 Found 4220 hits with Last Name = 'richard' and Initial = 's'
Found 4220 hits with Last Name = 'richard' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0558 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183152

(CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-7-9-21-10-8-16)12-14-3-5-15(6-4-14)17(18,19)20/h3-6,13,16,21H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194072

(CHEMBL220476 | syn-7-(6-chloro-pyridin-3-yl)-2-aza...)Show SMILES Clc1ccc(cn1)C1C2CCC1NC2 |THB:4:7:10.9:13.12| Show InChI InChI=1S/C11H13ClN2/c12-10-4-2-8(6-14-10)11-7-1-3-9(11)13-5-7/h2,4,6-7,9,11,13H,1,3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391708

(CHEMBL1767136)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22ClN3O/c1-24-12-4-5-17(24)14-25-21(26)19-7-3-2-6-18(19)20(23-25)13-15-8-10-16(22)11-9-15/h2-3,6-11,17H,4-5,12-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50341447
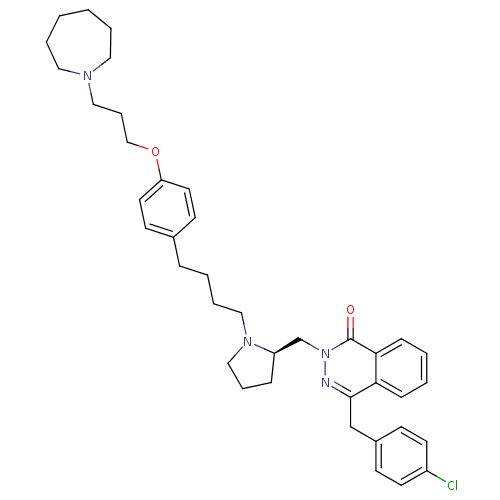
(4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H49ClN4O2/c40-33-19-15-32(16-20-33)29-38-36-13-3-4-14-37(36)39(45)44(41-38)30-34-12-9-27-43(34)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-42-23-6-1-2-7-24-42/h3-4,13-22,34H,1-2,5-12,23-30H2/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391698
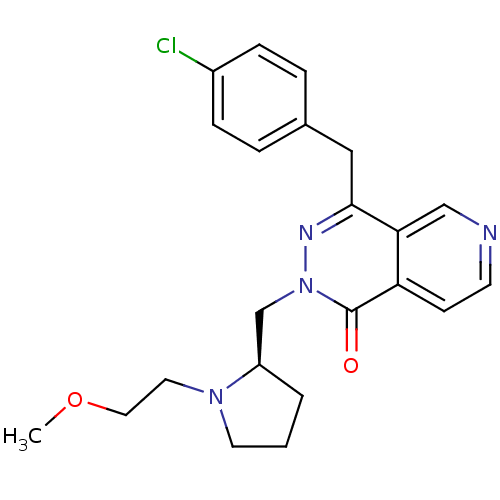
(CHEMBL2146801)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C22H25ClN4O2/c1-29-12-11-26-10-2-3-18(26)15-27-22(28)19-8-9-24-14-20(19)21(25-27)13-16-4-6-17(23)7-5-16/h4-9,14,18H,2-3,10-13,15H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183124
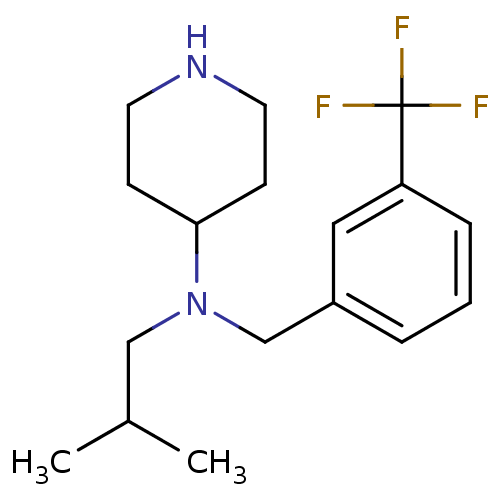
(CHEMBL381373 | N-(3-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-6-8-21-9-7-16)12-14-4-3-5-15(10-14)17(18,19)20/h3-5,10,13,16,21H,6-9,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183122

