

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50046728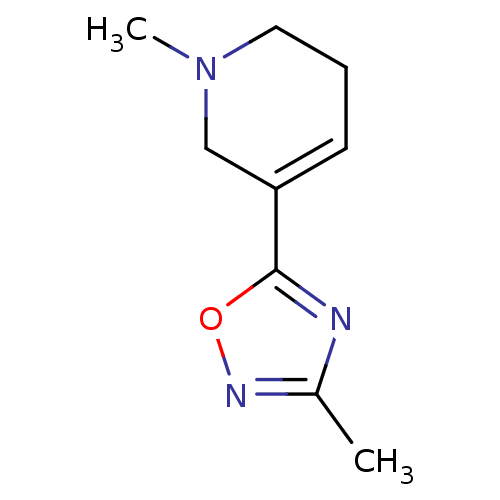 (1-Methyl-5-(3-methyl-[1,2,4]oxadiazol-5-yl)-1,2,3,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50046728 (1-Methyl-5-(3-methyl-[1,2,4]oxadiazol-5-yl)-1,2,3,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity of [3H]-(R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the presence of 300 uM GTP-gamma-S | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50046723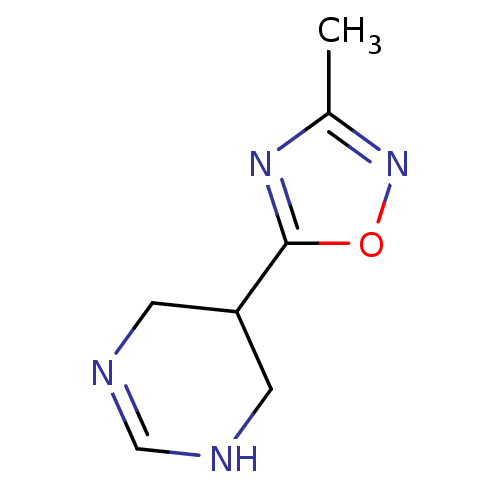 (5-(3-Methyl-[1,2,4]oxadiazol-5-yl)-1,4,5,6-tetrahy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50046723 (5-(3-Methyl-[1,2,4]oxadiazol-5-yl)-1,4,5,6-tetrahy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity of [3H]-(R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the presence of 300 uM GTP-gamma-S | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50039843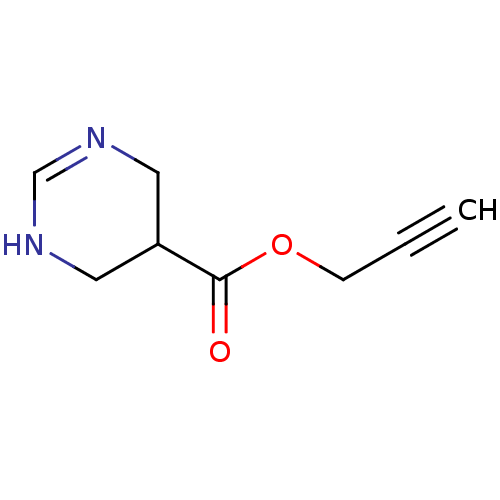 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid pr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM46858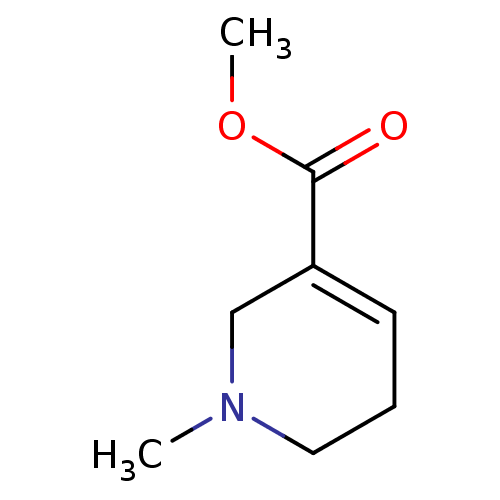 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM46858 