 Found 35 hits with Last Name = 'qian' and Initial = 'p'
Found 35 hits with Last Name = 'qian' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185416
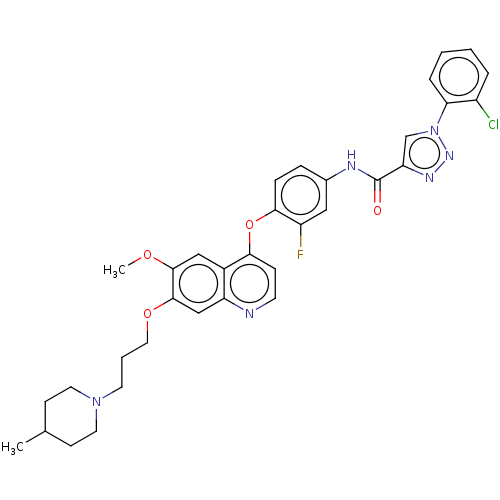
(CHEMBL3823902)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCC(C)CC1 Show InChI InChI=1S/C34H34ClFN6O4/c1-22-11-15-41(16-12-22)14-5-17-45-33-20-27-24(19-32(33)44-2)30(10-13-37-27)46-31-9-8-23(18-26(31)36)38-34(43)28-21-42(40-39-28)29-7-4-3-6-25(29)35/h3-4,6-10,13,18-22H,5,11-12,14-17H2,1-2H3,(H,38,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185386
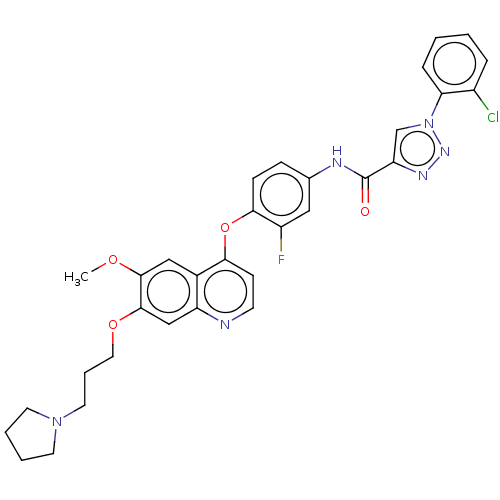
(CHEMBL3824101)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCC1 Show InChI InChI=1S/C32H30ClFN6O4/c1-42-30-18-22-25(19-31(30)43-16-6-15-39-13-4-5-14-39)35-12-11-28(22)44-29-10-9-21(17-24(29)34)36-32(41)26-20-40(38-37-26)27-8-3-2-7-23(27)33/h2-3,7-12,17-20H,4-6,13-16H2,1H3,(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185387
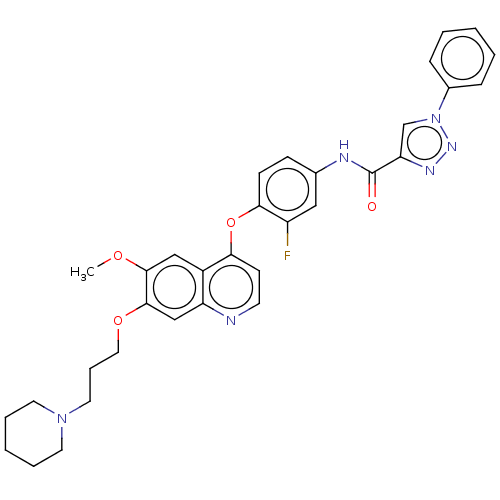
(CHEMBL3823944)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H33FN6O4/c1-42-31-20-25-27(21-32(31)43-18-8-17-39-15-6-3-7-16-39)35-14-13-29(25)44-30-12-11-23(19-26(30)34)36-33(41)28-22-40(38-37-28)24-9-4-2-5-10-24/h2,4-5,9-14,19-22H,3,6-8,15-18H2,1H3,(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-kit (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185417
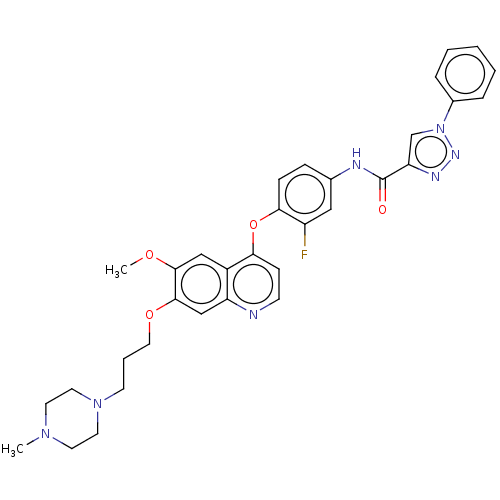
(CHEMBL3823376)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4)cc3F)ccnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C33H34FN7O4/c1-39-14-16-40(17-15-39)13-6-18-44-32-21-27-25(20-31(32)43-2)29(11-12-35-27)45-30-10-9-23(19-26(30)34)36-33(42)28-22-41(38-37-28)24-7-4-3-5-8-24/h3-5,7-12,19-22H,6,13-18H2,1-2H3,(H,36,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185418

(CHEMBL3823581)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C33H33ClFN7O4/c1-40-13-15-41(16-14-40)12-5-17-45-32-20-26-23(19-31(32)44-2)29(10-11-36-26)46-30-9-8-22(18-25(30)35)37-33(43)27-21-42(39-38-27)28-7-4-3-6-24(28)34/h3-4,6-11,18-21H,5,12-17H2,1-2H3,(H,37,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424201
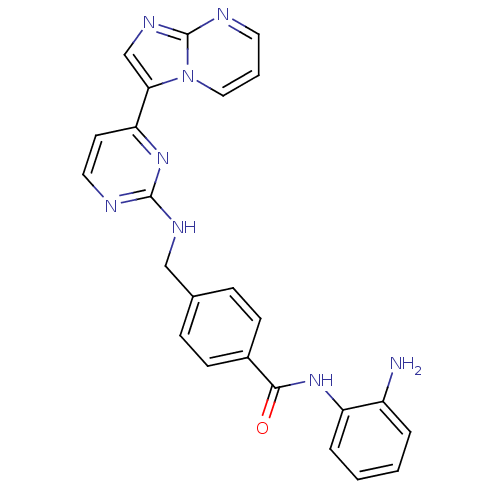
(CHEMBL2312466)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cnc3ncccn23)cc1 Show InChI InChI=1S/C24H20N8O/c25-18-4-1-2-5-19(18)30-22(33)17-8-6-16(7-9-17)14-28-23-26-12-10-20(31-23)21-15-29-24-27-11-3-13-32(21)24/h1-13,15H,14,25H2,(H,30,33)(H,26,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424202

(CHEMBL2312465)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C25H21N7O/c26-19-5-1-2-6-20(19)30-24(33)18-10-8-17(9-11-18)15-29-25-27-13-12-21(31-25)22-16-28-23-7-3-4-14-32(22)23/h1-14,16H,15,26H2,(H,30,33)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424197
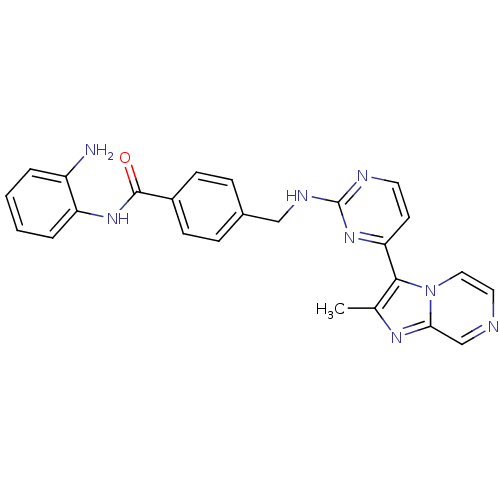
(CHEMBL2312459)Show SMILES Cc1nc2cnccn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2ccccc2N)n1 Show InChI InChI=1S/C25H22N8O/c1-16-23(33-13-12-27-15-22(33)30-16)21-10-11-28-25(32-21)29-14-17-6-8-18(9-7-17)24(34)31-20-5-3-2-4-19(20)26/h2-13,15H,14,26H2,1H3,(H,31,34)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424194
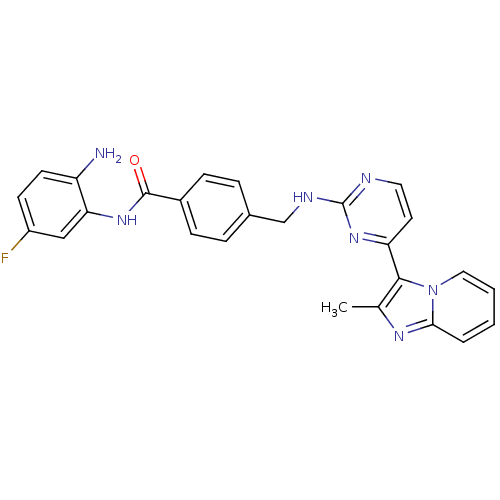
(CHEMBL2312462)Show SMILES Cc1nc2ccccn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2cc(F)ccc2N)n1 Show InChI InChI=1S/C26H22FN7O/c1-16-24(34-13-3-2-4-23(34)31-16)21-11-12-29-26(33-21)30-15-17-5-7-18(8-6-17)25(35)32-22-14-19(27)9-10-20(22)28/h2-14H,15,28H2,1H3,(H,32,35)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424199

(CHEMBL2312457)Show SMILES Cc1nc2ccccn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2ccccc2N)n1 Show InChI InChI=1S/C26H23N7O/c1-17-24(33-15-5-4-8-23(33)30-17)22-13-14-28-26(32-22)29-16-18-9-11-19(12-10-18)25(34)31-21-7-3-2-6-20(21)27/h2-15H,16,27H2,1H3,(H,31,34)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424200

(CHEMBL2312456)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cnc3cnccn23)cc1 Show InChI InChI=1S/C24H20N8O/c25-18-3-1-2-4-19(18)30-23(33)17-7-5-16(6-8-17)13-29-24-27-10-9-20(31-24)21-14-28-22-15-26-11-12-32(21)22/h1-12,14-15H,13,25H2,(H,30,33)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096258
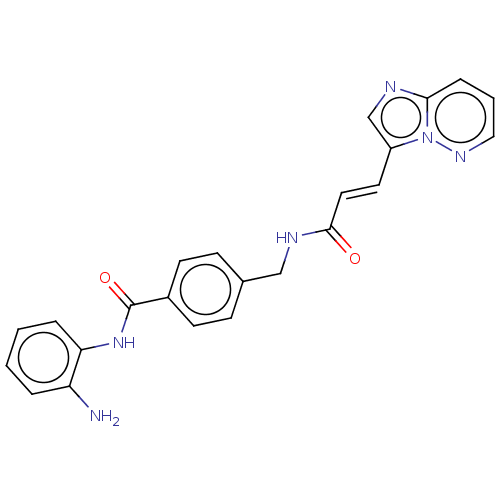
(CHEMBL3593303)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cnc3cccnn23)cc1 Show InChI InChI=1S/C23H20N6O2/c24-19-4-1-2-5-20(19)28-23(31)17-9-7-16(8-10-17)14-26-22(30)12-11-18-15-25-21-6-3-13-27-29(18)21/h1-13,15H,14,24H2,(H,26,30)(H,28,31)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096264
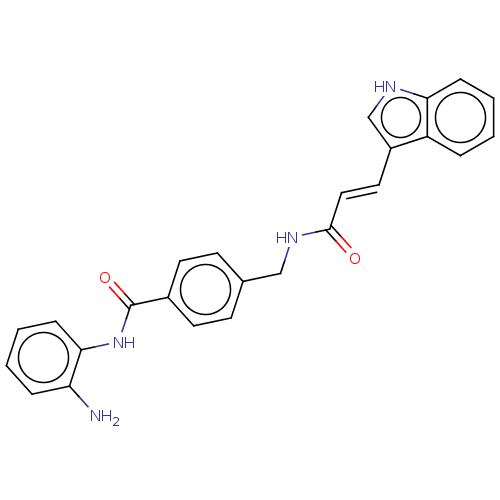
(CHEMBL3593308)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C25H22N4O2/c26-21-6-2-4-8-23(21)29-25(31)18-11-9-17(10-12-18)15-28-24(30)14-13-19-16-27-22-7-3-1-5-20(19)22/h1-14,16,27H,15,26H2,(H,28,30)(H,29,31)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424192
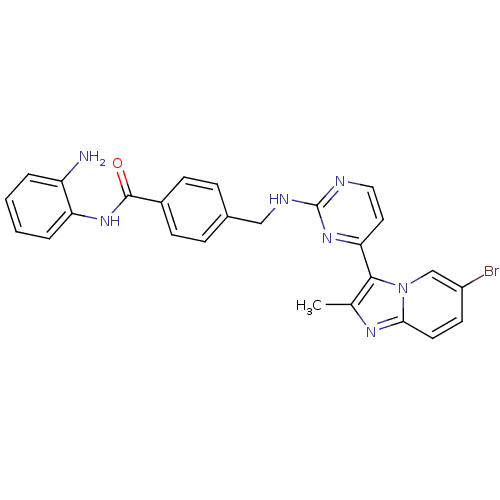
(CHEMBL2312464)Show SMILES Cc1nc2ccc(Br)cn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2ccccc2N)n1 Show InChI InChI=1S/C26H22BrN7O/c1-16-24(34-15-19(27)10-11-23(34)31-16)22-12-13-29-26(33-22)30-14-17-6-8-18(9-7-17)25(35)32-21-5-3-2-4-20(21)28/h2-13,15H,14,28H2,1H3,(H,32,35)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424198