(4-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-7-9-19-10-8-17)13-16-5-3-15(11-18)4-6-16/h3-6,14,17,19H,7-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183121
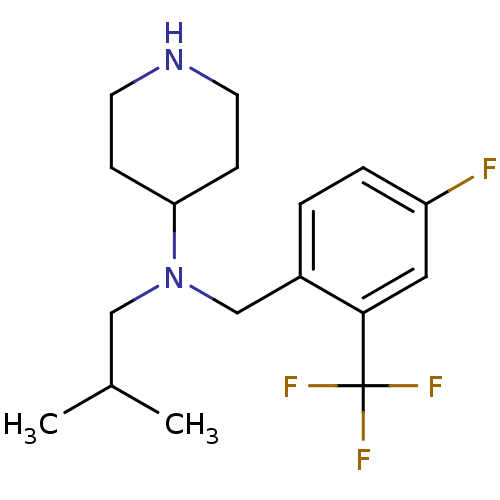
(CHEMBL441358 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-12(2)10-23(15-5-7-22-8-6-15)11-13-3-4-14(18)9-16(13)17(19,20)21/h3-4,9,12,15,22H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391702
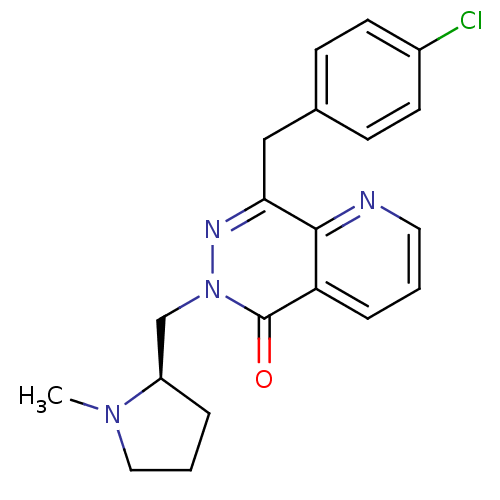
(CHEMBL2146809)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ncccc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-11-3-4-16(24)13-25-20(26)17-5-2-10-22-19(17)18(23-25)12-14-6-8-15(21)9-7-14/h2,5-10,16H,3-4,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194070

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES Clc1ccc(cn1)C1CC2CCCC1N2 |THB:4:7:14:12.11.10| Show InChI InChI=1S/C12H15ClN2/c13-12-5-4-8(7-14-12)10-6-9-2-1-3-11(10)15-9/h4-5,7,9-11,15H,1-3,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194070

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES Clc1ccc(cn1)C1CC2CCCC1N2 |THB:4:7:14:12.11.10| Show InChI InChI=1S/C12H15ClN2/c13-12-5-4-8(7-14-12)10-6-9-2-1-3-11(10)15-9/h4-5,7,9-11,15H,1-3,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391707
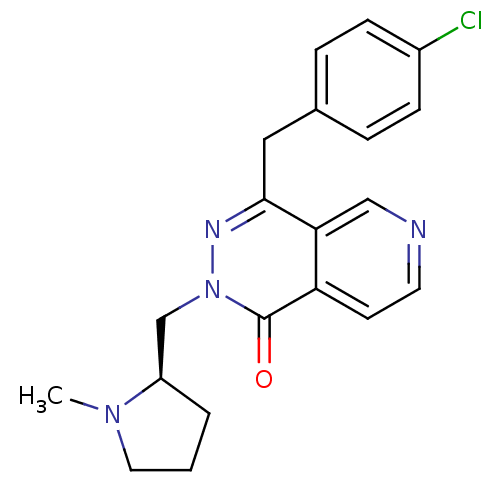
(CHEMBL2146805)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-10-2-3-16(24)13-25-20(26)17-8-9-22-12-18(17)19(23-25)11-14-4-6-15(21)7-5-14/h4-9,12,16H,2-3,10-11,13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391699
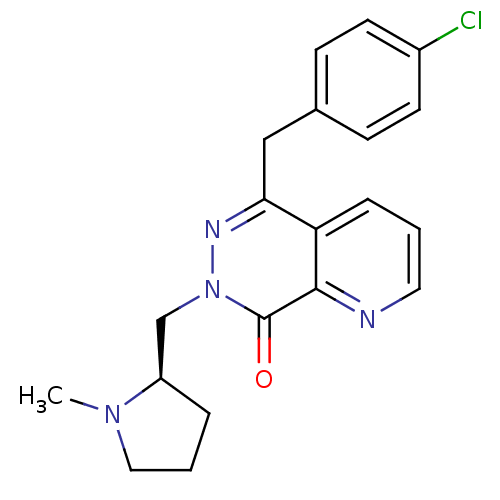
(CHEMBL2146484)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cccnc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-11-3-4-16(24)13-25-20(26)19-17(5-2-10-22-19)18(23-25)12-14-6-8-15(21)9-7-14/h2,5-10,16H,3-4,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183126