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity of [3H]-(R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the presence of 300 uM GTP-gamma-S | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057400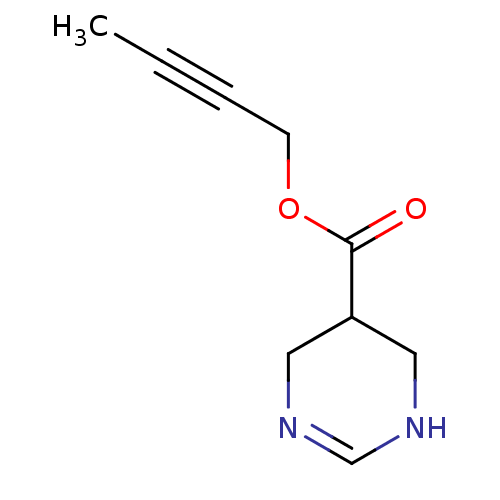 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid bu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057400 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid bu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057397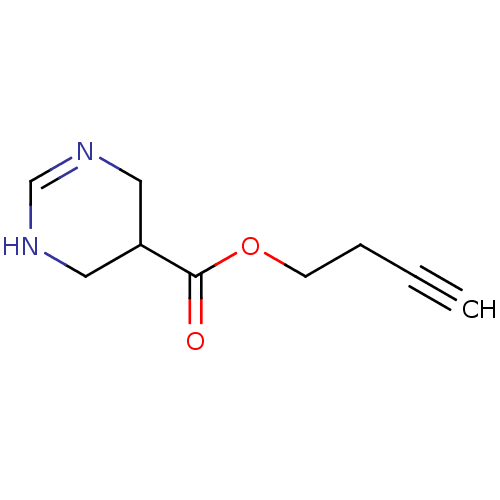 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid bu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057403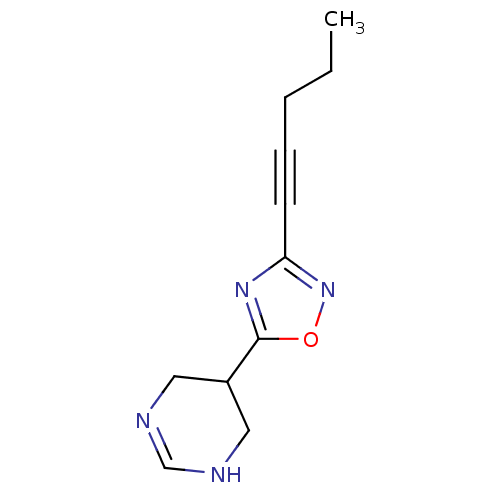 (5-(3-Pent-1-ynyl-[1,2,4]oxadiazol-5-yl)-1,4,5,6-te...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057403 (5-(3-Pent-1-ynyl-[1,2,4]oxadiazol-5-yl)-1,4,5,6-te...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50046723 (5-(3-Methyl-[1,2,4]oxadiazol-5-yl)-1,4,5,6-tetrahy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50039839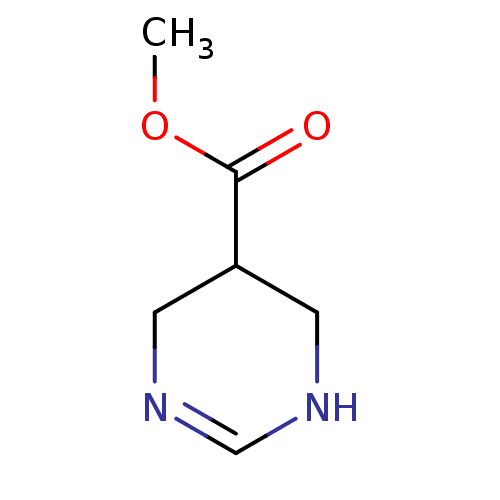 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50039839 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (low) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma-... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50057397 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid bu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the presence of 300 uM GTP-gamma-S | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50039843 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid pr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50039839 (1,4,5,6-Tetrahydro-pyrimidine-5-carboxylic acid me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Mus musculus) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Binding affinity (high) of [3H](R)-QNB binding to Muscarinic acetylcholine receptor M1 expressed in A9 L cell line in the absence of 300 uM GTP-gamma... | J Med Chem 40: 1230-46 (1997) Article DOI: 10.1021/jm960467d BindingDB Entry DOI: 10.7270/Q28916HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064365 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064373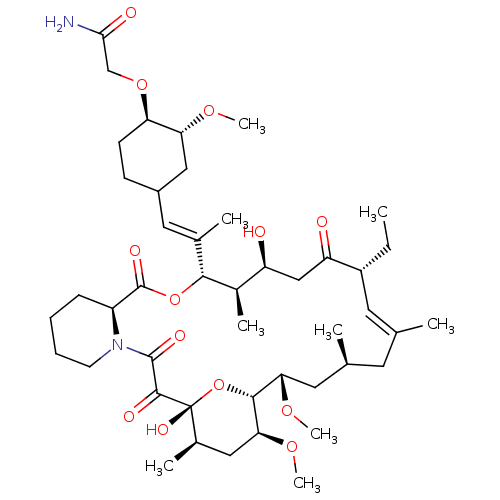 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064359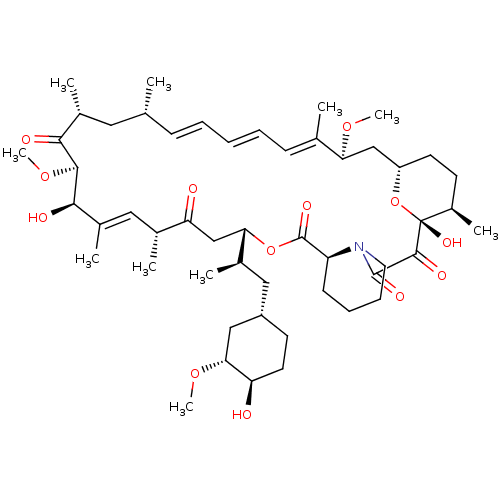 (1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064361 (CHEMBL48863 | N-Cyclohexyl-2-{4-[2-(17-ethyl-1,14-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50068939 ((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064375 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064355 ((E)-(1R,9S,12S,13R,14S,17R,21S,23S,25S,27R)-17-All...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064374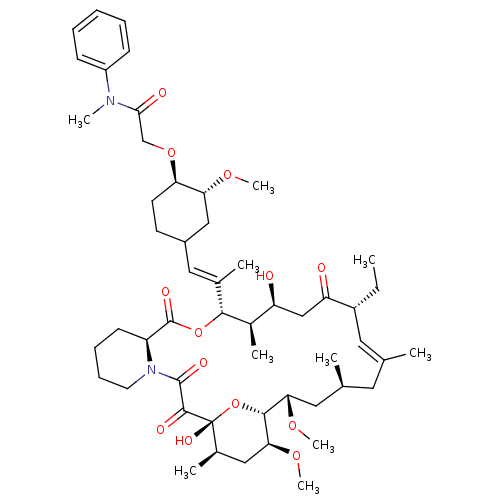 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064351 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064350 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064353 (CHEMBL51516 | {4-[2-(17-Ethyl-1,14-dihydroxy-23,25...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064352 (CHEMBL266912 | N-Benzyl-2-{4-[2-(17-ethyl-1,14-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064362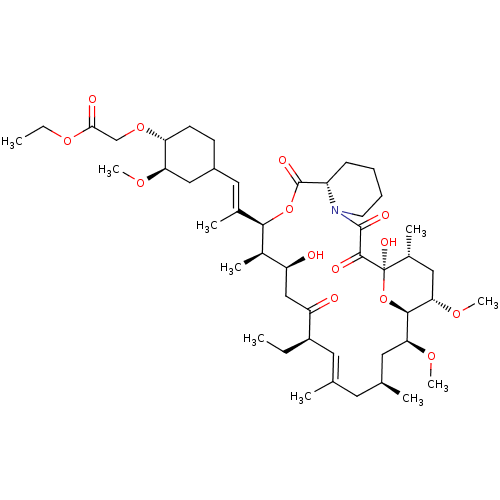 (CHEMBL299146 | {4-[2-(17-Ethyl-1,14-dihydroxy-23,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064363 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064376 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064367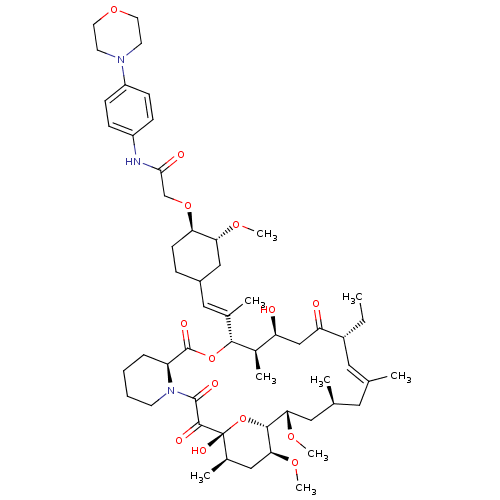 