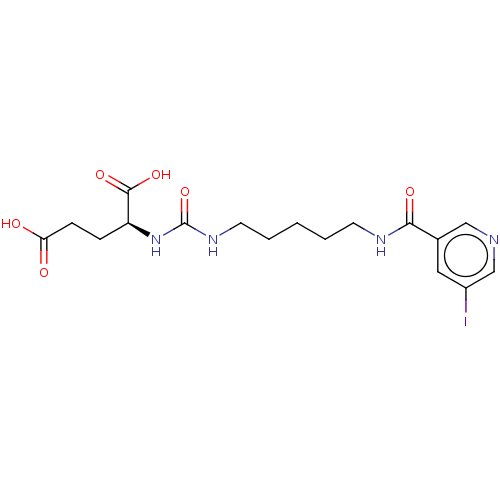 (US10736974, Compound C8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246935 ((S)-2-(3-((S)-1-carboxy-5-(3-iodonicotinamido)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456921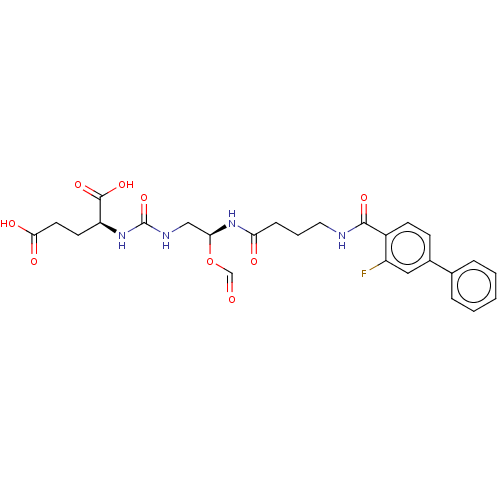 (US10736974, Entry 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578391 (US11478558, Compound 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578395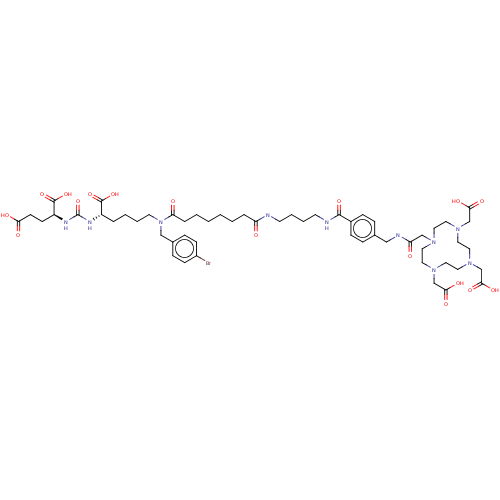 (US11478558, Compound L5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578397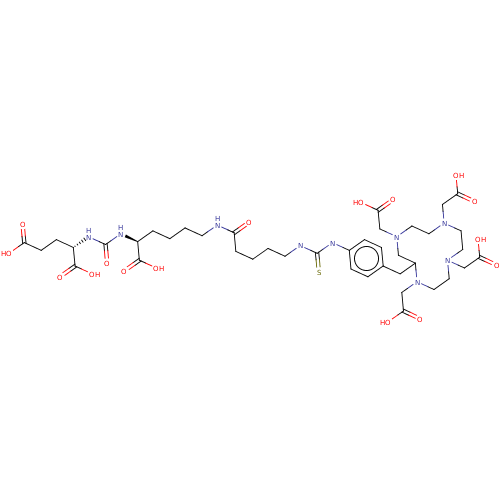 (US11478558, Compound L7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246935 ((S)-2-(3-((S)-1-carboxy-5-(3-iodonicotinamido)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.557 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578396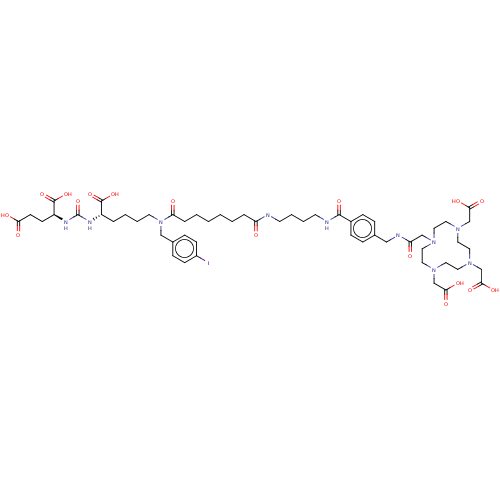 (US11478558, Compound L6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304738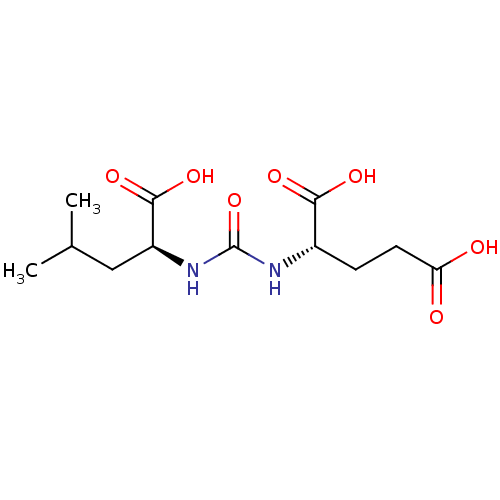 (2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of human cloned glutamate carboxypeptidase 2 | Bioorg Med Chem Lett 20: 392-7 (2010) Article DOI: 10.1016/j.bmcl.2009.10.061 BindingDB Entry DOI: 10.7270/Q2SN092X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578400 (US11478558, Compound L10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456906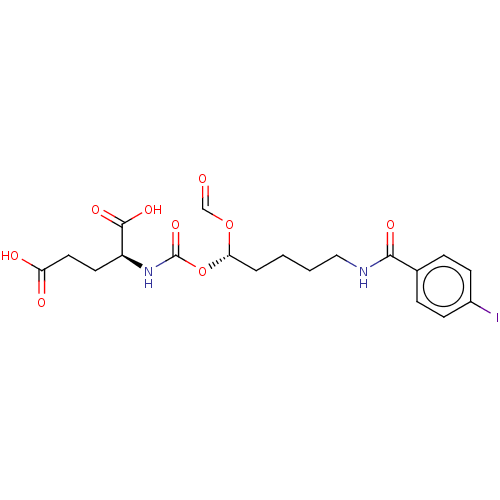 (US10736974, Compound XY-26 | US10736974, Entry 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456906 (US10736974, Compound XY-26 | US10736974, Entry 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456926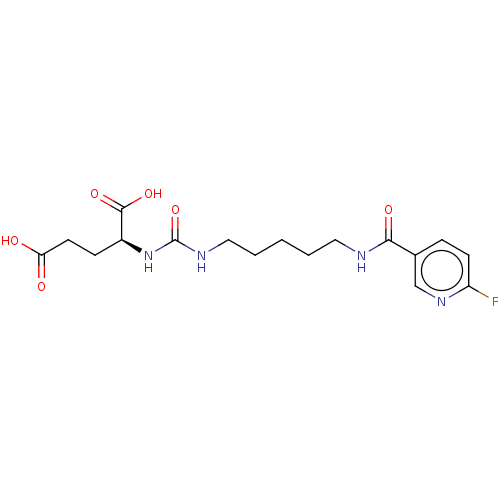 (US10736974, Compound DCFPyL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578389 (US11478558, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578390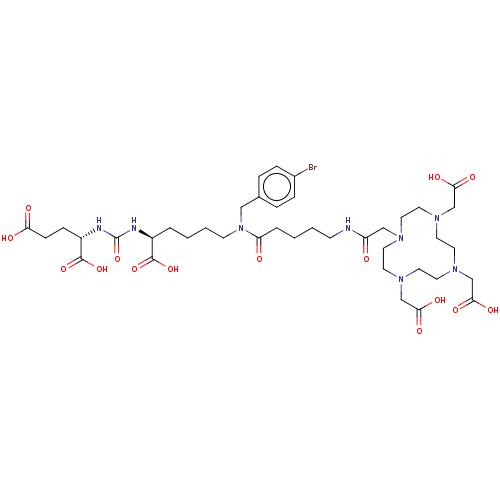 (US11478558, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578401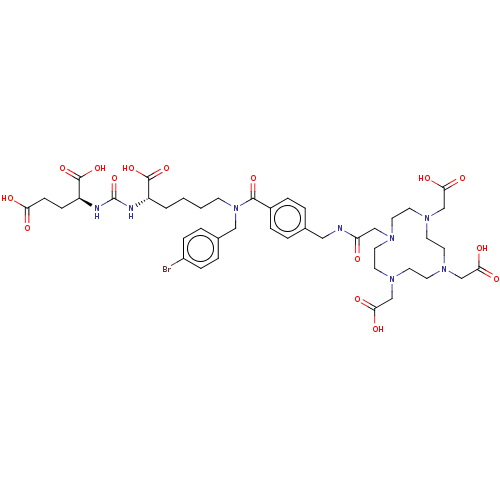 (US11478558, Compound L12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298754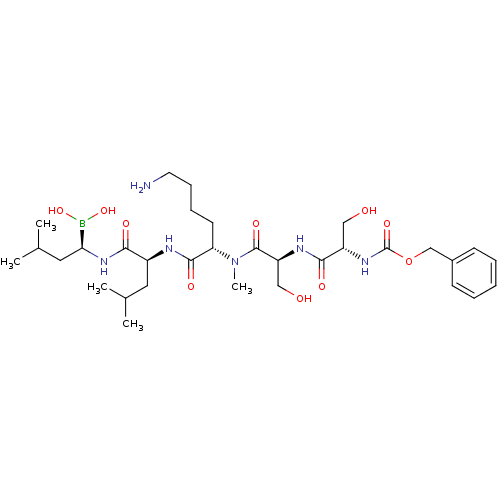 ((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298760 (CHEMBL574766 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298738 (CHEMBL583783 | benzyl(2S,5S,8S,11S,14S)-8-(3-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578402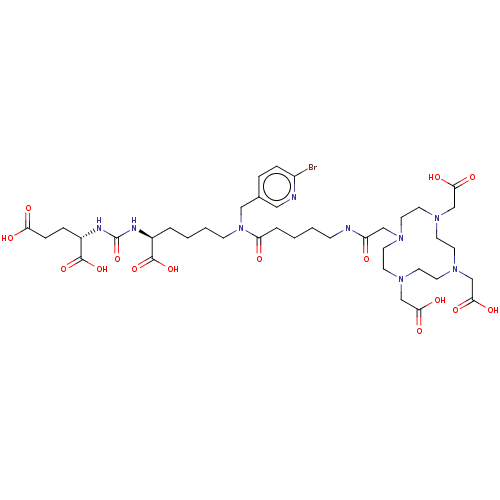 (US11478558, Compound L13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298761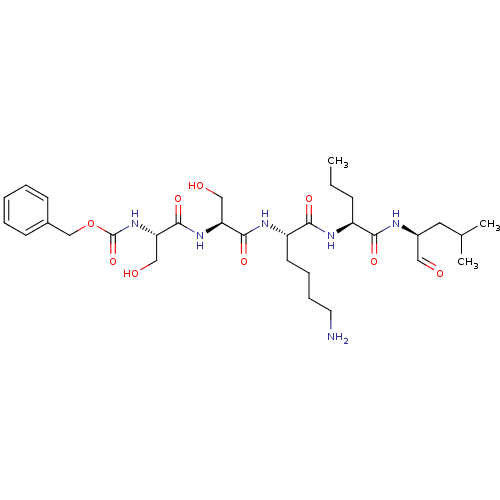 (CHEMBL574788 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456908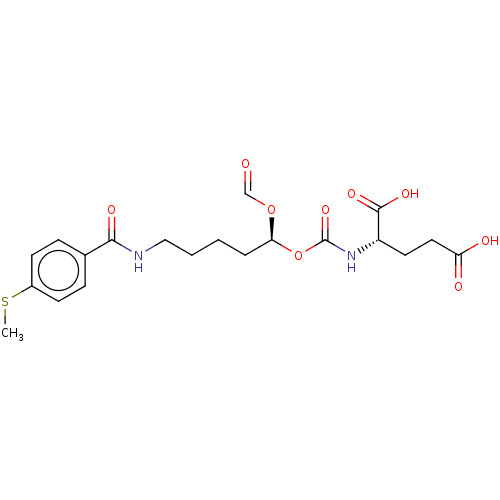 (US10736974, Compound XY-28 | US10736974, Entry 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456908 (US10736974, Compound XY-28 | US10736974, Entry 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304731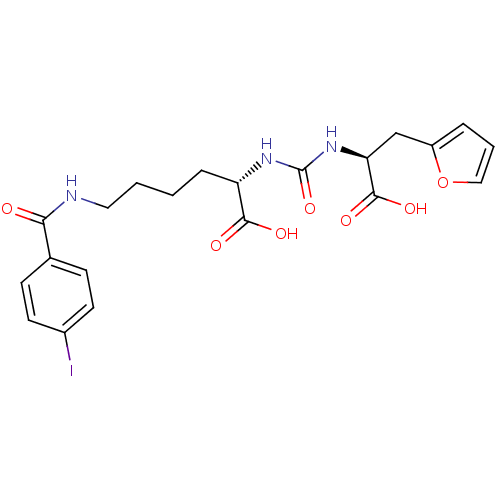 ((S)-2-(3-((S)-1-Carboxy-2-(furan-2-yl)ethyl)ureido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of human cloned glutamate carboxypeptidase 2 | Bioorg Med Chem Lett 20: 392-7 (2010) Article DOI: 10.1016/j.bmcl.2009.10.061 BindingDB Entry DOI: 10.7270/Q2SN092X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304722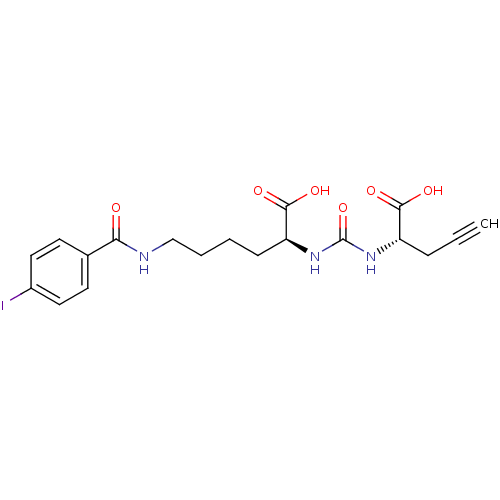 ((S)-2-(3-((S)-1-Carboxybut-3-ynyl)ureido)-6-(4-iod...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of human cloned glutamate carboxypeptidase 2 | Bioorg Med Chem Lett 20: 392-7 (2010) Article DOI: 10.1016/j.bmcl.2009.10.061 BindingDB Entry DOI: 10.7270/Q2SN092X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298757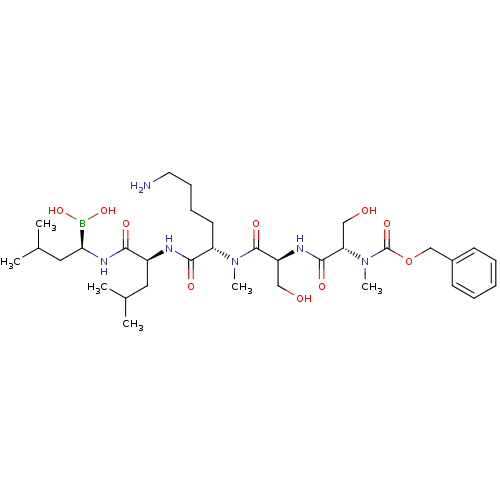 ((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456900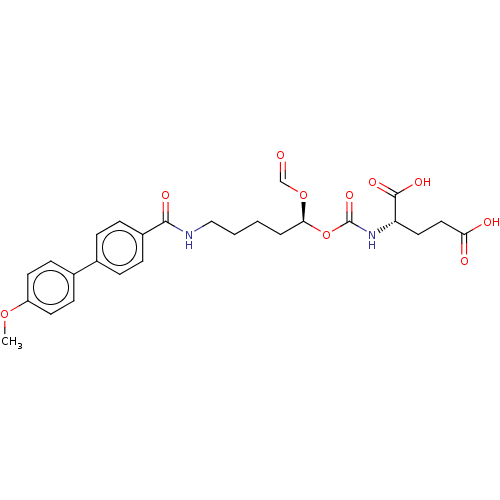 (US10736974, Entry 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298762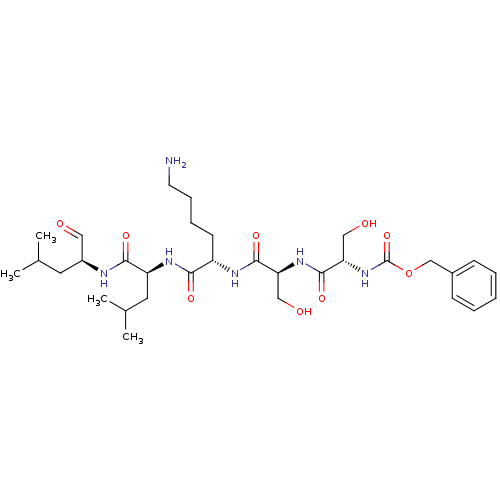 (CHEMBL574924 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298739 (CHEMBL583784 | benzyl(2S,5S,8S,11S,14S)-14-formyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456911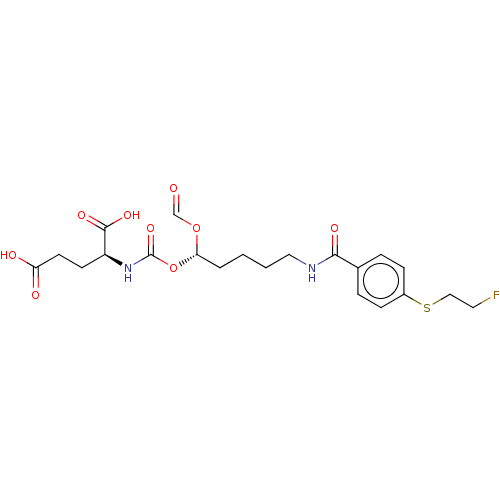 (US10736974, Entry 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostate-specific antigen (Homo sapiens (Human)) | BDBM50298740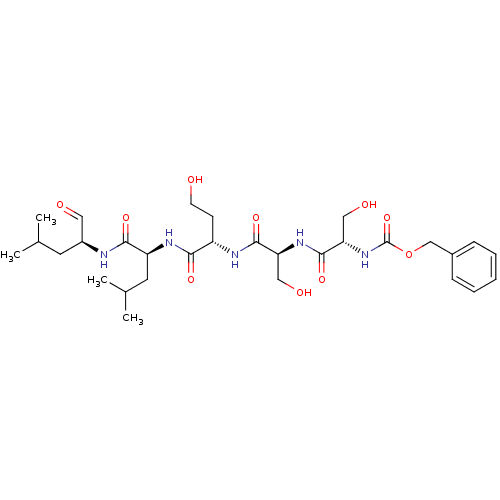 (CHEMBL574324 | benzyl(2S,5S,8S,11S,14S)-14-formyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay | Bioorg Med Chem 17: 4888-93 (2009) Article DOI: 10.1016/j.bmc.2009.06.012 BindingDB Entry DOI: 10.7270/Q2T43T49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 211 total ) | Next | Last >> |