(CHEMBL207374 | N-(2,4-dimethylbenzyl)-N-isobutylpi...)Show InChI InChI=1S/C18H30N2/c1-14(2)12-20(18-7-9-19-10-8-18)13-17-6-5-15(3)11-16(17)4/h5-6,11,14,18-19H,7-10,12-13H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391697
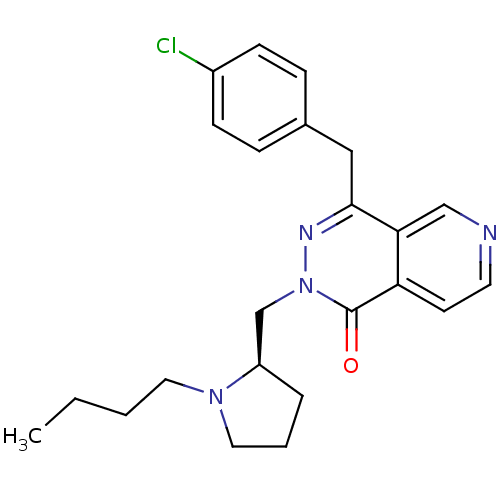
(CHEMBL2146806)Show SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C23H27ClN4O/c1-2-3-12-27-13-4-5-19(27)16-28-23(29)20-10-11-25-15-21(20)22(26-28)14-17-6-8-18(24)9-7-17/h6-11,15,19H,2-5,12-14,16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183177

(4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C17H21F4N3/c18-14-4-3-13(16(11-14)17(19,20)21)12-24(10-2-1-7-22)15-5-8-23-9-6-15/h3-4,11,15,23H,1-2,5-6,8-10,12H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183154

(CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...)Show InChI InChI=1S/C17H22F4N2/c18-14-4-3-13(16(9-14)17(19,20)21)11-23(10-12-1-2-12)15-5-7-22-8-6-15/h3-4,9,12,15,22H,1-2,5-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183141

