

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051895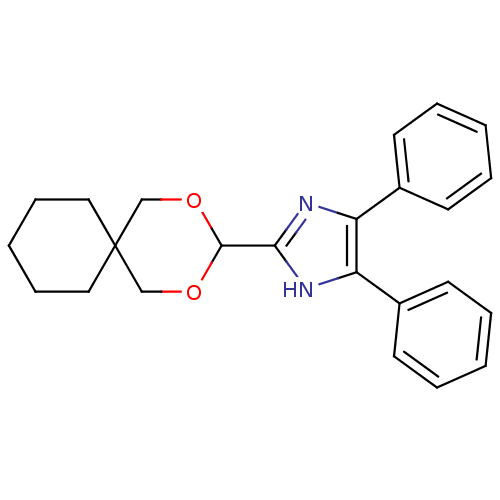 (2-(2,4-Dioxa-spiro[5.5]undec-3-yl)-4,5-diphenyl-1H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase 1 from rat liver. | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085853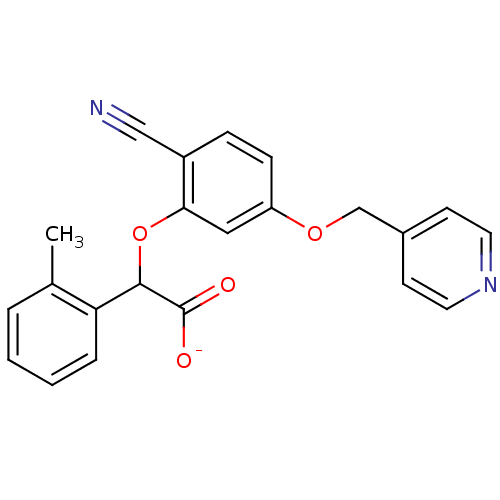 (CHEMBL173251 | Sodium; [2-cyano-5-(pyridin-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065516 ((R)-4-[2-Cyano-5-(pyridin-3-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065508 ((R)-4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085845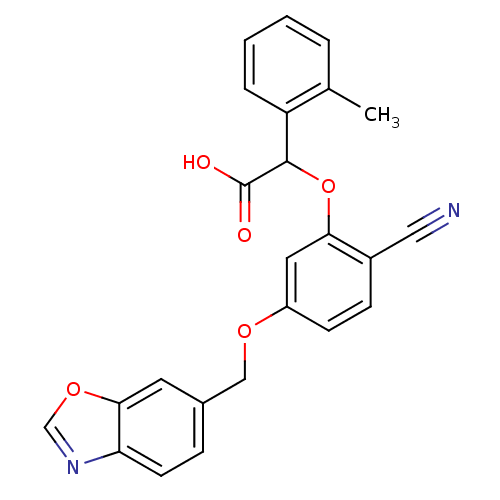 (CHEMBL174235 | [5-(Benzooxazol-6-ylmethoxy)-2-cyan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085859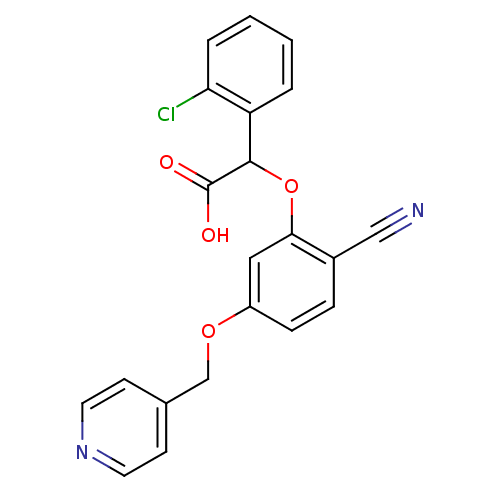 ((2-Chloro-phenyl)-[2-cyano-5-(pyridin-4-ylmethoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065518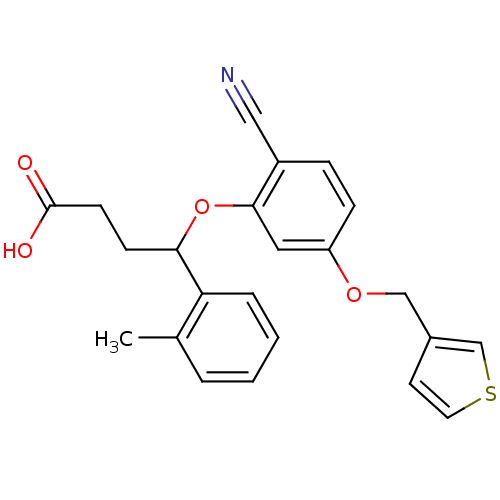 (4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065523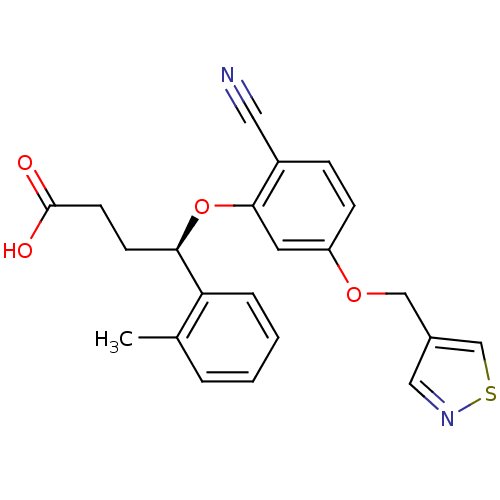 ((R)-4-[2-Cyano-5-(isothiazol-4-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065524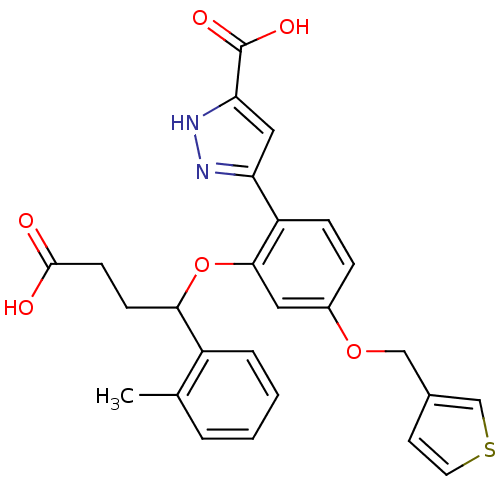 (5-[2-(3-Carboxy-1-o-tolyl-propoxy)-4-(thiophen-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085833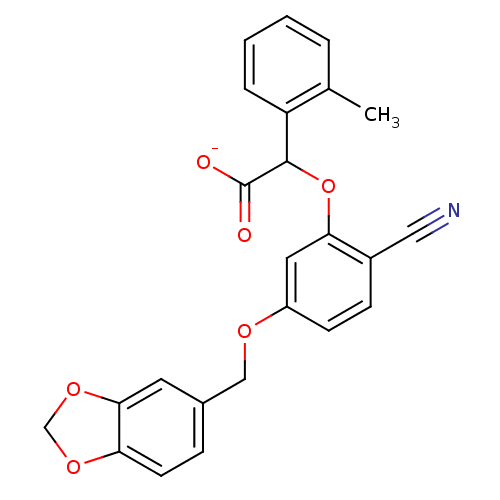 (CHEMBL172144 | Sodium; [5-(benzo[1,3]dioxol-5-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065518 (4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Concentration required to inhibit 50% Et-1 binding to endothelin A receptor in rat A-10 cells. | J Med Chem 41: 2745-53 (1998) Article DOI: 10.1021/jm970847e BindingDB Entry DOI: 10.7270/Q2PN94SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085861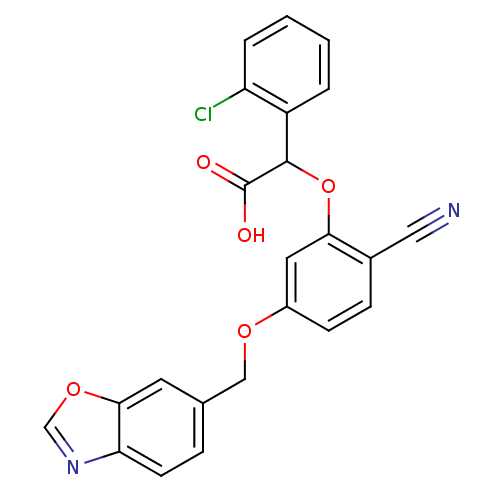 (CHEMBL170681 | [5-(Benzooxazol-6-ylmethoxy)-2-cyan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085831 (CHEMBL174092 | [2-Cyano-5-(pyridin-4-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051894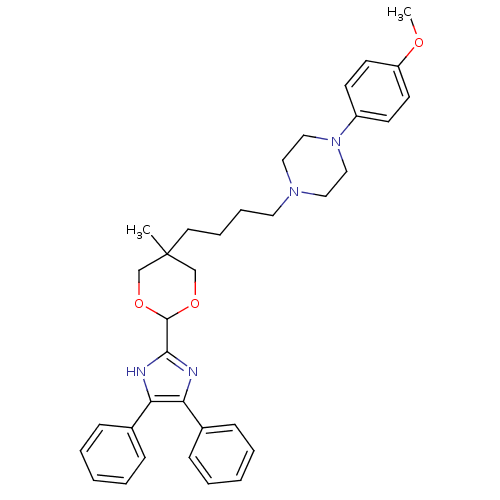 (1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the Acyl coenzyme A:cholesterol acyltransferase 1 ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085830 (CHEMBL173792 | [5-(Benzo[1,3]dioxol-5-ylmethoxy)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085838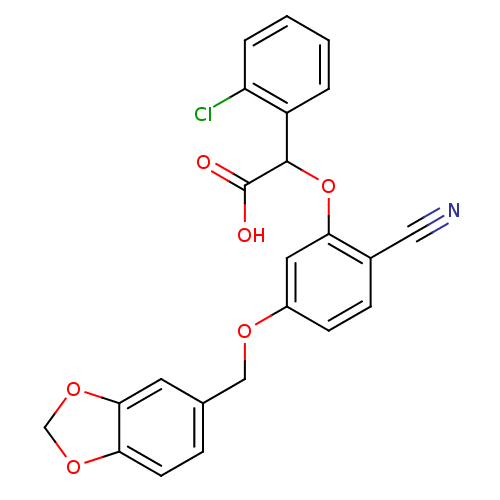 (CHEMBL352536 | [5-(Benzo[1,3]dioxol-5-ylmethoxy)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50329850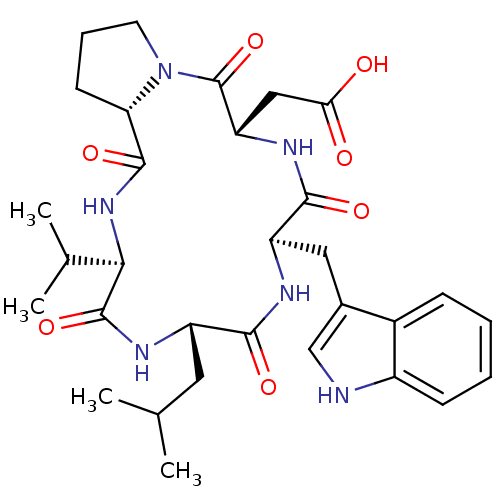 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051897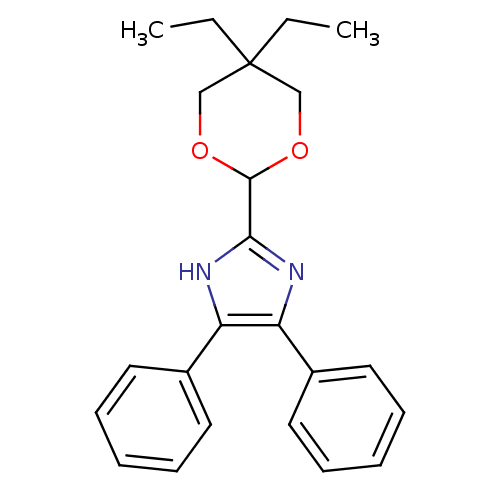 (2-(5,5-Diethyl-[1,3]dioxan-2-yl)-4,5-diphenyl-1H-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase 1 from rat liver. | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085855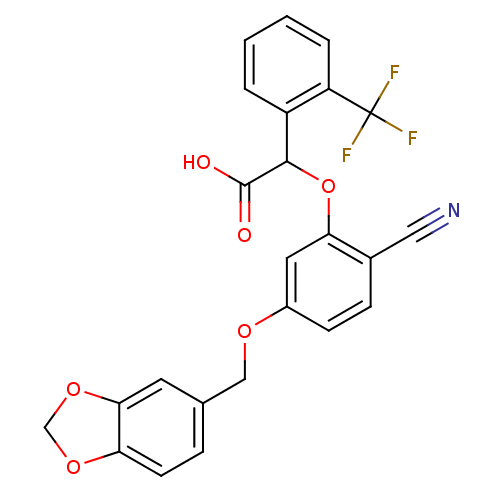 (CHEMBL170202 | [5-(Benzo[1,3]dioxol-5-ylmethoxy)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085840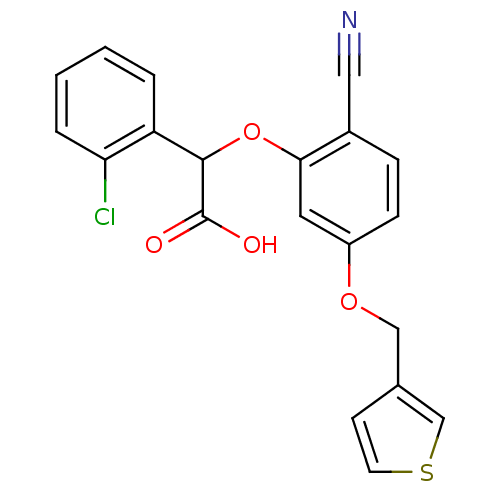 ((2-Chloro-phenyl)-[2-cyano-5-(thiophen-3-ylmethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051881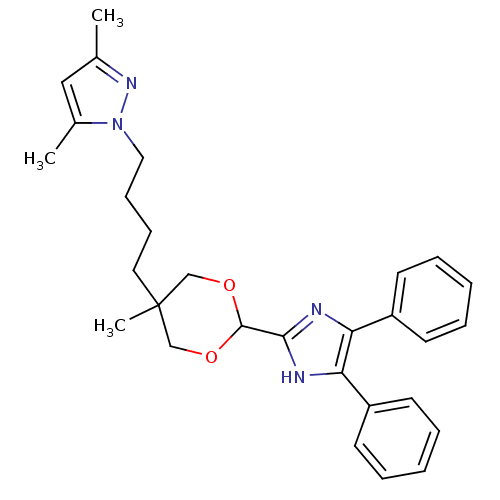 (1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% activity of the rabbit liver Acyl coenzyme A:choleste... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065525 (4-[2-(1H-Pyrazol-3-yl)-5-(thiophen-3-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085835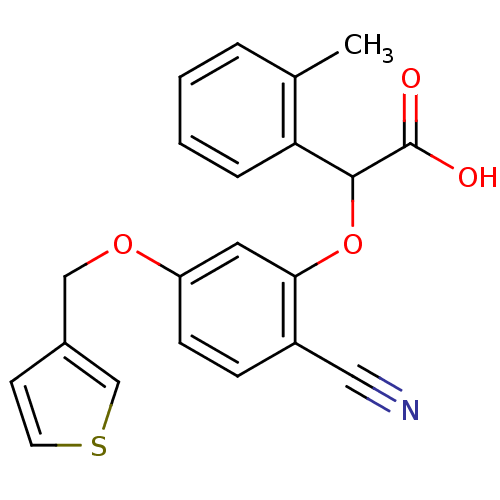 (CHEMBL427294 | [2-Cyano-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065513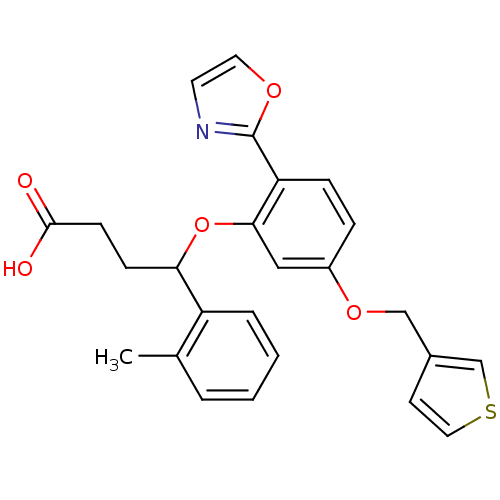 (4-[2-Oxazol-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065515 ((R)-4-[2-Cyano-5-(pyridin-4-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085854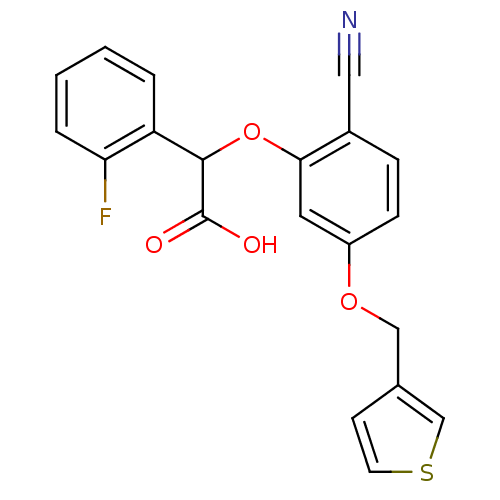 (CHEMBL171638 | [2-Cyano-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085832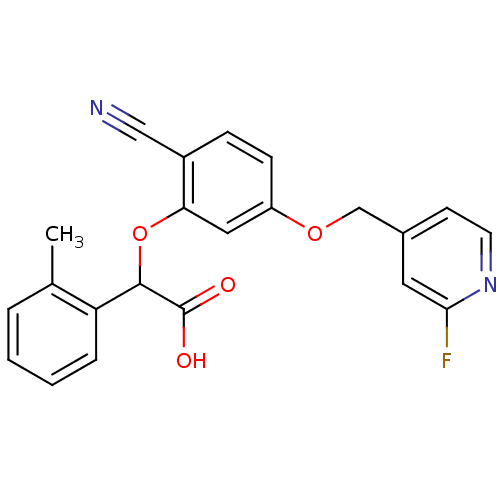 (CHEMBL171513 | [2-Cyano-5-(2-fluoro-pyridin-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051881 (1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% activity of the rabbit artery Acyl coenzyme A:cholest... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase 1 from rat liver. | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065521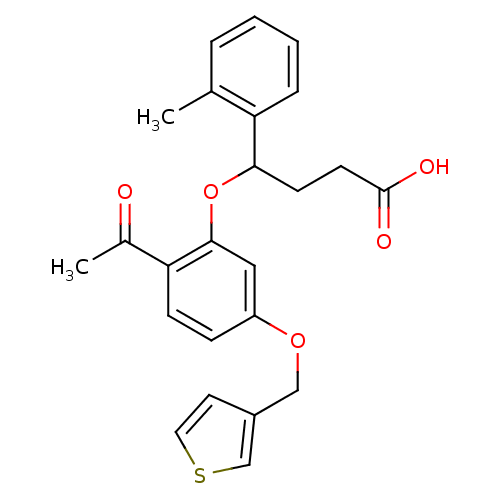 (4-[2-Acetyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065512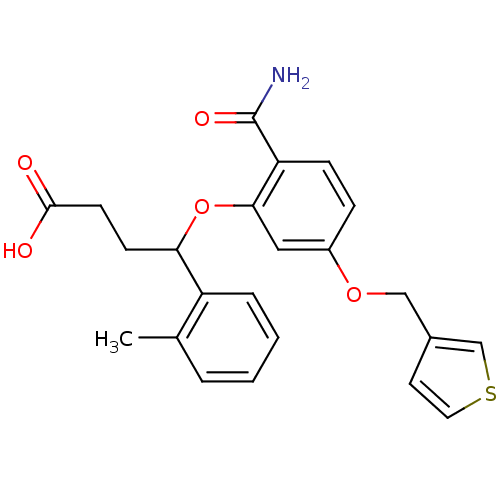 (4-[2-Carbamoyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085864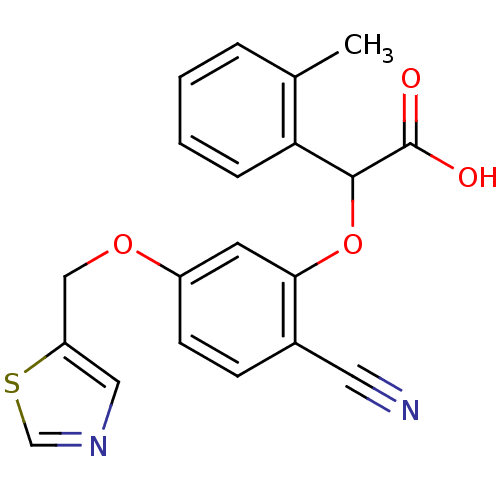 (CHEMBL354740 | [2-Cyano-5-(thiazol-5-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085839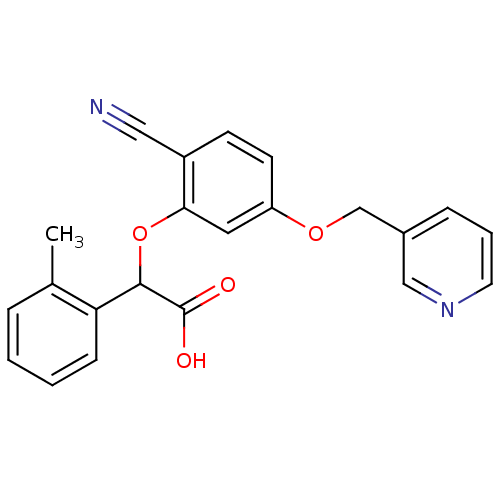 (CHEMBL170348 | [2-Cyano-5-(pyridin-3-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% activity of the rabbit artery Acyl coenzyme A:cholest... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the Acyl coenzyme A:cholesterol acyltransferase 1 ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065520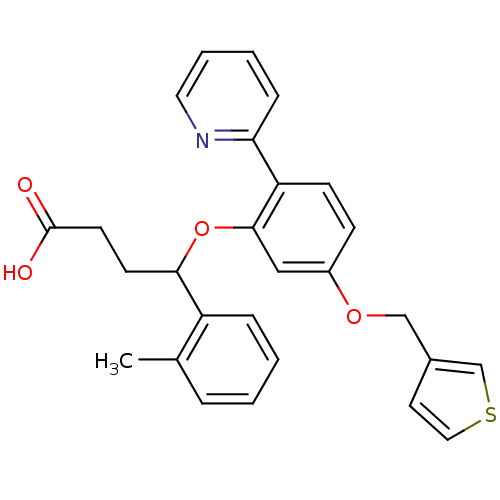 (4-[2-Pyridin-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% activity of the rabbit liver Acyl coenzyme A:choleste... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085856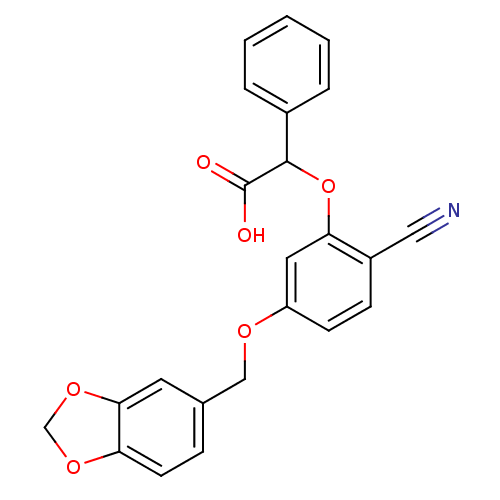 (CHEMBL355413 | [5-(Benzo[1,3]dioxol-5-ylmethoxy)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085858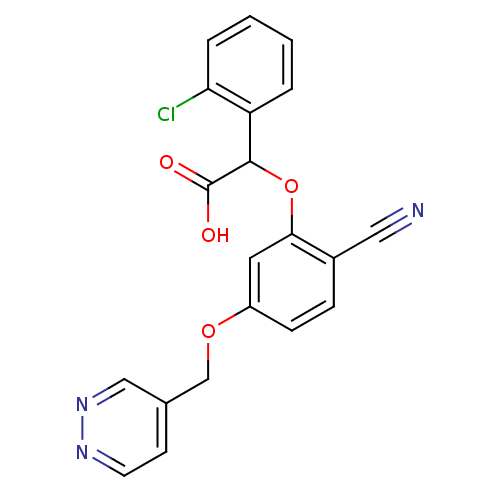 ((2-Chloro-phenyl)-[2-cyano-5-(pyridazin-4-ylmethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085860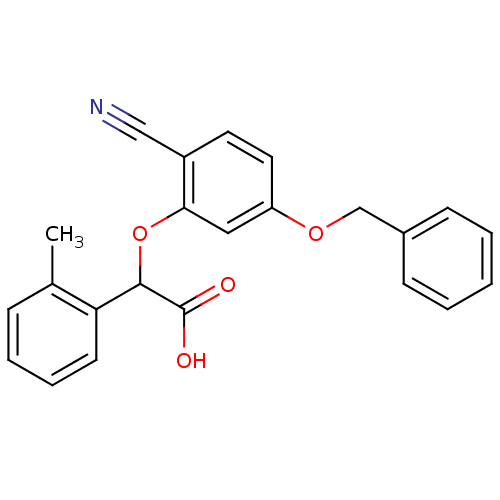 ((5-Benzyloxy-2-cyano-phenoxy)-o-tolyl-acetic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051888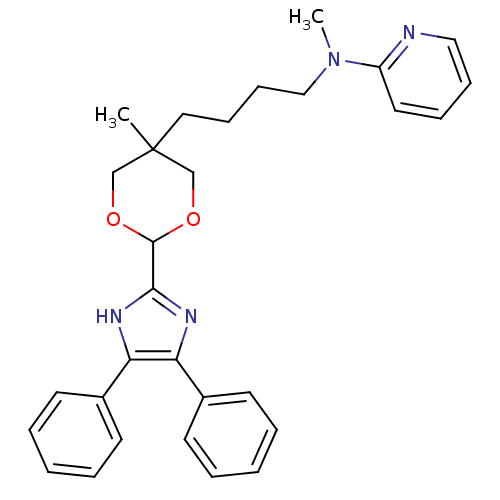 (CHEMBL284378 | {4-[2-(4,5-Diphenyl-1H-imidazol-2-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the Acyl coenzyme A:cholesterol acyltransferase 1 ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051898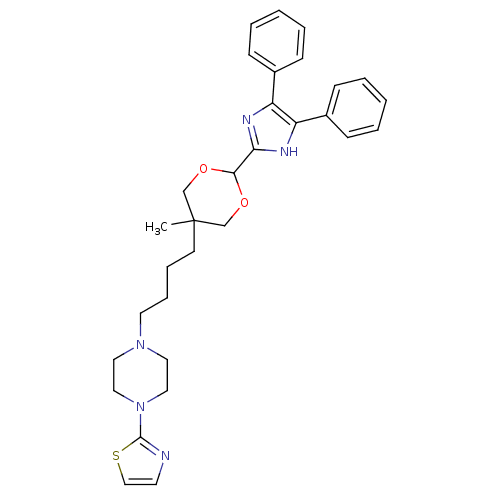 (1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the Acyl coenzyme A:cholesterol acyltransferase 1 ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085844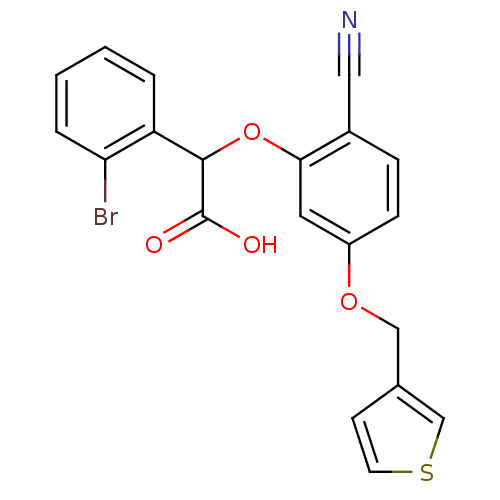 ((2-Bromo-phenyl)-[2-cyano-5-(thiophen-3-ylmethoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085837 (CHEMBL170715 | [2-Cyano-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051883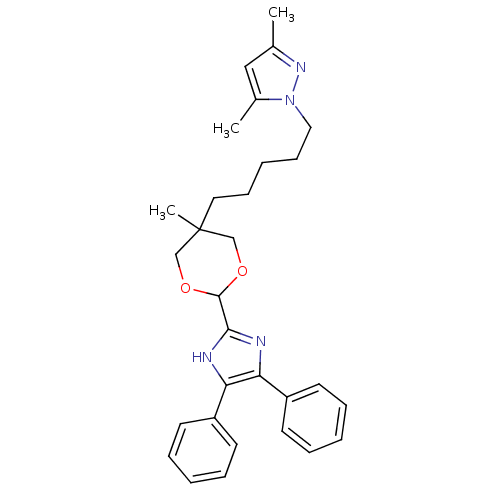 (1-{5-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the activity of human Acyl coenzyme A:cholesterol ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051890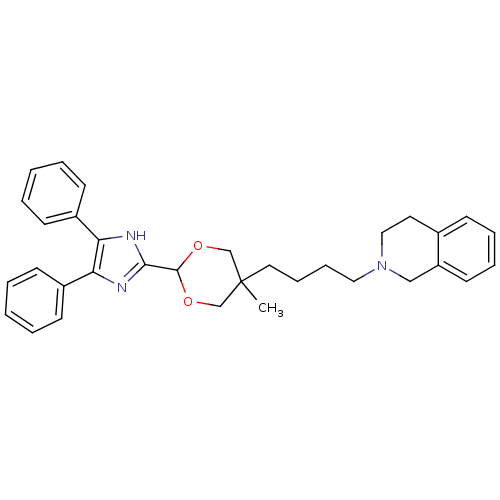 (2-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the Acyl coenzyme A:cholesterol acyltransferase 1 ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085836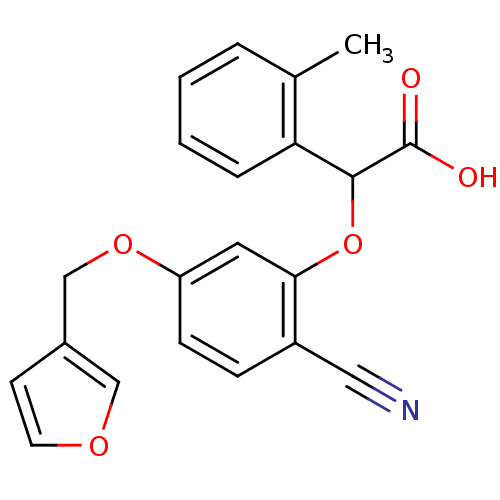 (CHEMBL172412 | [2-Cyano-5-(furan-3-ylmethoxy)-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065522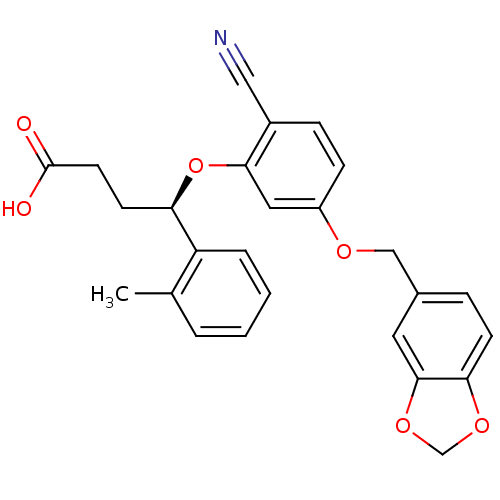 ((R)-4-[5-(Benzo[1,3]dioxol-5-ylmethoxy)-2-cyano-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085852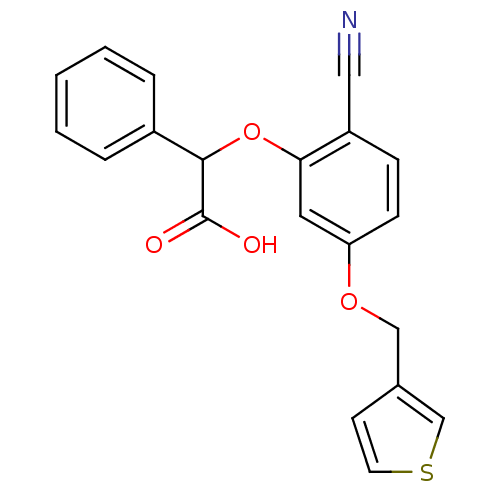 (CHEMBL174229 | [2-Cyano-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051891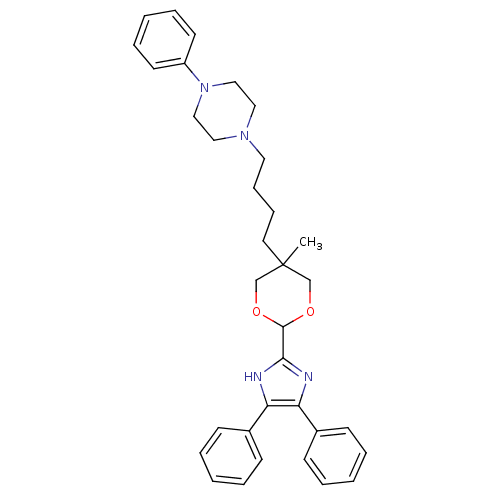 (1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated as concentration required to inhibit 50% of the activity of human Acyl coenzyme A:cholesterol ... | J Med Chem 39: 1423-32 (1996) Article DOI: 10.1021/jm9505876 BindingDB Entry DOI: 10.7270/Q2TB160C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 243 total ) | Next | Last >> |