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064357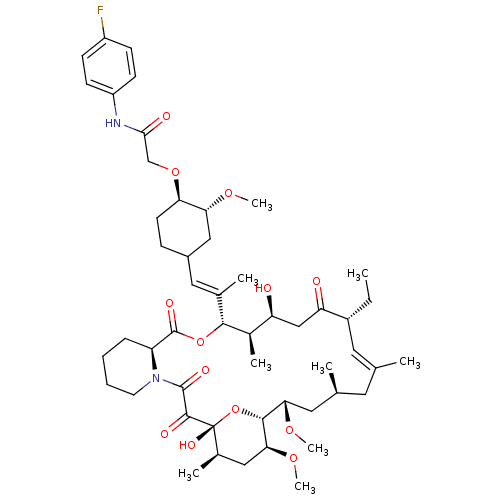 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064358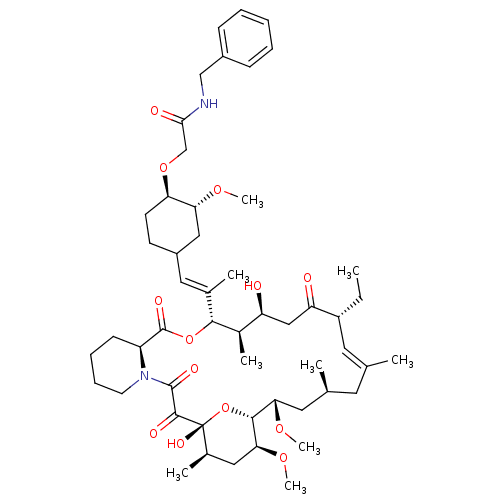 (CHEMBL51523 | N-Benzyl-2-{4-[2-(17-ethyl-1,14-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064369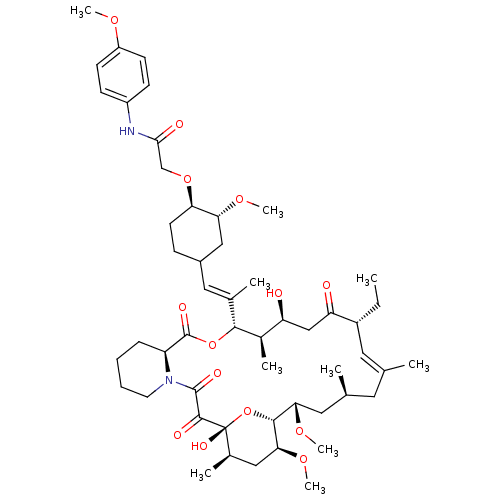 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064360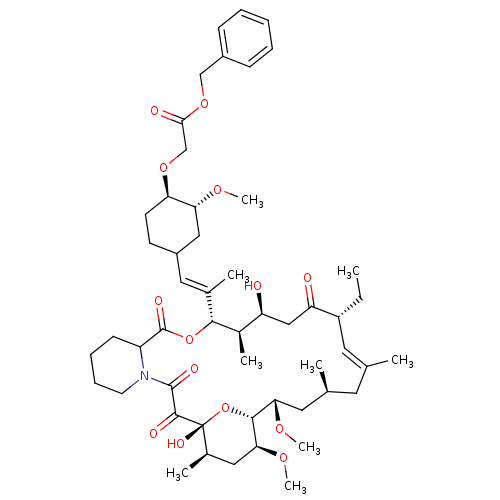 (CHEMBL296160 | {4-[2-(17-Ethyl-1,14-dihydroxy-23,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064364 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064371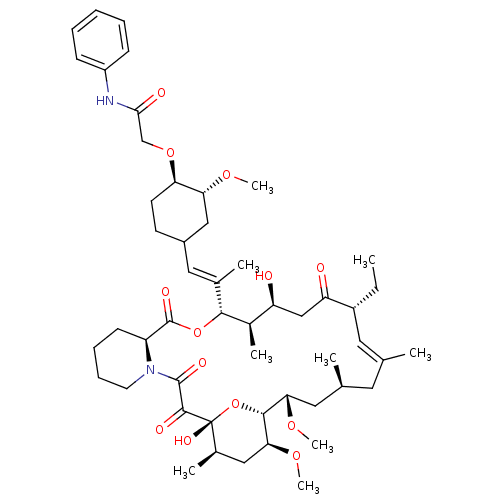 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064372 (CHEMBL299549 | N-(4-Chloro-phenyl)-2-{4-[2-(17-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064368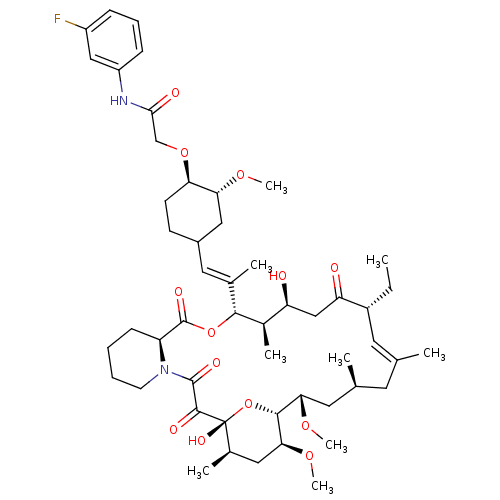 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064370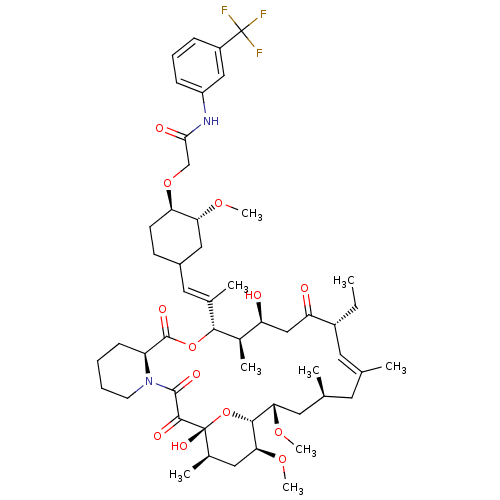 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064354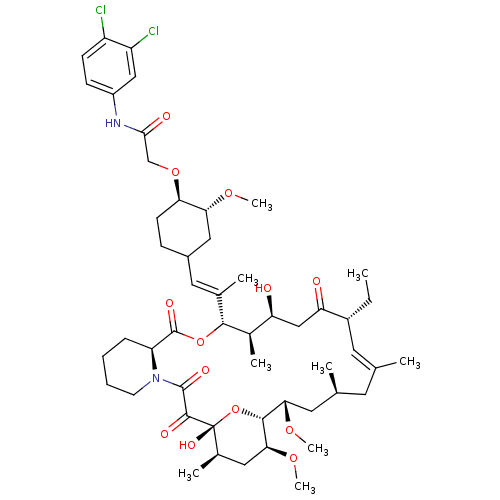 (CHEMBL411841 | N-(3,4-Dichloro-phenyl)-2-{4-[2-(17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50064366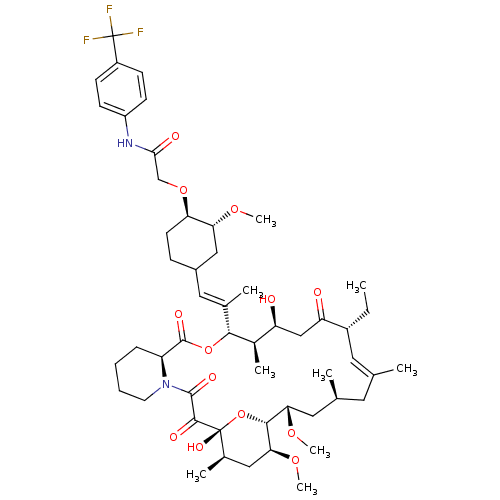 (2-{4-[2-(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory binding activity against human Immunophilin-FK506 binding protein 12 | J Med Chem 41: 1764-76 (1998) Article DOI: 10.1021/jm960066y BindingDB Entry DOI: 10.7270/Q2319V0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50039841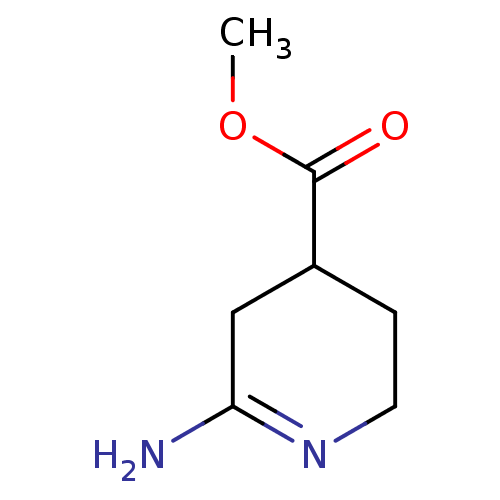 (6-Amino-2,3,4,5-tetrahydro-pyridine-4-carboxylic a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Affinity for muscarinic receptor M1 in rat brain was measured by inhibition of [3H](R)-quinuclidinyl benzilate (QNB) binding | J Med Chem 37: 2774-82 (1994) BindingDB Entry DOI: 10.7270/Q28S4QJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50039836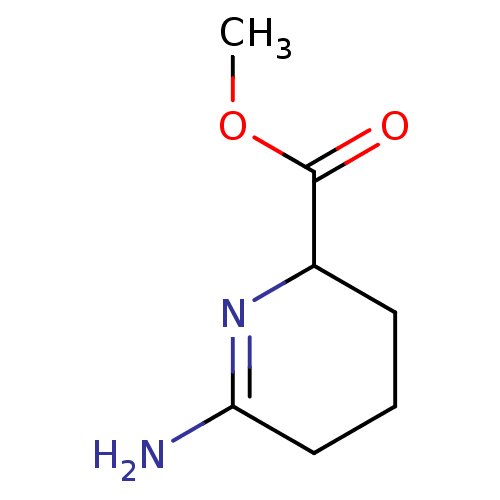 (6-Amino-2,3,4,5-tetrahydro-pyridine-2-carboxylic a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Affinity for muscarinic receptor M1 in rat brain was measured by inhibition of [3H](R)-quinuclidinyl benzilate (QNB) binding | J Med Chem 37: 2774-82 (1994) BindingDB Entry DOI: 10.7270/Q28S4QJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |