 Found 37 hits with Last Name = 'reineke' and Initial = 'u'
Found 37 hits with Last Name = 'reineke' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466499
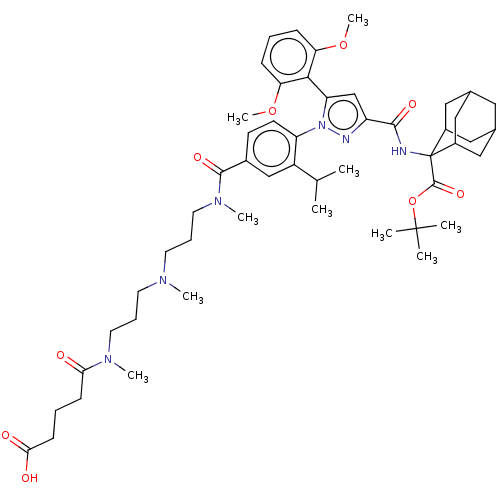
(US10799605, Example 4)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCCC(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |TLB:48:49:51:54.55.53,THB:49:50:57.58.56:53,49:57:51.50.55:53,56:57:51:54.55.53,56:54:51:49.57.58,59:49:51:54.55.53,(-8.99,4.14,;-7.9,3.05,;-8.3,1.56,;-9.79,1.16,;-10.19,-.33,;-9.1,-1.42,;-7.61,-1.02,;-6.52,-2.11,;-6.92,-3.59,;-7.21,.47,;-5.73,.87,;-5.25,2.33,;-3.71,2.33,;-3.24,.87,;-4.48,-.04,;-4.48,-1.58,;-5.81,-2.35,;-5.81,-3.89,;-4.48,-4.66,;-3.15,-3.89,;-3.15,-2.35,;-1.81,-1.58,;-.48,-2.35,;-1.81,-.04,;-4.48,-6.2,;-5.81,-6.97,;-3.15,-6.97,;-3.15,-8.51,;-1.81,-6.2,;-.48,-6.97,;.85,-6.2,;2.19,-6.97,;2.19,-8.51,;3.52,-6.2,;4.85,-6.97,;6.19,-6.2,;7.52,-6.97,;7.52,-8.51,;8.86,-6.2,;10.19,-6.97,;8.86,-4.66,;7.52,-3.89,;7.52,-2.35,;6.19,-1.58,;6.19,-.04,;4.85,-2.35,;-2.62,3.42,;-3.02,4.91,;-1.13,3.02,;-.05,4.11,;-.69,5.51,;-1.96,6.39,;-1.45,7.84,;-.06,8.51,;1.27,7.86,;.63,6.46,;1.78,6.41,;.46,5.57,;-.8,6.44,;1.29,3.34,;2.62,4.11,;1.29,1.8,;2.62,1.03,;2.62,-.51,;3.96,1.8,;3.96,.26,)| Show InChI InChI=1S/C51H72N6O9/c1-32(2)38-30-35(48(62)56(8)24-14-22-54(6)21-13-23-55(7)44(58)17-12-18-45(59)60)19-20-40(38)57-41(46-42(64-9)15-11-16-43(46)65-10)31-39(53-57)47(61)52-51(49(63)66-50(3,4)5)36-26-33-25-34(28-36)29-37(51)27-33/h11,15-16,19-20,30-34,36-37H,12-14,17-18,21-29H2,1-10H3,(H,52,61)(H,59,60) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466500
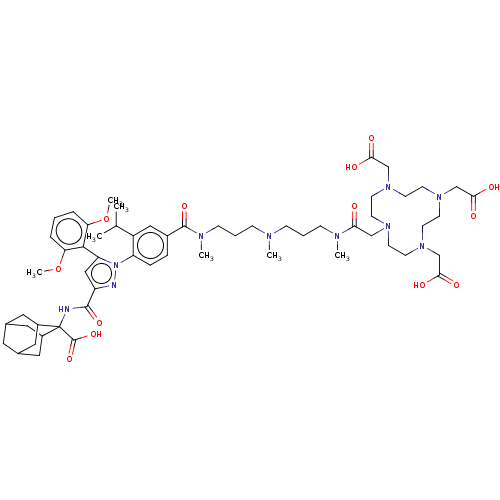
(US10799605, Example 5)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:67:68:70:73.74.72,THB:75:76:70:73.74.72,75:73:70:68.76.77,78:68:70:73.74.72,68:69:76.77.75:72,68:76:70.69.74:72,(-13.42,6.92,;-12.33,5.83,;-12.73,4.34,;-14.22,3.94,;-14.62,2.45,;-13.53,1.36,;-12.04,1.76,;-10.95,.67,;-11.35,-.81,;-11.64,3.25,;-10.15,3.65,;-9.68,5.11,;-8.14,5.11,;-7.66,3.65,;-8.91,2.74,;-8.91,1.2,;-10.24,.43,;-10.24,-1.11,;-8.91,-1.88,;-7.57,-1.11,;-7.57,.43,;-6.24,1.2,;-4.91,.43,;-6.24,2.74,;-8.91,-3.42,;-10.24,-4.19,;-7.57,-4.19,;-7.57,-5.73,;-6.24,-3.42,;-4.91,-4.19,;-3.57,-3.42,;-2.24,-4.19,;-2.24,-5.73,;-.91,-3.42,;.43,-4.19,;1.76,-3.42,;3.1,-4.19,;3.1,-5.73,;4.43,-3.42,;4.43,-1.88,;5.97,-3.42,;7.3,-4.19,;8.1,-2.87,;9.68,-2.86,;10.4,-4.18,;11.17,-2.85,;10.4,-1.51,;8.86,-1.51,;11.17,-.18,;11.7,-4.95,;11.72,-6.52,;10.41,-7.28,;11.74,-8.05,;13.08,-7.28,;13.08,-5.74,;14.62,-7.28,;9.59,-8.6,;8.01,-8.61,;7.31,-7.29,;6.54,-8.62,;7.31,-9.95,;8.85,-9.95,;6.54,-11.29,;6.03,-6.49,;6.01,-4.91,;-7.05,6.2,;-7.45,7.69,;-5.56,5.8,;-4.47,6.89,;-5.12,8.29,;-6.38,9.17,;-5.87,10.62,;-4.48,11.29,;-3.16,10.64,;-3.8,9.24,;-2.64,9.19,;-3.96,8.35,;-5.23,9.22,;-3.14,6.12,;-3.14,4.58,;-1.81,6.89,)| Show InChI InChI=1S/C58H84N10O13/c1-38(2)44-32-41(13-14-46(44)68-47(54-48(80-6)11-8-12-49(54)81-7)33-45(60-68)55(76)59-58(57(78)79)42-28-39-27-40(30-42)31-43(58)29-39)56(77)63(5)18-10-16-61(3)15-9-17-62(4)50(69)34-64-19-21-65(35-51(70)71)23-25-67(37-53(74)75)26-24-66(22-20-64)36-52(72)73/h8,11-14,32-33,38-40,42-43H,9-10,15-31,34-37H2,1-7H3,(H,59,76)(H,70,71)(H,72,73)(H,74,75)(H,78,79) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466502
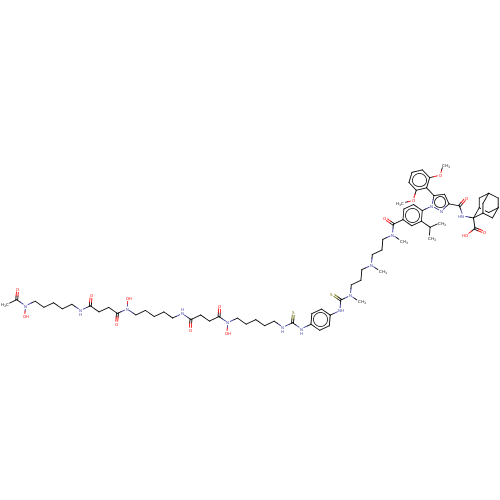
(US10799605, Example 13)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=S)Nc1ccc(NC(=S)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(C)=O)cc1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:91:92:94:97.98.96,THB:92:93:100.101.99:96,92:100:94.93.98:96,99:100:94:97.98.96,99:97:94:92.100.101,102:92:94:97.98.96,(-20.33,6.06,;-19.24,4.97,;-19.64,3.48,;-21.13,3.09,;-21.53,1.6,;-20.44,.51,;-18.95,.91,;-17.86,-.18,;-18.26,-1.67,;-18.55,2.39,;-17.06,2.79,;-16.59,4.26,;-15.05,4.26,;-14.57,2.79,;-15.82,1.89,;-15.82,.35,;-17.15,-.42,;-17.15,-1.96,;-15.82,-2.73,;-14.48,-1.96,;-14.48,-.42,;-13.15,.35,;-11.82,-.42,;-13.15,1.89,;-15.82,-4.27,;-17.15,-5.04,;-14.48,-5.04,;-14.48,-6.58,;-13.15,-4.27,;-11.82,-5.04,;-10.48,-4.27,;-9.15,-5.04,;-9.15,-6.58,;-7.82,-4.27,;-6.48,-5.04,;-5.15,-4.27,;-3.81,-5.04,;-3.81,-6.58,;-2.48,-4.27,;-2.48,-2.73,;-1.15,-5.04,;.19,-4.27,;1.52,-5.04,;2.85,-4.27,;2.85,-2.73,;4.19,-1.96,;5.52,-2.73,;5.52,-4.27,;6.86,-1.96,;8.19,-1.19,;9.52,-1.96,;10.86,-1.19,;12.19,-1.96,;12.19,-3.5,;13.52,-4.27,;14.86,-3.5,;13.52,-5.81,;12.19,-6.58,;14.86,-6.58,;14.86,-8.12,;16.19,-8.89,;16.19,-10.43,;17.52,-8.12,;18.86,-8.89,;20.19,-8.12,;20.19,-6.58,;21.53,-5.81,;21.53,-4.27,;20.19,-3.5,;18.86,-4.27,;20.19,-1.96,;21.53,-1.19,;18.86,-1.19,;18.86,.35,;20.19,1.12,;21.53,.35,;20.19,2.66,;18.86,3.43,;17.52,2.66,;16.19,3.43,;14.86,2.66,;14.86,1.12,;13.52,.35,;13.52,-1.19,;12.19,1.12,;10.86,.35,;12.19,2.66,;1.52,-1.96,;.19,-2.73,;-13.96,5.35,;-14.36,6.83,;-12.47,4.95,;-11.38,6.04,;-12.03,7.44,;-13.29,8.31,;-12.78,9.77,;-11.39,10.43,;-10.07,9.78,;-10.71,8.38,;-9.55,8.33,;-10.87,7.49,;-12.14,8.37,;-10.05,5.27,;-10.05,3.62,;-8.71,6.04,)| Show InChI InChI=1S/C75H110N14O14S2/c1-50(2)59-48-54(23-28-61(59)89-62(69-63(102-7)21-18-22-64(69)103-8)49-60(82-89)70(95)81-75(72(97)98)55-44-52-43-53(46-55)47-56(75)45-52)71(96)84(5)38-19-36-83(4)37-20-39-85(6)74(105)80-58-26-24-57(25-27-58)79-73(104)78-35-14-11-17-42-88(101)68(94)32-30-66(92)77-34-13-10-16-41-87(100)67(93)31-29-65(91)76-33-12-9-15-40-86(99)51(3)90/h18,21-28,48-50,52-53,55-56,99-101H,9-17,19-20,29-47H2,1-8H3,(H,76,91)(H,77,92)(H,80,105)(H,81,95)(H,97,98)(H2,78,79,104) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466505
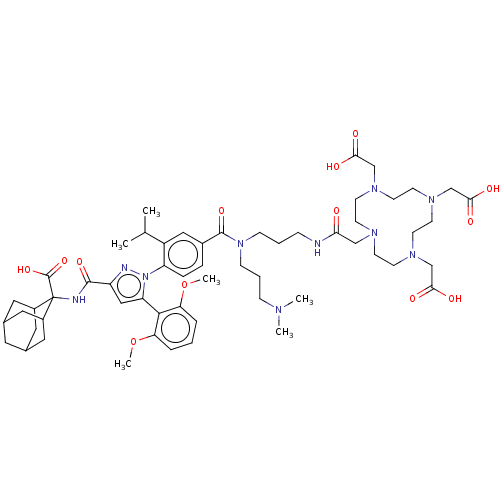
(US10799605, Example 19)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:77:67:76:72.74.71,73:72:76:69.67.68,73:68:76:72.74.71,THB:66:67:76:72.74.71,67:75:69.73.68:71,67:68:76.74.75:71,(-10.5,7.02,;-9.41,5.93,;-9.8,4.44,;-11.29,4.04,;-11.69,2.56,;-10.6,1.47,;-9.11,1.86,;-8.03,.78,;-8.42,-.71,;-8.72,3.35,;-7.23,3.75,;-6.75,5.22,;-5.21,5.22,;-4.74,3.75,;-5.98,2.85,;-5.98,1.31,;-7.32,.54,;-7.32,-1,;-5.98,-1.77,;-4.65,-1,;-4.65,.54,;-3.32,1.31,;-3.32,2.85,;-1.98,.54,;-5.98,-3.31,;-7.32,-4.08,;-4.65,-4.08,;-3.32,-3.31,;-1.98,-4.08,;-.65,-3.31,;.69,-4.08,;2.02,-3.31,;2.02,-1.77,;3.56,-3.31,;4.65,-4.4,;5.42,-3.07,;6.96,-3.07,;7.73,-4.4,;9.06,-3.63,;8.29,-2.3,;6.75,-2.3,;8.69,-.81,;9.06,-5.17,;9.06,-6.71,;7.73,-7.48,;8.82,-8.57,;10.15,-7.8,;10.15,-6.26,;11.69,-7.8,;6.96,-8.82,;5.42,-8.82,;4.65,-7.48,;3.88,-8.82,;4.65,-10.15,;6.19,-10.15,;3.88,-11.48,;3.31,-6.71,;3.31,-5.17,;-4.65,-5.62,;-3.32,-6.39,;-3.32,-7.93,;-1.98,-8.7,;-1.98,-10.24,;-.65,-7.93,;-4.12,6.3,;-4.52,7.79,;-2.64,5.91,;-1.55,6.99,;-1.23,8.49,;-2.5,9.25,;-3.25,10.59,;-2.16,11.48,;-.86,10.92,;-.09,9.58,;-1.23,9.42,;-2.32,8.33,;-3.65,9.11,;-.21,6.22,;-.21,4.68,;1.12,6.99,)| Show InChI InChI=1S/C57H82N10O13/c1-37(2)43-31-40(12-13-45(43)67-46(53-47(79-5)10-7-11-48(53)80-6)32-44(60-67)54(75)59-57(56(77)78)41-27-38-26-39(29-41)30-42(57)28-38)55(76)66(17-9-15-61(3)4)16-8-14-58-49(68)33-62-18-20-63(34-50(69)70)22-24-65(36-52(73)74)25-23-64(21-19-62)35-51(71)72/h7,10-13,31-32,37-39,41-42H,8-9,14-30,33-36H2,1-6H3,(H,58,68)(H,59,75)(H,69,70)(H,71,72)(H,73,74)(H,77,78) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466501
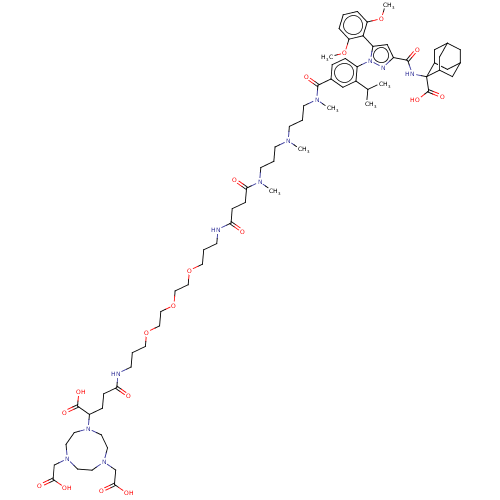
(US10799605, Example 12)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCC(=O)NCCCOCCOCCOCCCNC(=O)CCC(N1CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:86:87:89:92.93.91,THB:87:88:95.96.94:91,87:95:89.88.93:91,94:95:89:92.93.91,94:92:89:87.95.96,97:87:89:92.93.91,(-12.72,2.45,;-11.95,1.11,;-12.72,-.22,;-14.26,-.22,;-15.03,-1.56,;-14.26,-2.89,;-12.72,-2.89,;-11.95,-4.22,;-12.72,-5.56,;-11.95,-1.56,;-10.41,-1.56,;-9.94,-.09,;-8.4,-.09,;-7.92,-1.56,;-9.17,-2.46,;-9.17,-4,;-10.5,-4.77,;-10.5,-6.31,;-9.17,-7.08,;-7.84,-6.31,;-7.84,-4.77,;-6.5,-4,;-5.17,-4.77,;-6.5,-2.46,;-9.17,-8.62,;-10.5,-9.39,;-7.84,-9.39,;-7.84,-10.93,;-6.5,-8.62,;-5.17,-9.39,;-3.83,-8.62,;-2.5,-9.39,;-2.5,-10.93,;-1.17,-8.62,;.17,-9.39,;1.5,-8.62,;2.83,-9.39,;2.83,-10.93,;4.17,-8.62,;5.5,-9.39,;4.17,-7.08,;5.5,-6.31,;6.84,-7.08,;6.84,-8.62,;8.17,-6.31,;8.17,-4.77,;6.84,-4,;5.5,-4.77,;4.17,-4,;2.83,-4.77,;1.5,-4,;1.5,-2.46,;2.83,-1.69,;4.17,-2.46,;5.5,-1.69,;6.84,-2.46,;8.17,-1.69,;9.5,-2.46,;10.84,-1.69,;10.84,-.15,;12.38,-.15,;10.07,1.18,;8.53,1.18,;7.76,2.52,;8.52,3.85,;7.18,4.62,;7.17,6.16,;8.5,6.93,;7.72,8.26,;8.49,9.6,;7.71,10.93,;10.03,9.6,;9.83,7.71,;11.17,6.95,;11.18,5.41,;12.72,5.42,;13.49,4.09,;15.03,4.09,;12.73,2.75,;11.19,3.87,;9.86,3.09,;6.22,2.53,;5.45,1.19,;5.44,3.85,;-7.31,1,;-7.71,2.49,;-5.82,.6,;-4.73,1.69,;-5.38,3.09,;-6.64,3.96,;-6.13,5.42,;-4.74,6.08,;-3.42,5.43,;-4.06,4.03,;-2.9,3.98,;-4.22,3.14,;-5.49,4.02,;-3.4,.92,;-2.63,-.42,;-2.07,1.69,)| Show InChI InChI=1S/C71H107N11O18/c1-48(2)54-44-51(15-16-56(54)82-58(66-59(96-6)13-8-14-60(66)97-7)45-55(75-82)67(90)74-71(70(94)95)52-40-49-39-50(42-52)43-53(71)41-49)68(91)78(5)26-12-24-76(3)23-11-25-77(4)63(85)20-19-62(84)73-22-10-34-99-36-38-100-37-35-98-33-9-21-72-61(83)18-17-57(69(92)93)81-31-29-79(46-64(86)87)27-28-80(30-32-81)47-65(88)89/h8,13-16,44-45,48-50,52-53,57H,9-12,17-43,46-47H2,1-7H3,(H,72,83)(H,73,84)(H,74,90)(H,86,87)(H,88,89)(H,92,93)(H,94,95) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466504
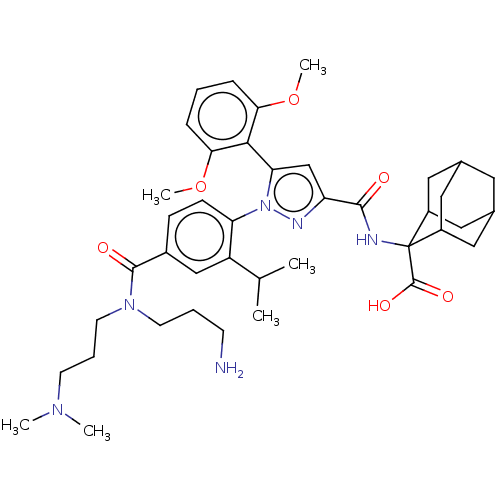
(US10799605, Example 18)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCN)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:50:40:49:45.47.44,46:45:49:42.40.41,46:41:49:45.47.44,THB:39:40:49:45.47.44,40:48:42.46.41:44,40:41:49.47.48:44,(-5.21,6.4,;-4.12,5.31,;-4.52,3.82,;-6.01,3.42,;-6.41,1.94,;-5.32,.85,;-3.83,1.24,;-2.74,.16,;-3.14,-1.33,;-3.43,2.73,;-1.94,3.13,;-1.47,4.6,;.07,4.6,;.55,3.13,;-.7,2.23,;-.7,.69,;-2.03,-.08,;-2.03,-1.62,;-.7,-2.39,;.64,-1.62,;.64,-.08,;1.97,.69,;1.97,2.23,;3.3,-.08,;-.7,-3.93,;-2.03,-4.7,;.64,-4.7,;1.97,-3.93,;3.3,-4.7,;4.64,-3.93,;5.97,-4.7,;.64,-6.24,;1.97,-7.01,;1.97,-8.55,;3.3,-9.32,;3.3,-10.86,;4.64,-8.55,;1.16,5.68,;.76,7.17,;2.65,5.29,;3.74,6.37,;4.05,7.87,;2.79,8.63,;2.04,9.97,;3.13,10.86,;4.43,10.3,;5.2,8.96,;4.05,8.8,;2.97,7.71,;1.64,8.49,;5.07,5.6,;5.07,4.06,;6.41,6.37,)| Show InChI InChI=1S/C41H56N6O6/c1-25(2)31-23-28(39(49)46(16-8-14-42)17-9-15-45(3)4)12-13-33(31)47-34(37-35(52-5)10-7-11-36(37)53-6)24-32(44-47)38(48)43-41(40(50)51)29-19-26-18-27(21-29)22-30(41)20-26/h7,10-13,23-27,29-30H,8-9,14-22,42H2,1-6H3,(H,43,48)(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466499