(CHEMBL2312458)Show SMILES Cc1nc2ncccn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2ccccc2N)n1 Show InChI InChI=1S/C25H22N8O/c1-16-22(33-14-4-12-28-25(33)30-16)21-11-13-27-24(32-21)29-15-17-7-9-18(10-8-17)23(34)31-20-6-3-2-5-19(20)26/h2-14H,15,26H2,1H3,(H,31,34)(H,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096260
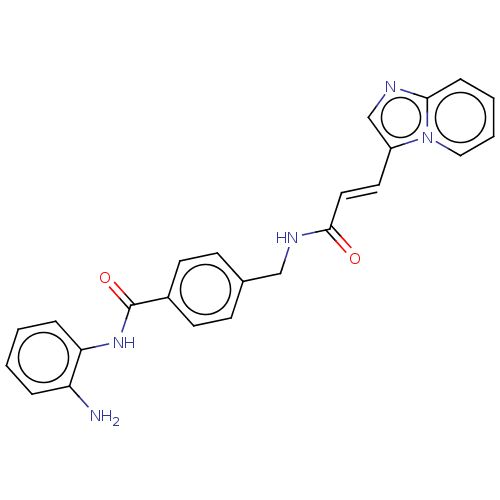
(CHEMBL3593305)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cnc3ccccn23)cc1 Show InChI InChI=1S/C24H21N5O2/c25-20-5-1-2-6-21(20)28-24(31)18-10-8-17(9-11-18)15-27-23(30)13-12-19-16-26-22-7-3-4-14-29(19)22/h1-14,16H,15,25H2,(H,27,30)(H,28,31)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096266

(CHEMBL3593310)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cnc3cccnn23)cc1 Show InChI InChI=1S/C23H19FN6O2/c24-17-7-9-20(19(25)12-17)29-23(32)16-5-3-15(4-6-16)13-27-22(31)10-8-18-14-26-21-2-1-11-28-30(18)21/h1-12,14H,13,25H2,(H,27,31)(H,29,32)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424196

(CHEMBL2312460)Show SMILES Cc1nc2ccccn2c1-c1ccnc(NCCc2ccc(cc2)C(=O)Nc2ccccc2N)n1 Show InChI InChI=1S/C27H25N7O/c1-18-25(34-17-5-4-8-24(34)31-18)23-14-16-30-27(33-23)29-15-13-19-9-11-20(12-10-19)26(35)32-22-7-3-2-6-21(22)28/h2-12,14,16-17H,13,15,28H2,1H3,(H,32,35)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424193
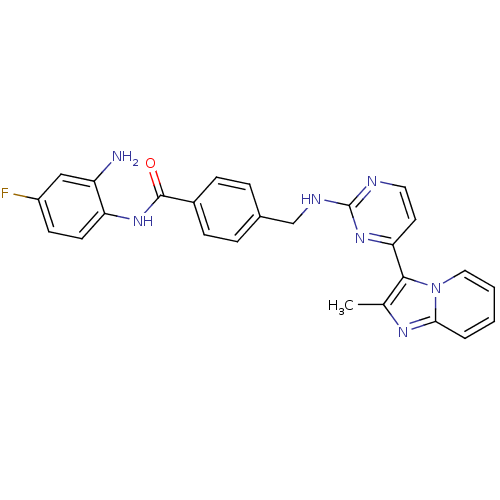
(CHEMBL2312463)Show SMILES Cc1nc2ccccn2c1-c1ccnc(NCc2ccc(cc2)C(=O)Nc2ccc(F)cc2N)n1 Show InChI InChI=1S/C26H22FN7O/c1-16-24(34-13-3-2-4-23(34)31-16)22-11-12-29-26(33-22)30-15-17-5-7-18(8-6-17)25(35)32-21-10-9-19(27)14-20(21)28/h2-14H,15,28H2,1H3,(H,32,35)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096267

(CHEMBL3593311)Show SMILES Nc1ccc(F)cc1NC(=O)c1ccc(CNC(=O)\C=C\c2cnc3cccnn23)cc1 Show InChI InChI=1S/C23H19FN6O2/c24-17-7-9-19(25)20(12-17)29-23(32)16-5-3-15(4-6-16)13-27-22(31)10-8-18-14-26-21-2-1-11-28-30(18)21/h1-12,14H,13,25H2,(H,27,31)(H,29,32)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096259
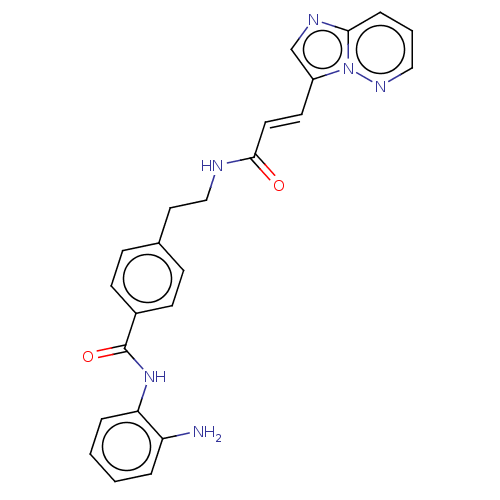
(CHEMBL3593304)Show SMILES Nc1ccccc1NC(=O)c1ccc(CCNC(=O)\C=C\c2cnc3cccnn23)cc1 Show InChI InChI=1S/C24H22N6O2/c25-20-4-1-2-5-21(20)29-24(32)18-9-7-17(8-10-18)13-15-26-23(31)12-11-19-16-27-22-6-3-14-28-30(19)22/h1-12,14,16H,13,15,25H2,(H,26,31)(H,29,32)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50424195

(CHEMBL2312461)Show SMILES Nc1ccccc1NC(=O)c1ccc(CCNc2nccc(n2)-c2cnc3ncccn23)cc1 Show InChI InChI=1S/C25H22N8O/c26-19-4-1-2-5-20(19)31-23(34)18-8-6-17(7-9-18)10-13-27-24-28-14-11-21(32-24)22-16-30-25-29-12-3-15-33(22)25/h1-9,11-12,14-16H,10,13,26H2,(H,31,34)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096265

(CHEMBL3593309)Show SMILES Nc1ccccc1NC(=O)c1ccc(CCNC(=O)\C=C\c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C26H24N4O2/c27-22-6-2-4-8-24(22)30-26(32)19-11-9-18(10-12-19)15-16-28-25(31)14-13-20-17-29-23-7-3-1-5-21(20)23/h1-14,17,29H,15-16,27H2,(H,28,31)(H,30,32)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096262
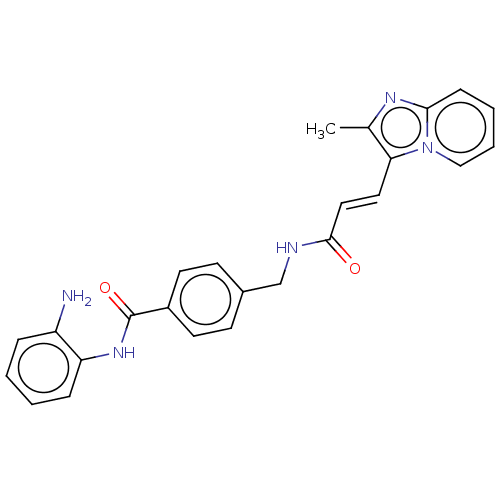
(CHEMBL3593246)Show SMILES Cc1nc2ccccn2c1\C=C\C(=O)NCc1ccc(cc1)C(=O)Nc1ccccc1N Show InChI InChI=1S/C25H23N5O2/c1-17-22(30-15-5-4-8-23(30)28-17)13-14-24(31)27-16-18-9-11-19(12-10-18)25(32)29-21-7-3-2-6-20(21)26/h2-15H,16,26H2,1H3,(H,27,31)(H,29,32)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096263

(CHEMBL3593307)Show SMILES Cc1nc2ccccn2c1\C=C\C(=O)NCCc1ccc(cc1)C(=O)Nc1ccccc1N Show InChI InChI=1S/C26H25N5O2/c1-18-23(31-17-5-4-8-24(31)29-18)13-14-25(32)28-16-15-19-9-11-20(12-10-19)26(33)30-22-7-3-2-6-21(22)27/h2-14,17H,15-16,27H2,1H3,(H,28,32)(H,30,33)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24624
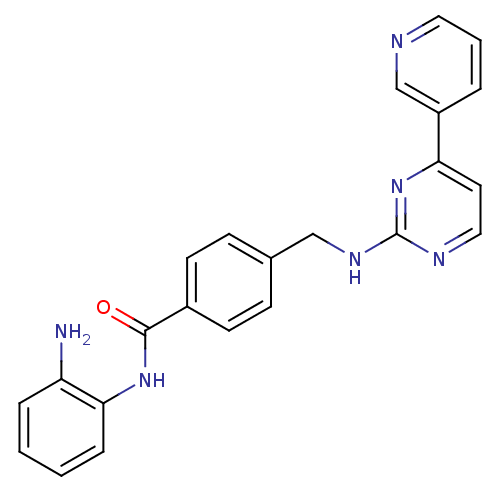
(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital of Chinese People's Liberation Army
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 23: 179-82 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.114
BindingDB Entry DOI: 10.7270/Q29K4CJH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50096261
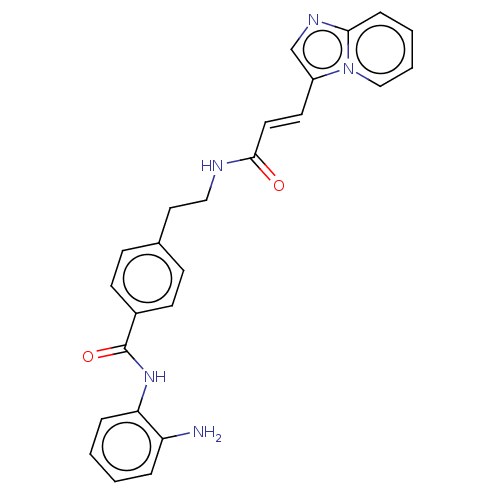
(CHEMBL3593306)Show SMILES Nc1ccccc1NC(=O)c1ccc(CCNC(=O)\C=C\c2cnc3ccccn23)cc1 Show InChI InChI=1S/C25H23N5O2/c26-21-5-1-2-6-22(21)29-25(32)19-10-8-18(9-11-19)14-15-27-24(31)13-12-20-17-28-23-7-3-4-16-30(20)23/h1-13,16-17H,14-15,26H2,(H,27,31)(H,29,32)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Military Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 5 mins followed by fluorogenic substrate addition measured after 30 mins by microplate reader a... |
Eur J Med Chem 100: 270-6 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.045
BindingDB Entry DOI: 10.7270/Q2GT5PXH |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of Ron (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50185415

(CHEMBL3823520)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(nn4)-c4ccccc4Cl)cc3F)ccnc2cc1OCCCN1CCCCC1 Show InChI InChI=1S/C33H32ClFN6O4/c1-43-31-19-23-26(20-32(31)44-17-7-16-40-14-5-2-6-15-40)36-13-12-29(23)45-30-11-10-22(18-25(30)35)37-33(42)27-21-41(39-38-27)28-9-4-3-8-24(28)34/h3-4,8-13,18-21H,2,5-7,14-17H2,1H3,(H,37,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 529 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 30 mins by HTRF assay |
Eur J Med Chem 119: 96-108 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.035
BindingDB Entry DOI: 10.7270/Q24J0H2G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data