(US10799605, Example 4)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCCC(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |TLB:48:49:51:54.55.53,THB:49:50:57.58.56:53,49:57:51.50.55:53,56:57:51:54.55.53,56:54:51:49.57.58,59:49:51:54.55.53,(-8.99,4.14,;-7.9,3.05,;-8.3,1.56,;-9.79,1.16,;-10.19,-.33,;-9.1,-1.42,;-7.61,-1.02,;-6.52,-2.11,;-6.92,-3.59,;-7.21,.47,;-5.73,.87,;-5.25,2.33,;-3.71,2.33,;-3.24,.87,;-4.48,-.04,;-4.48,-1.58,;-5.81,-2.35,;-5.81,-3.89,;-4.48,-4.66,;-3.15,-3.89,;-3.15,-2.35,;-1.81,-1.58,;-.48,-2.35,;-1.81,-.04,;-4.48,-6.2,;-5.81,-6.97,;-3.15,-6.97,;-3.15,-8.51,;-1.81,-6.2,;-.48,-6.97,;.85,-6.2,;2.19,-6.97,;2.19,-8.51,;3.52,-6.2,;4.85,-6.97,;6.19,-6.2,;7.52,-6.97,;7.52,-8.51,;8.86,-6.2,;10.19,-6.97,;8.86,-4.66,;7.52,-3.89,;7.52,-2.35,;6.19,-1.58,;6.19,-.04,;4.85,-2.35,;-2.62,3.42,;-3.02,4.91,;-1.13,3.02,;-.05,4.11,;-.69,5.51,;-1.96,6.39,;-1.45,7.84,;-.06,8.51,;1.27,7.86,;.63,6.46,;1.78,6.41,;.46,5.57,;-.8,6.44,;1.29,3.34,;2.62,4.11,;1.29,1.8,;2.62,1.03,;2.62,-.51,;3.96,1.8,;3.96,.26,)| Show InChI InChI=1S/C51H72N6O9/c1-32(2)38-30-35(48(62)56(8)24-14-22-54(6)21-13-23-55(7)44(58)17-12-18-45(59)60)19-20-40(38)57-41(46-42(64-9)15-11-16-43(46)65-10)31-39(53-57)47(61)52-51(49(63)66-50(3,4)5)36-26-33-25-34(28-36)29-37(51)27-33/h11,15-16,19-20,30-34,36-37H,12-14,17-18,21-29H2,1-10H3,(H,52,61)(H,59,60) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.54 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466504

(US10799605, Example 18)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCN)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:50:40:49:45.47.44,46:45:49:42.40.41,46:41:49:45.47.44,THB:39:40:49:45.47.44,40:48:42.46.41:44,40:41:49.47.48:44,(-5.21,6.4,;-4.12,5.31,;-4.52,3.82,;-6.01,3.42,;-6.41,1.94,;-5.32,.85,;-3.83,1.24,;-2.74,.16,;-3.14,-1.33,;-3.43,2.73,;-1.94,3.13,;-1.47,4.6,;.07,4.6,;.55,3.13,;-.7,2.23,;-.7,.69,;-2.03,-.08,;-2.03,-1.62,;-.7,-2.39,;.64,-1.62,;.64,-.08,;1.97,.69,;1.97,2.23,;3.3,-.08,;-.7,-3.93,;-2.03,-4.7,;.64,-4.7,;1.97,-3.93,;3.3,-4.7,;4.64,-3.93,;5.97,-4.7,;.64,-6.24,;1.97,-7.01,;1.97,-8.55,;3.3,-9.32,;3.3,-10.86,;4.64,-8.55,;1.16,5.68,;.76,7.17,;2.65,5.29,;3.74,6.37,;4.05,7.87,;2.79,8.63,;2.04,9.97,;3.13,10.86,;4.43,10.3,;5.2,8.96,;4.05,8.8,;2.97,7.71,;1.64,8.49,;5.07,5.6,;5.07,4.06,;6.41,6.37,)| Show InChI InChI=1S/C41H56N6O6/c1-25(2)31-23-28(39(49)46(16-8-14-42)17-9-15-45(3)4)12-13-33(31)47-34(37-35(52-5)10-7-11-36(37)53-6)24-32(44-47)38(48)43-41(40(50)51)29-19-26-18-27(21-29)22-30(41)20-26/h7,10-13,23-27,29-30H,8-9,14-22,42H2,1-6H3,(H,43,48)(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.95 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466505

(US10799605, Example 19)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:77:67:76:72.74.71,73:72:76:69.67.68,73:68:76:72.74.71,THB:66:67:76:72.74.71,67:75:69.73.68:71,67:68:76.74.75:71,(-10.5,7.02,;-9.41,5.93,;-9.8,4.44,;-11.29,4.04,;-11.69,2.56,;-10.6,1.47,;-9.11,1.86,;-8.03,.78,;-8.42,-.71,;-8.72,3.35,;-7.23,3.75,;-6.75,5.22,;-5.21,5.22,;-4.74,3.75,;-5.98,2.85,;-5.98,1.31,;-7.32,.54,;-7.32,-1,;-5.98,-1.77,;-4.65,-1,;-4.65,.54,;-3.32,1.31,;-3.32,2.85,;-1.98,.54,;-5.98,-3.31,;-7.32,-4.08,;-4.65,-4.08,;-3.32,-3.31,;-1.98,-4.08,;-.65,-3.31,;.69,-4.08,;2.02,-3.31,;2.02,-1.77,;3.56,-3.31,;4.65,-4.4,;5.42,-3.07,;6.96,-3.07,;7.73,-4.4,;9.06,-3.63,;8.29,-2.3,;6.75,-2.3,;8.69,-.81,;9.06,-5.17,;9.06,-6.71,;7.73,-7.48,;8.82,-8.57,;10.15,-7.8,;10.15,-6.26,;11.69,-7.8,;6.96,-8.82,;5.42,-8.82,;4.65,-7.48,;3.88,-8.82,;4.65,-10.15,;6.19,-10.15,;3.88,-11.48,;3.31,-6.71,;3.31,-5.17,;-4.65,-5.62,;-3.32,-6.39,;-3.32,-7.93,;-1.98,-8.7,;-1.98,-10.24,;-.65,-7.93,;-4.12,6.3,;-4.52,7.79,;-2.64,5.91,;-1.55,6.99,;-1.23,8.49,;-2.5,9.25,;-3.25,10.59,;-2.16,11.48,;-.86,10.92,;-.09,9.58,;-1.23,9.42,;-2.32,8.33,;-3.65,9.11,;-.21,6.22,;-.21,4.68,;1.12,6.99,)| Show InChI InChI=1S/C57H82N10O13/c1-37(2)43-31-40(12-13-45(43)67-46(53-47(79-5)10-7-11-48(53)80-6)32-44(60-67)54(75)59-57(56(77)78)41-27-38-26-39(29-41)30-42(57)28-38)55(76)66(17-9-15-61(3)4)16-8-14-58-49(68)33-62-18-20-63(34-50(69)70)22-24-65(36-52(73)74)25-23-64(21-19-62)35-51(71)72/h7,10-13,31-32,37-39,41-42H,8-9,14-30,33-36H2,1-6H3,(H,58,68)(H,59,75)(H,69,70)(H,71,72)(H,73,74)(H,77,78) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466502

(US10799605, Example 13)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=S)Nc1ccc(NC(=S)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(C)=O)cc1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:91:92:94:97.98.96,THB:92:93:100.101.99:96,92:100:94.93.98:96,99:100:94:97.98.96,99:97:94:92.100.101,102:92:94:97.98.96,(-20.33,6.06,;-19.24,4.97,;-19.64,3.48,;-21.13,3.09,;-21.53,1.6,;-20.44,.51,;-18.95,.91,;-17.86,-.18,;-18.26,-1.67,;-18.55,2.39,;-17.06,2.79,;-16.59,4.26,;-15.05,4.26,;-14.57,2.79,;-15.82,1.89,;-15.82,.35,;-17.15,-.42,;-17.15,-1.96,;-15.82,-2.73,;-14.48,-1.96,;-14.48,-.42,;-13.15,.35,;-11.82,-.42,;-13.15,1.89,;-15.82,-4.27,;-17.15,-5.04,;-14.48,-5.04,;-14.48,-6.58,;-13.15,-4.27,;-11.82,-5.04,;-10.48,-4.27,;-9.15,-5.04,;-9.15,-6.58,;-7.82,-4.27,;-6.48,-5.04,;-5.15,-4.27,;-3.81,-5.04,;-3.81,-6.58,;-2.48,-4.27,;-2.48,-2.73,;-1.15,-5.04,;.19,-4.27,;1.52,-5.04,;2.85,-4.27,;2.85,-2.73,;4.19,-1.96,;5.52,-2.73,;5.52,-4.27,;6.86,-1.96,;8.19,-1.19,;9.52,-1.96,;10.86,-1.19,;12.19,-1.96,;12.19,-3.5,;13.52,-4.27,;14.86,-3.5,;13.52,-5.81,;12.19,-6.58,;14.86,-6.58,;14.86,-8.12,;16.19,-8.89,;16.19,-10.43,;17.52,-8.12,;18.86,-8.89,;20.19,-8.12,;20.19,-6.58,;21.53,-5.81,;21.53,-4.27,;20.19,-3.5,;18.86,-4.27,;20.19,-1.96,;21.53,-1.19,;18.86,-1.19,;18.86,.35,;20.19,1.12,;21.53,.35,;20.19,2.66,;18.86,3.43,;17.52,2.66,;16.19,3.43,;14.86,2.66,;14.86,1.12,;13.52,.35,;13.52,-1.19,;12.19,1.12,;10.86,.35,;12.19,2.66,;1.52,-1.96,;.19,-2.73,;-13.96,5.35,;-14.36,6.83,;-12.47,4.95,;-11.38,6.04,;-12.03,7.44,;-13.29,8.31,;-12.78,9.77,;-11.39,10.43,;-10.07,9.78,;-10.71,8.38,;-9.55,8.33,;-10.87,7.49,;-12.14,8.37,;-10.05,5.27,;-10.05,3.62,;-8.71,6.04,)| Show InChI InChI=1S/C75H110N14O14S2/c1-50(2)59-48-54(23-28-61(59)89-62(69-63(102-7)21-18-22-64(69)103-8)49-60(82-89)70(95)81-75(72(97)98)55-44-52-43-53(46-55)47-56(75)45-52)71(96)84(5)38-19-36-83(4)37-20-39-85(6)74(105)80-58-26-24-57(25-27-58)79-73(104)78-35-14-11-17-42-88(101)68(94)32-30-66(92)77-34-13-10-16-41-87(100)67(93)31-29-65(91)76-33-12-9-15-40-86(99)51(3)90/h18,21-28,48-50,52-53,55-56,99-101H,9-17,19-20,29-47H2,1-8H3,(H,76,91)(H,77,92)(H,80,105)(H,81,95)(H,97,98)(H2,78,79,104) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466500

(US10799605, Example 5)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:67:68:70:73.74.72,THB:75:76:70:73.74.72,75:73:70:68.76.77,78:68:70:73.74.72,68:69:76.77.75:72,68:76:70.69.74:72,(-13.42,6.92,;-12.33,5.83,;-12.73,4.34,;-14.22,3.94,;-14.62,2.45,;-13.53,1.36,;-12.04,1.76,;-10.95,.67,;-11.35,-.81,;-11.64,3.25,;-10.15,3.65,;-9.68,5.11,;-8.14,5.11,;-7.66,3.65,;-8.91,2.74,;-8.91,1.2,;-10.24,.43,;-10.24,-1.11,;-8.91,-1.88,;-7.57,-1.11,;-7.57,.43,;-6.24,1.2,;-4.91,.43,;-6.24,2.74,;-8.91,-3.42,;-10.24,-4.19,;-7.57,-4.19,;-7.57,-5.73,;-6.24,-3.42,;-4.91,-4.19,;-3.57,-3.42,;-2.24,-4.19,;-2.24,-5.73,;-.91,-3.42,;.43,-4.19,;1.76,-3.42,;3.1,-4.19,;3.1,-5.73,;4.43,-3.42,;4.43,-1.88,;5.97,-3.42,;7.3,-4.19,;8.1,-2.87,;9.68,-2.86,;10.4,-4.18,;11.17,-2.85,;10.4,-1.51,;8.86,-1.51,;11.17,-.18,;11.7,-4.95,;11.72,-6.52,;10.41,-7.28,;11.74,-8.05,;13.08,-7.28,;13.08,-5.74,;14.62,-7.28,;9.59,-8.6,;8.01,-8.61,;7.31,-7.29,;6.54,-8.62,;7.31,-9.95,;8.85,-9.95,;6.54,-11.29,;6.03,-6.49,;6.01,-4.91,;-7.05,6.2,;-7.45,7.69,;-5.56,5.8,;-4.47,6.89,;-5.12,8.29,;-6.38,9.17,;-5.87,10.62,;-4.48,11.29,;-3.16,10.64,;-3.8,9.24,;-2.64,9.19,;-3.96,8.35,;-5.23,9.22,;-3.14,6.12,;-3.14,4.58,;-1.81,6.89,)| Show InChI InChI=1S/C58H84N10O13/c1-38(2)44-32-41(13-14-46(44)68-47(54-48(80-6)11-8-12-49(54)81-7)33-45(60-68)55(76)59-58(57(78)79)42-28-39-27-40(30-42)31-43(58)29-39)56(77)63(5)18-10-16-61(3)15-9-17-62(4)50(69)34-64-19-21-65(35-51(70)71)23-25-67(37-53(74)75)26-24-66(22-20-64)36-52(72)73/h8,11-14,32-33,38-40,42-43H,9-10,15-31,34-37H2,1-7H3,(H,59,76)(H,70,71)(H,72,73)(H,74,75)(H,78,79) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466501

(US10799605, Example 12)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCC(=O)NCCCOCCOCCOCCCNC(=O)CCC(N1CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:86:87:89:92.93.91,THB:87:88:95.96.94:91,87:95:89.88.93:91,94:95:89:92.93.91,94:92:89:87.95.96,97:87:89:92.93.91,(-12.72,2.45,;-11.95,1.11,;-12.72,-.22,;-14.26,-.22,;-15.03,-1.56,;-14.26,-2.89,;-12.72,-2.89,;-11.95,-4.22,;-12.72,-5.56,;-11.95,-1.56,;-10.41,-1.56,;-9.94,-.09,;-8.4,-.09,;-7.92,-1.56,;-9.17,-2.46,;-9.17,-4,;-10.5,-4.77,;-10.5,-6.31,;-9.17,-7.08,;-7.84,-6.31,;-7.84,-4.77,;-6.5,-4,;-5.17,-4.77,;-6.5,-2.46,;-9.17,-8.62,;-10.5,-9.39,;-7.84,-9.39,;-7.84,-10.93,;-6.5,-8.62,;-5.17,-9.39,;-3.83,-8.62,;-2.5,-9.39,;-2.5,-10.93,;-1.17,-8.62,;.17,-9.39,;1.5,-8.62,;2.83,-9.39,;2.83,-10.93,;4.17,-8.62,;5.5,-9.39,;4.17,-7.08,;5.5,-6.31,;6.84,-7.08,;6.84,-8.62,;8.17,-6.31,;8.17,-4.77,;6.84,-4,;5.5,-4.77,;4.17,-4,;2.83,-4.77,;1.5,-4,;1.5,-2.46,;2.83,-1.69,;4.17,-2.46,;5.5,-1.69,;6.84,-2.46,;8.17,-1.69,;9.5,-2.46,;10.84,-1.69,;10.84,-.15,;12.38,-.15,;10.07,1.18,;8.53,1.18,;7.76,2.52,;8.52,3.85,;7.18,4.62,;7.17,6.16,;8.5,6.93,;7.72,8.26,;8.49,9.6,;7.71,10.93,;10.03,9.6,;9.83,7.71,;11.17,6.95,;11.18,5.41,;12.72,5.42,;13.49,4.09,;15.03,4.09,;12.73,2.75,;11.19,3.87,;9.86,3.09,;6.22,2.53,;5.45,1.19,;5.44,3.85,;-7.31,1,;-7.71,2.49,;-5.82,.6,;-4.73,1.69,;-5.38,3.09,;-6.64,3.96,;-6.13,5.42,;-4.74,6.08,;-3.42,5.43,;-4.06,4.03,;-2.9,3.98,;-4.22,3.14,;-5.49,4.02,;-3.4,.92,;-2.63,-.42,;-2.07,1.69,)| Show InChI InChI=1S/C71H107N11O18/c1-48(2)54-44-51(15-16-56(54)82-58(66-59(96-6)13-8-14-60(66)97-7)45-55(75-82)67(90)74-71(70(94)95)52-40-49-39-50(42-52)43-53(71)41-49)68(91)78(5)26-12-24-76(3)23-11-25-77(4)63(85)20-19-62(84)73-22-10-34-99-36-38-100-37-35-98-33-9-21-72-61(83)18-17-57(69(92)93)81-31-29-79(46-64(86)87)27-28-80(30-32-81)47-65(88)89/h8,13-16,44-45,48-50,52-53,57H,9-12,17-43,46-47H2,1-7H3,(H,72,83)(H,73,84)(H,74,90)(H,86,87)(H,88,89)(H,92,93)(H,94,95) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141994
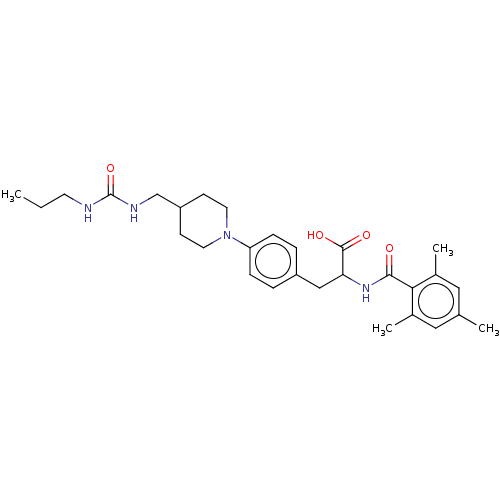
(US8927534, 269)Show SMILES CCCNC(=O)NCC1CCN(CC1)c1ccc(CC(NC(=O)c2c(C)cc(C)cc2C)C(O)=O)cc1 Show InChI InChI=1S/C29H40N4O4/c1-5-12-30-29(37)31-18-23-10-13-33(14-11-23)24-8-6-22(7-9-24)17-25(28(35)36)32-27(34)26-20(3)15-19(2)16-21(26)4/h6-9,15-16,23,25H,5,10-14,17-18H2,1-4H3,(H,32,34)(H,35,36)(H2,30,31,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141990
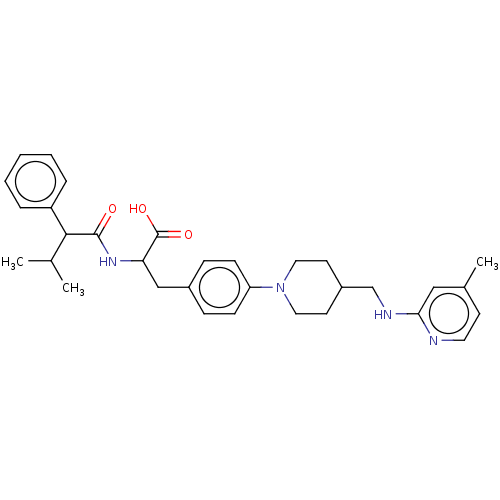
(US8927534, 266)Show SMILES CC(C)C(C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2cc(C)ccn2)CC1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C32H40N4O3/c1-22(2)30(26-7-5-4-6-8-26)31(37)35-28(32(38)39)20-24-9-11-27(12-10-24)36-17-14-25(15-18-36)21-34-29-19-23(3)13-16-33-29/h4-13,16,19,22,25,28,30H,14-15,17-18,20-21H2,1-3H3,(H,33,34)(H,35,37)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141986

(US8927534, 262)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3ccccc3)C(O)=O)cc2)c1 Show InChI InChI=1S/C28H32N4O3/c1-20-11-14-29-26(17-20)30-19-22-12-15-32(16-13-22)24-9-7-21(8-10-24)18-25(28(34)35)31-27(33)23-5-3-2-4-6-23/h2-11,14,17,22,25H,12-13,15-16,18-19H2,1H3,(H,29,30)(H,31,33)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM50423736

(CHEMBL591543)Show SMILES COc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O4/c1-20-15-21(2)29(22(3)16-20)30(36)34-27(31(37)38)17-23-5-7-25(8-6-23)35-13-10-24(11-14-35)19-33-28-18-26(39-4)9-12-32-28/h5-9,12,15-16,18,24,27H,10-11,13-14,17,19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141981
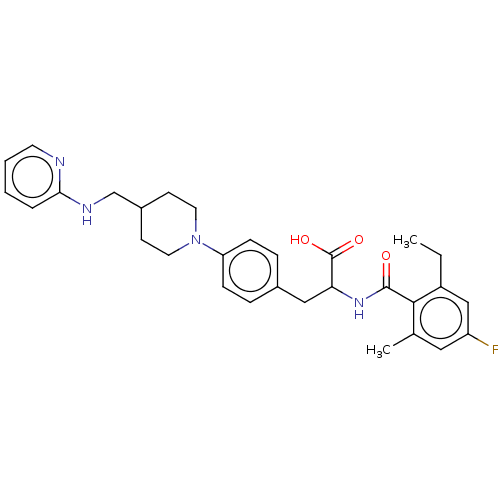
(US8927534, 120)Show SMILES CCc1cc(F)cc(C)c1C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O Show InChI InChI=1S/C30H35FN4O3/c1-3-23-18-24(31)16-20(2)28(23)29(36)34-26(30(37)38)17-21-7-9-25(10-8-21)35-14-11-22(12-15-35)19-33-27-6-4-5-13-32-27/h4-10,13,16,18,22,26H,3,11-12,14-15,17,19H2,1-2H3,(H,32,33)(H,34,36)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141973
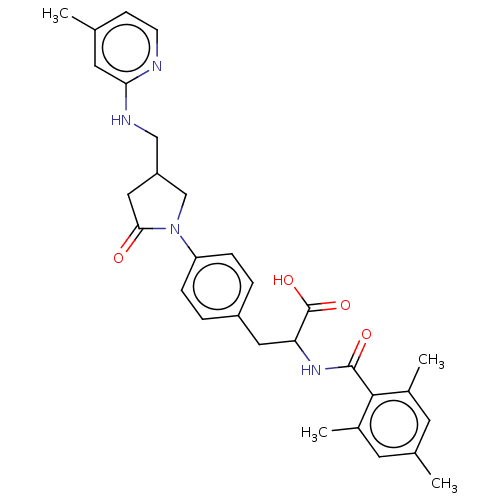
(US8927534, 114 | US8927534, 83)Show SMILES Cc1ccnc(NCC2CN(C(=O)C2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C30H34N4O4/c1-18-9-10-31-26(13-18)32-16-23-15-27(35)34(17-23)24-7-5-22(6-8-24)14-25(30(37)38)33-29(36)28-20(3)11-19(2)12-21(28)4/h5-13,23,25H,14-17H2,1-4H3,(H,31,32)(H,33,36)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141979
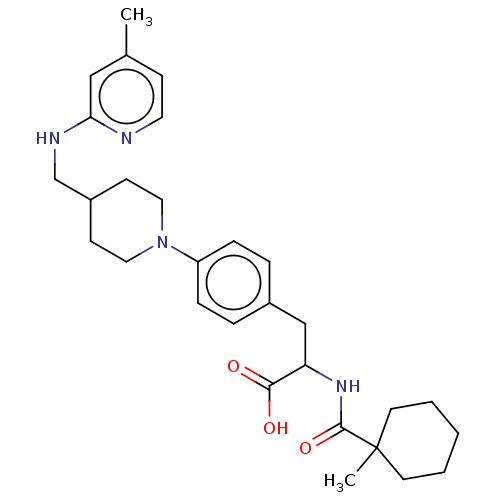
(US8927534, 75)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)C3(C)CCCCC3)C(O)=O)cc2)c1 Show InChI InChI=1S/C29H40N4O3/c1-21-10-15-30-26(18-21)31-20-23-11-16-33(17-12-23)24-8-6-22(7-9-24)19-25(27(34)35)32-28(36)29(2)13-4-3-5-14-29/h6-10,15,18,23,25H,3-5,11-14,16-17,19-20H2,1-2H3,(H,30,31)(H,32,36)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141978
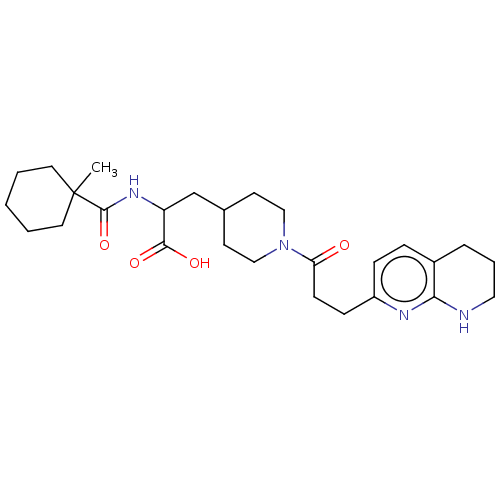
(US8927534, 29)Show SMILES CC1(CCCCC1)C(=O)NC(CC1CCN(CC1)C(=O)CCc1ccc2CCCNc2n1)C(O)=O Show InChI InChI=1S/C27H40N4O4/c1-27(13-3-2-4-14-27)26(35)30-22(25(33)34)18-19-11-16-31(17-12-19)23(32)10-9-21-8-7-20-6-5-15-28-24(20)29-21/h7-8,19,22H,2-6,9-18H2,1H3,(H,28,29)(H,30,35)(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141976

(US8927534, 23)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CCC(CC2)c2ccc3CCCNc3n2)C(O)=O)c(C)c1 Show InChI InChI=1S/C32H38N4O3/c1-20-17-21(2)29(22(3)18-20)31(37)35-28(32(38)39)19-23-6-9-26(10-7-23)36-15-12-24(13-16-36)27-11-8-25-5-4-14-33-30(25)34-27/h6-11,17-18,24,28H,4-5,12-16,19H2,1-3H3,(H,33,34)(H,35,37)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141975
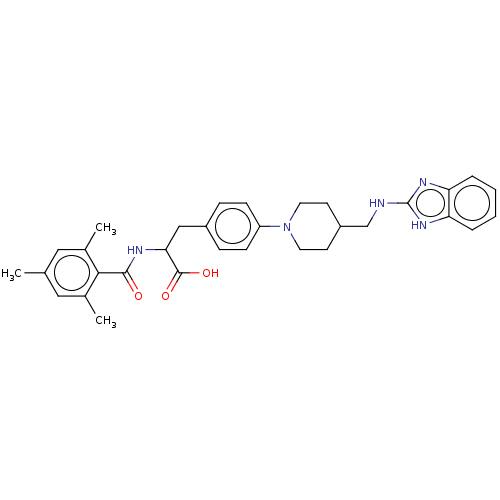
(US8927534, 20)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CCC(CNc3nc4ccccc4[nH]3)CC2)C(O)=O)c(C)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM50423721
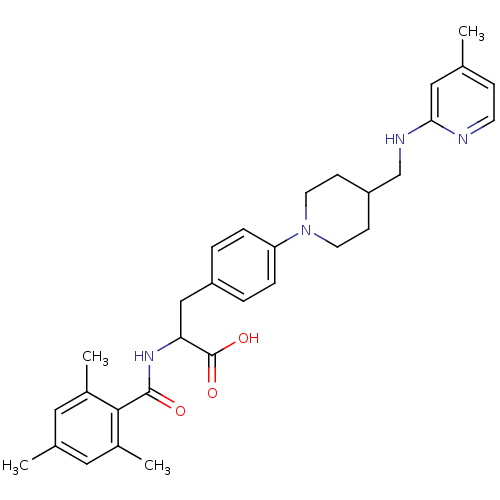
(CHEMBL595105)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O3/c1-20-9-12-32-28(17-20)33-19-25-10-13-35(14-11-25)26-7-5-24(6-8-26)18-27(31(37)38)34-30(36)29-22(3)15-21(2)16-23(29)4/h5-9,12,15-17,25,27H,10-11,13-14,18-19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-5
(Homo sapiens (Human)) | BDBM141972
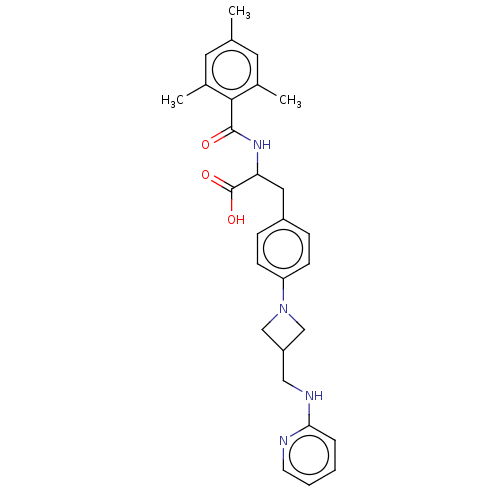
(US8927534, 11)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CC(CNc3ccccn3)C2)C(O)=O)c(C)c1 Show InChI InChI=1S/C28H32N4O3/c1-18-12-19(2)26(20(3)13-18)27(33)31-24(28(34)35)14-21-7-9-23(10-8-21)32-16-22(17-32)15-30-25-6-4-5-11-29-25/h4-13,22,24H,14-17H2,1-3H3,(H,29,30)(H,31,33)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibronectin was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates (Nalge Nu... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466506
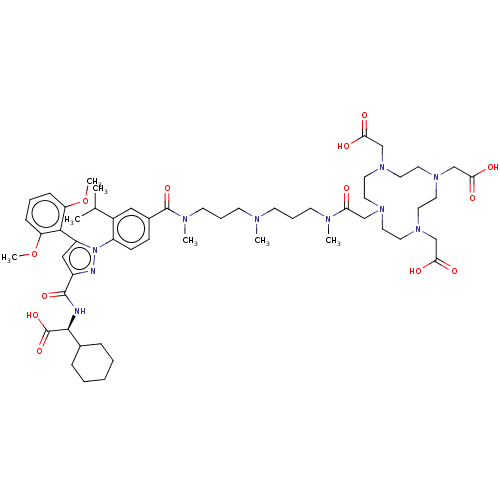
(US10799605, Example 22)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r,wU:68.71,(-13.16,6.68,;-12.07,5.59,;-12.47,4.1,;-13.96,3.7,;-14.36,2.22,;-13.27,1.13,;-11.78,1.53,;-10.69,.44,;-11.09,-1.05,;-11.38,3.01,;-9.9,3.41,;-9.42,4.88,;-7.88,4.88,;-7.4,3.41,;-8.65,2.51,;-8.65,.97,;-9.98,.2,;-9.98,-1.34,;-8.65,-2.11,;-7.32,-1.34,;-7.32,.2,;-5.98,.97,;-5.98,2.51,;-4.65,.2,;-8.65,-3.65,;-9.98,-4.42,;-7.32,-4.42,;-7.32,-5.96,;-5.98,-3.65,;-4.65,-4.42,;-3.32,-3.65,;-1.98,-4.42,;-1.98,-5.96,;-.65,-3.65,;.69,-4.42,;2.02,-3.65,;3.35,-4.42,;3.35,-5.96,;4.69,-3.65,;3.92,-2.32,;6.23,-3.65,;7.32,-4.74,;8.09,-3.41,;9.63,-3.41,;10.4,-4.74,;11.73,-3.97,;10.96,-2.64,;9.42,-2.64,;11.36,-1.15,;11.73,-5.51,;11.73,-7.05,;10.4,-7.82,;11.48,-8.91,;12.82,-8.14,;12.82,-6.6,;14.36,-8.14,;9.63,-9.16,;8.09,-9.16,;7.32,-7.82,;6.55,-9.16,;7.32,-10.49,;8.86,-10.49,;6.55,-11.82,;5.98,-7.05,;5.98,-5.51,;-7.08,6.11,;-7.48,7.6,;-5.59,6.11,;-4.5,7.2,;-4.5,8.74,;-3.17,9.51,;-3.17,11.05,;-4.5,11.82,;-5.84,11.05,;-5.84,9.51,;-3.17,6.43,;-3.17,4.89,;-1.84,7.2,)| Show InChI InChI=1S/C55H82N10O13/c1-38(2)41-32-40(18-19-43(41)65-44(51-45(77-6)16-11-17-46(51)78-7)33-42(57-65)53(73)56-52(55(75)76)39-14-9-8-10-15-39)54(74)60(5)23-13-21-58(3)20-12-22-59(4)47(66)34-61-24-26-62(35-48(67)68)28-30-64(37-50(71)72)31-29-63(27-25-61)36-49(69)70/h11,16-19,32-33,38-39,52H,8-10,12-15,20-31,34-37H2,1-7H3,(H,56,73)(H,67,68)(H,69,70)(H,71,72)(H,75,76)/t52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141986

(US8927534, 262)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3ccccc3)C(O)=O)cc2)c1 Show InChI InChI=1S/C28H32N4O3/c1-20-11-14-29-26(17-20)30-19-22-12-15-32(16-13-22)24-9-7-21(8-10-24)18-25(28(34)35)31-27(33)23-5-3-2-4-6-23/h2-11,14,17,22,25H,12-13,15-16,18-19H2,1H3,(H,29,30)(H,31,33)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM50423736

(CHEMBL591543)Show SMILES COc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O4/c1-20-15-21(2)29(22(3)16-20)30(36)34-27(31(37)38)17-23-5-7-25(8-6-23)35-13-10-24(11-14-35)19-33-28-18-26(39-4)9-12-32-28/h5-9,12,15-16,18,24,27H,10-11,13-14,17,19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141981

(US8927534, 120)Show SMILES CCc1cc(F)cc(C)c1C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O Show InChI InChI=1S/C30H35FN4O3/c1-3-23-18-24(31)16-20(2)28(23)29(36)34-26(30(37)38)17-21-7-9-25(10-8-21)35-14-11-22(12-15-35)19-33-27-6-4-5-13-32-27/h4-10,13,16,18,22,26H,3,11-12,14-15,17,19H2,1-2H3,(H,32,33)(H,34,36)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141973

(US8927534, 114 | US8927534, 83)Show SMILES Cc1ccnc(NCC2CN(C(=O)C2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C30H34N4O4/c1-18-9-10-31-26(13-18)32-16-23-15-27(35)34(17-23)24-7-5-22(6-8-24)14-25(30(37)38)33-29(36)28-20(3)11-19(2)12-21(28)4/h5-13,23,25H,14-17H2,1-4H3,(H,31,32)(H,33,36)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141979

(US8927534, 75)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)C3(C)CCCCC3)C(O)=O)cc2)c1 Show InChI InChI=1S/C29H40N4O3/c1-21-10-15-30-26(18-21)31-20-23-11-16-33(17-12-23)24-8-6-22(7-9-24)19-25(27(34)35)32-28(36)29(2)13-4-3-5-14-29/h6-10,15,18,23,25H,3-5,11-14,16-17,19-20H2,1-2H3,(H,30,31)(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141994

(US8927534, 269)Show SMILES CCCNC(=O)NCC1CCN(CC1)c1ccc(CC(NC(=O)c2c(C)cc(C)cc2C)C(O)=O)cc1 Show InChI InChI=1S/C29H40N4O4/c1-5-12-30-29(37)31-18-23-10-13-33(14-11-23)24-8-6-22(7-9-24)17-25(28(35)36)32-27(34)26-20(3)15-19(2)16-21(26)4/h6-9,15-16,23,25H,5,10-14,17-18H2,1-4H3,(H,32,34)(H,35,36)(H2,30,31,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141976

(US8927534, 23)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CCC(CC2)c2ccc3CCCNc3n2)C(O)=O)c(C)c1 Show InChI InChI=1S/C32H38N4O3/c1-20-17-21(2)29(22(3)18-20)31(37)35-28(32(38)39)19-23-6-9-26(10-7-23)36-15-12-24(13-16-36)27-11-8-25-5-4-14-33-30(25)34-27/h6-11,17-18,24,28H,4-5,12-16,19H2,1-3H3,(H,33,34)(H,35,37)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141975

(US8927534, 20)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CCC(CNc3nc4ccccc4[nH]3)CC2)C(O)=O)c(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141972

(US8927534, 11)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CC(CNc3ccccn3)C2)C(O)=O)c(C)c1 Show InChI InChI=1S/C28H32N4O3/c1-18-12-19(2)26(20(3)13-18)27(33)31-24(28(34)35)14-21-7-9-23(10-8-21)32-16-22(17-32)15-30-25-6-4-5-11-29-25/h4-13,22,24H,14-17H2,1-3H3,(H,29,30)(H,31,33)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM50423721

(CHEMBL595105)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O3/c1-20-9-12-32-28(17-20)33-19-25-10-13-35(14-11-25)26-7-5-24(6-8-26)18-27(31(37)38)34-30(36)29-22(3)15-21(2)16-23(29)4/h5-9,12,15-17,25,27H,10-11,13-14,18-19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141990

(US8927534, 266)Show SMILES CC(C)C(C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2cc(C)ccn2)CC1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C32H40N4O3/c1-22(2)30(26-7-5-4-6-8-26)31(37)35-28(32(38)39)20-24-9-11-27(12-10-24)36-17-14-25(15-18-36)21-34-29-19-23(3)13-16-33-29/h4-13,16,19,22,25,28,30H,14-15,17-18,20-21H2,1-3H3,(H,33,34)(H,35,37)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb
(Homo sapiens (Human)) | BDBM141978

(US8927534, 29)Show SMILES CC1(CCCCC1)C(=O)NC(CC1CCN(CC1)C(=O)CCc1ccc2CCCNc2n1)C(O)=O Show InChI InChI=1S/C27H40N4O4/c1-27(13-3-2-4-14-27)26(35)30-22(25(33)34)18-19-11-16-31(17-12-19)23(32)10-9-21-8-7-20-6-5-15-28-24(20)29-21/h7-8,19,22H,2-6,9-18H2,1H3,(H,28,29)(H,30,35)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 9.6 | 4 |
Shire Orphan Therapies GmbH
US Patent
| Assay Description
Fibrinogen was diluted with coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and coated with 100 uL/well to Nunc-Immuno maxisorp plates over night... |
US Patent US8927534 (2015)
BindingDB Entry DOI: 10.7270/Q2TQ607Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




