(3-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C16H19F4N3/c17-13-3-2-12(15(10-13)16(18,19)20)11-23(9-1-6-21)14-4-7-22-8-5-14/h2-3,10,14,22H,1,4-5,7-9,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183136
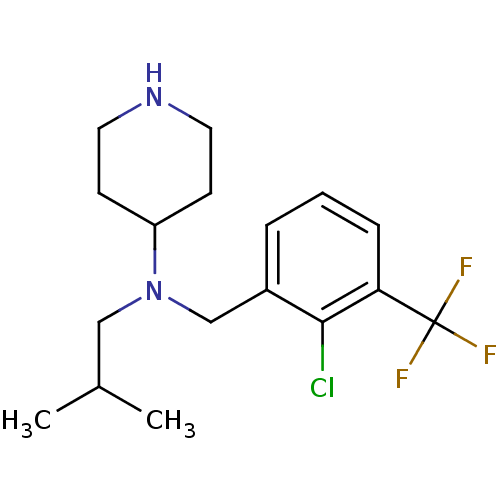
(CHEMBL208533 | N-(2-chloro-3-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24ClF3N2/c1-12(2)10-23(14-6-8-22-9-7-14)11-13-4-3-5-15(16(13)18)17(19,20)21/h3-5,12,14,22H,6-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183137
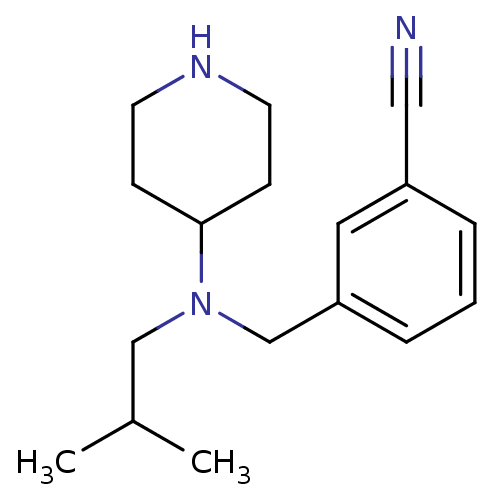
(1-(2-cyclohexyl-4-methylpentyl)-3-ethynylbenzene f...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-6-8-19-9-7-17)13-16-5-3-4-15(10-16)11-18/h3-5,10,14,17,19H,6-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391710
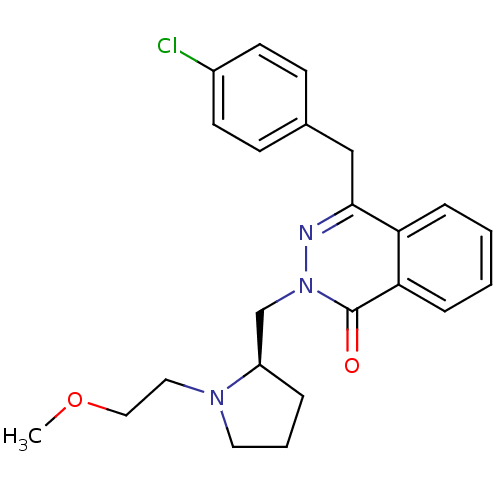
(CHEMBL2146803)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C23H26ClN3O2/c1-29-14-13-26-12-4-5-19(26)16-27-23(28)21-7-3-2-6-20(21)22(25-27)15-17-8-10-18(24)11-9-17/h2-3,6-11,19H,4-5,12-16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50555343
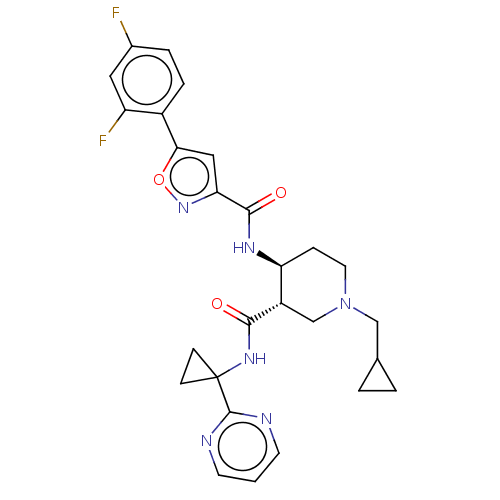
(CHEMBL4782111 | US11306078, Example 4.032)Show SMILES Fc1ccc(-c2cc(no2)C(=O)N[C@H]2CCN(CC3CC3)C[C@@H]2C(=O)NC2(CC2)c2ncccn2)c(F)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to SNAP-tag fused human CXCR7 expressed in HEK293 cells by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01588
BindingDB Entry DOI: 10.7270/Q20P13P3 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391704
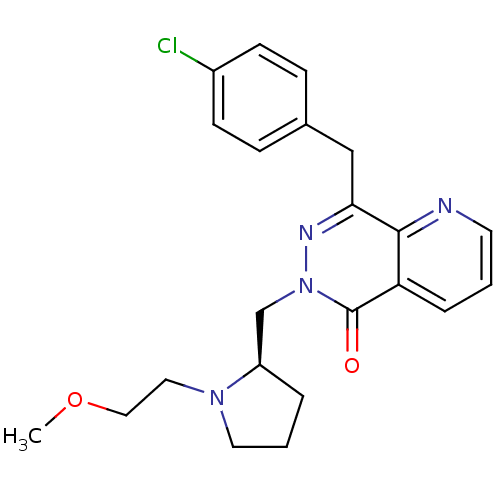
(CHEMBL2146811)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ncccc2c1=O |r| Show InChI InChI=1S/C22H25ClN4O2/c1-29-13-12-26-11-3-4-18(26)15-27-22(28)19-5-2-10-24-21(19)20(25-27)14-16-6-8-17(23)9-7-16/h2,5-10,18H,3-4,11-15H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50391696
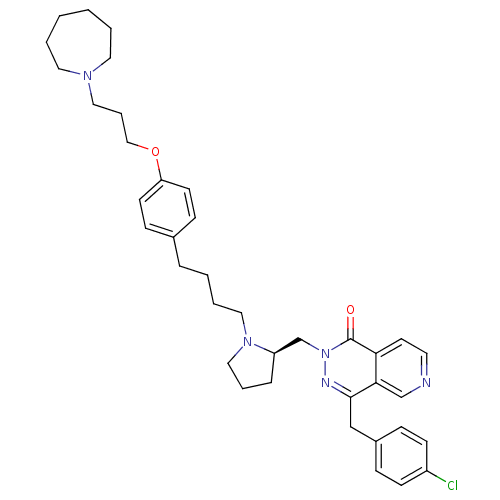
(CHEMBL2146813)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccncc23)cc1 |r| Show InChI InChI=1S/C38H48ClN5O2/c39-32-15-11-31(12-16-32)27-37-36-28-40-20-19-35(36)38(45)44(41-37)29-33-10-7-25-43(33)24-6-3-9-30-13-17-34(18-14-30)46-26-8-23-42-21-4-1-2-5-22-42/h11-20,28,33H,1-10,21-27,29H2/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183135
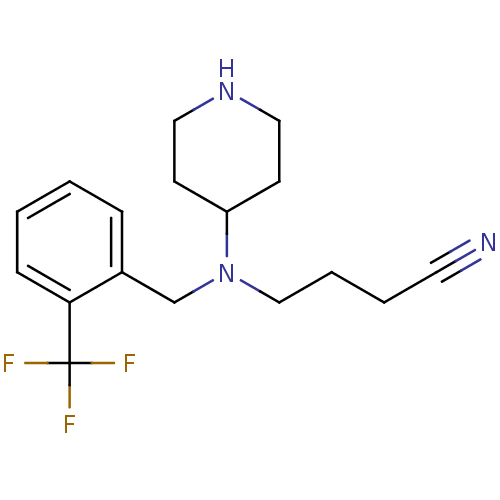
(4-((2-(trifluoromethyl)benzyl)(piperidin-4-yl)amin...)Show InChI InChI=1S/C17H22F3N3/c18-17(19,20)16-6-2-1-5-14(16)13-23(12-4-3-9-21)15-7-10-22-11-8-15/h1-2,5-6,15,22H,3-4,7-8,10-13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391700
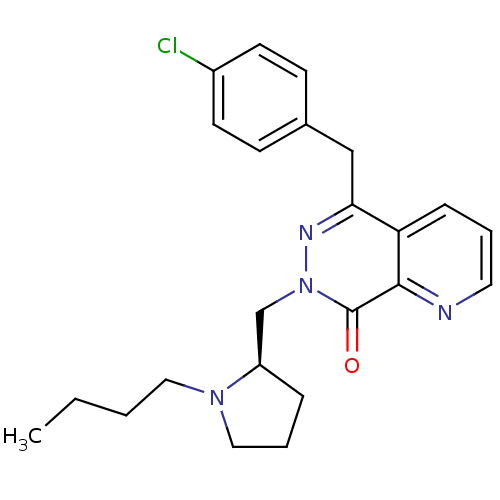
(CHEMBL2146807)Show SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cccnc2c1=O |r| Show InChI InChI=1S/C23H27ClN4O/c1-2-3-13-27-14-5-6-19(27)16-28-23(29)22-20(7-4-12-25-22)21(26-28)15-17-8-10-18(24)11-9-17/h4,7-12,19H,2-3,5-6,13-16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391703
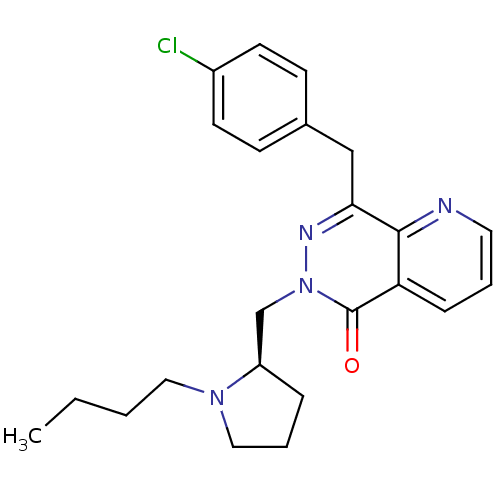
(CHEMBL2146810)Show SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ncccc2c1=O |r| Show InChI InChI=1S/C23H27ClN4O/c1-2-3-13-27-14-5-6-19(27)16-28-23(29)20-7-4-12-25-22(20)21(26-28)15-17-8-10-18(24)11-9-17/h4,7-12,19H,2-3,5-6,13-16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391706
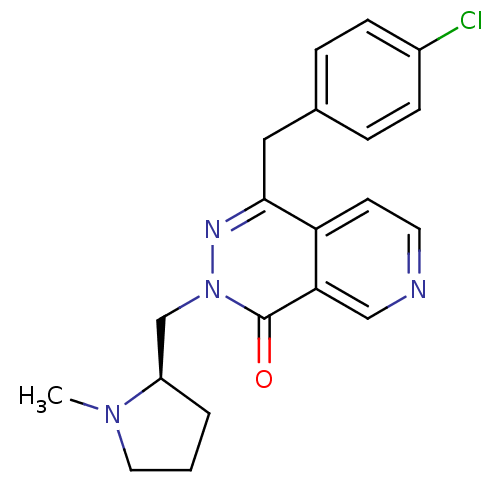
(CHEMBL2146804)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccncc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-10-2-3-16(24)13-25-20(26)18-12-22-9-8-17(18)19(23-25)11-14-4-6-15(21)7-5-14/h4-9,12,16H,2-3,10-11,13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183173
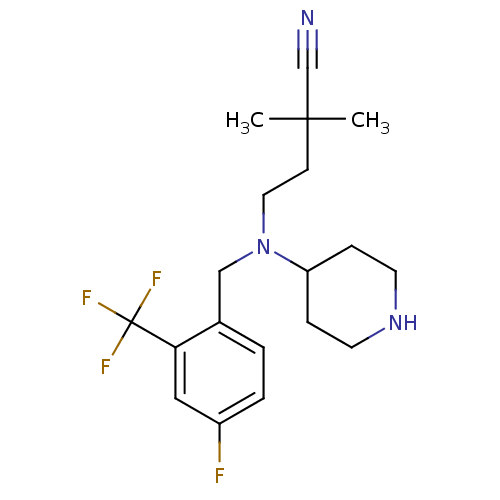
(4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show SMILES CC(C)(CCN(Cc1ccc(F)cc1C(F)(F)F)C1CCNCC1)C#N Show InChI InChI=1S/C19H25F4N3/c1-18(2,13-24)7-10-26(16-5-8-25-9-6-16)12-14-3-4-15(20)11-17(14)19(21,22)23/h3-4,11,16,25H,5-10,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194068
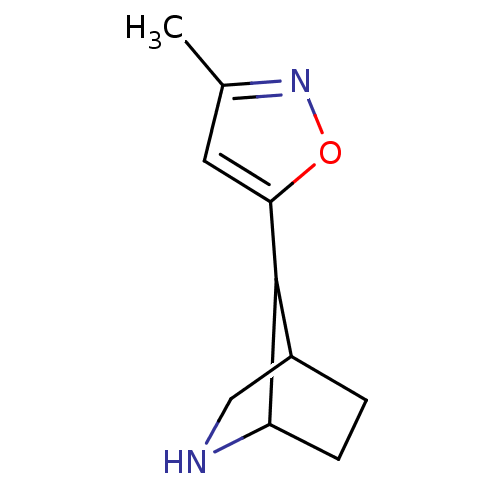
(7-(3-methylisoxazol-5-yl)-6-aza-bicyclo[2.2.1]hept...)Show InChI InChI=1S/C10H14N2O/c1-6-4-9(13-12-6)10-7-2-3-8(10)11-5-7/h4,7-8,10-11H,2-3,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.763 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194069
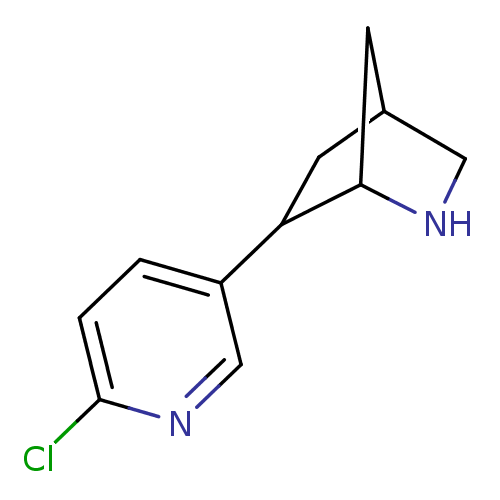
(2-(6-chloropyridin-3-yl)-6-aza-bicyclo[2.2.1]hepta...)Show InChI InChI=1S/C11H13ClN2/c12-11-2-1-8(6-14-11)9-3-7-4-10(9)13-5-7/h1-2,6-7,9-10,13H,3-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183154

(CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...)Show InChI InChI=1S/C17H22F4N2/c18-14-4-3-13(16(9-14)17(19,20)21)11-23(10-12-1-2-12)15-5-7-22-8-6-15/h3-4,9,12,15,22H,1-2,5-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183134
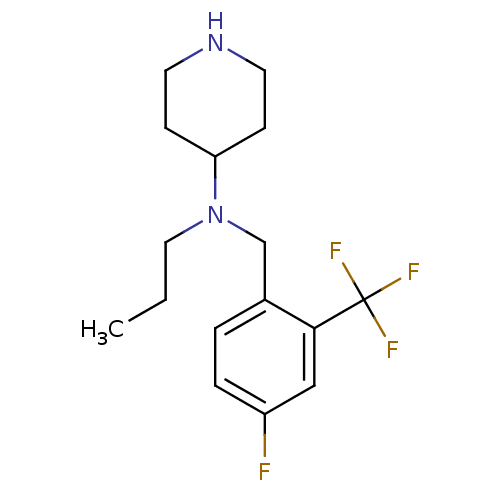
(CHEMBL207854 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C16H22F4N2/c1-2-9-22(14-5-7-21-8-6-14)11-12-3-4-13(17)10-15(12)16(18,19)20/h3-4,10,14,21H,2,5-9,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391709
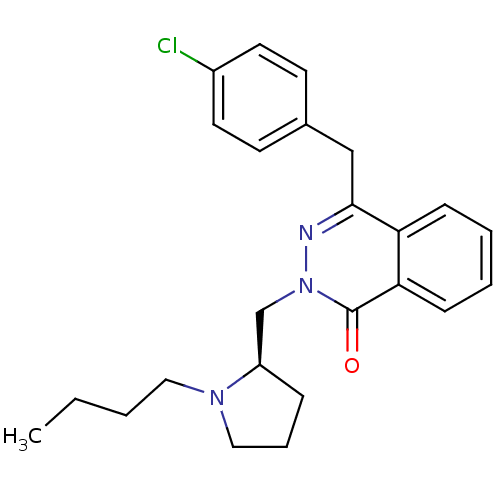
(CHEMBL2146802)Show SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C24H28ClN3O/c1-2-3-14-27-15-6-7-20(27)17-28-24(29)22-9-5-4-8-21(22)23(26-28)16-18-10-12-19(25)13-11-18/h4-5,8-13,20H,2-3,6-7,14-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183176
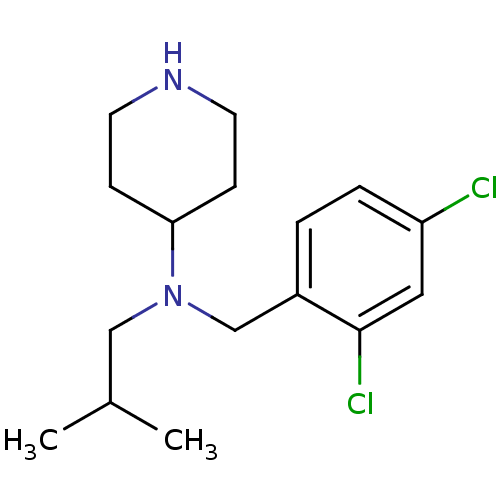
(CHEMBL207860 | N-(2,4-dichlorobenzyl)-N-isobutylpi...)Show InChI InChI=1S/C16H24Cl2N2/c1-12(2)10-20(15-5-7-19-8-6-15)11-13-3-4-14(17)9-16(13)18/h3-4,9,12,15,19H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183166
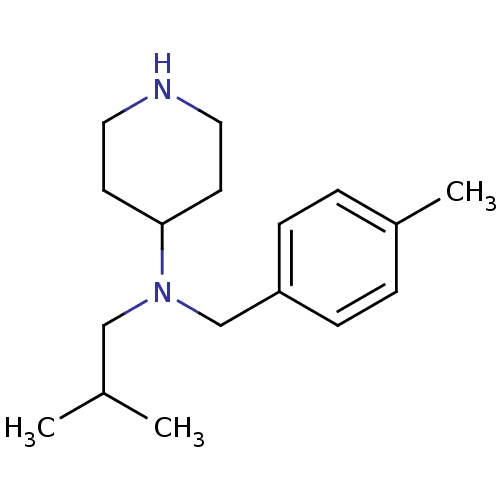
(CHEMBL380191 | isobutyl-(4-methyl-benzyl)-piperidi...)Show InChI InChI=1S/C17H28N2/c1-14(2)12-19(17-8-10-18-11-9-17)13-16-6-4-15(3)5-7-16/h4-7,14,17-18H,8-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12648
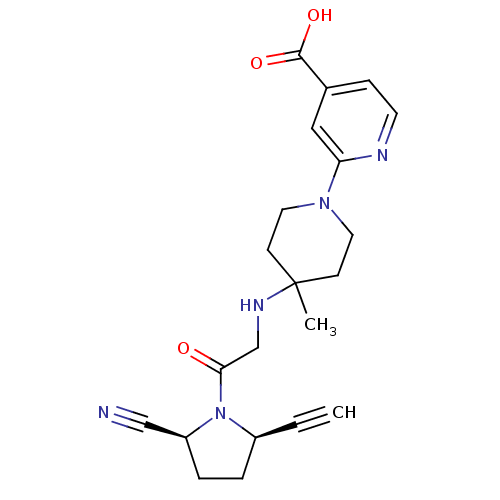
((2S,5R)-5-Ethynyl-1-{N-(4-methyl-1-(4-carboxy-pyri...)Show SMILES CC1(CCN(CC1)c1cc(ccn1)C(O)=O)NCC(=O)N1[C@H](CC[C@H]1C#N)C#C |r| Show InChI InChI=1S/C21H25N5O3/c1-3-16-4-5-17(13-22)26(16)19(27)14-24-21(2)7-10-25(11-8-21)18-12-15(20(28)29)6-9-23-18/h1,6,9,12,16-17,24H,4-5,7-8,10-11,14H2,2H3,(H,28,29)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 6416-20 (2006)
Article DOI: 10.1021/jm060777o
BindingDB Entry DOI: 10.7270/Q2M043M1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11644
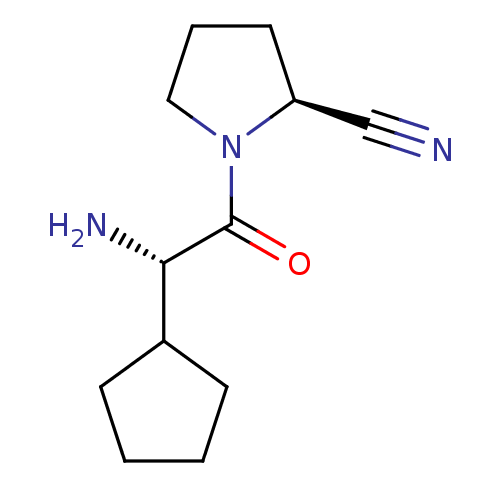
((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...)Show InChI InChI=1S/C12H19N3O/c13-8-10-6-3-7-15(10)12(16)11(14)9-4-1-2-5-9/h9-11H,1-7,14H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 6416-20 (2006)
Article DOI: 10.1021/jm060777o
BindingDB Entry DOI: 10.7270/Q2M043M1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391701

(CHEMBL2146808)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cccnc2c1=O |r| Show InChI InChI=1S/C22H25ClN4O2/c1-29-13-12-26-11-3-4-18(26)15-27-22(28)21-19(5-2-10-24-21)20(25-27)14-16-6-8-17(23)9-7-16/h2,5-10,18H,3-4,11-15H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50500893
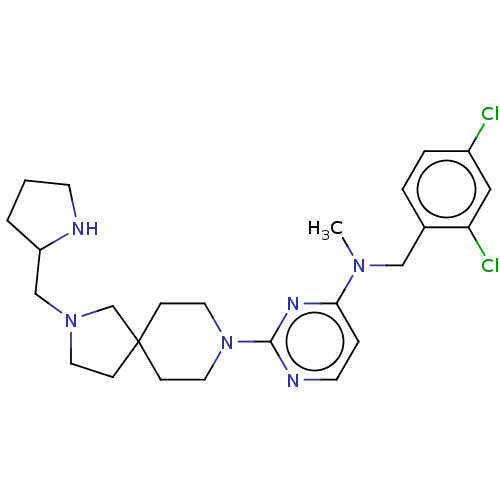
(CHEMBL3797662)Show SMILES CN(Cc1ccc(Cl)cc1Cl)c1ccnc(n1)N1CCC2(CCN(CC3CCCN3)C2)CC1 Show InChI InChI=1S/C25H34Cl2N6/c1-31(16-19-4-5-20(26)15-22(19)27)23-6-11-29-24(30-23)33-13-8-25(9-14-33)7-12-32(18-25)17-21-3-2-10-28-21/h4-6,11,15,21,28H,2-3,7-10,12-14,16-18H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [125I]-TARC from human recombinant CCR4 expressed in CHO cell membranes by scintillation counting method |
Eur J Med Chem 115: 14-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.058
BindingDB Entry DOI: 10.7270/Q24J0J4S |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183147
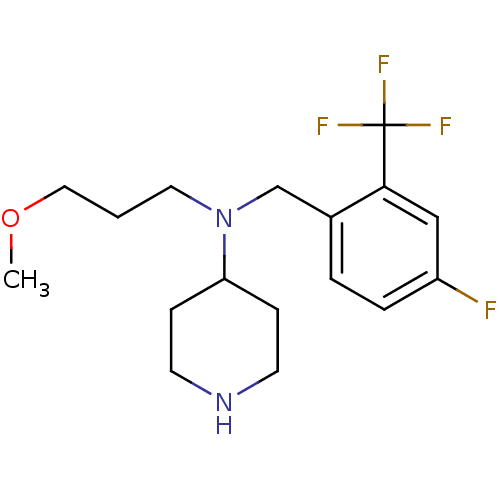
(CHEMBL207755 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2O/c1-24-10-2-9-23(15-5-7-22-8-6-15)12-13-3-4-14(18)11-16(13)17(19,20)21/h3-4,11,15,22H,2,5-10,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183165

(CHEMBL438059 | N-(2-(methylthio)benzyl)-N-isobutyl...)Show InChI InChI=1S/C17H28N2S/c1-14(2)12-19(16-8-10-18-11-9-16)13-15-6-4-5-7-17(15)20-3/h4-7,14,16,18H,8-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183160

(2-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-7-9-19-10-8-17)13-16-6-4-3-5-15(16)11-18/h3-6,14,17,19H,7-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50341448

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show SMILES CN1CCCC(CC1)n1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O Show InChI InChI=1S/C22H24ClN3O/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16/h2-3,6-11,18H,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194071

(3-(6-chloropyridin-3-yl)-6-aza-bicyclo[2.2.1]hepta...)Show InChI InChI=1S/C11H13ClN2/c12-11-2-1-7(5-14-11)10-4-9-3-8(10)6-13-9/h1-2,5,8-10,13H,3-4,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194071

(3-(6-chloropyridin-3-yl)-6-aza-bicyclo[2.2.1]hepta...)Show InChI InChI=1S/C11H13ClN2/c12-11-2-1-7(5-14-11)10-4-9-3-8(10)6-13-9/h1-2,5,8-10,13H,3-4,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391705
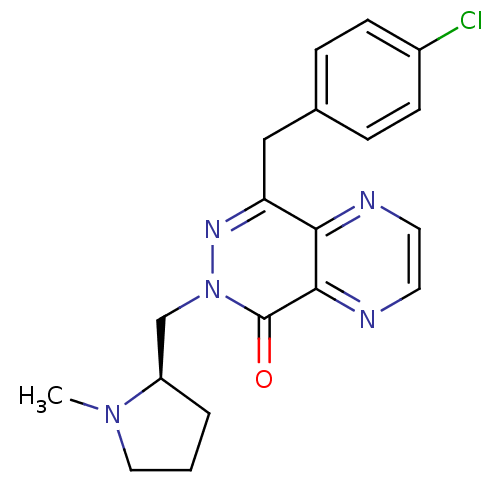
(CHEMBL2146812)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2nccnc2c1=O |r| Show InChI InChI=1S/C19H20ClN5O/c1-24-10-2-3-15(24)12-25-19(26)18-17(21-8-9-22-18)16(23-25)11-13-4-6-14(20)7-5-13/h4-9,15H,2-3,10-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183146
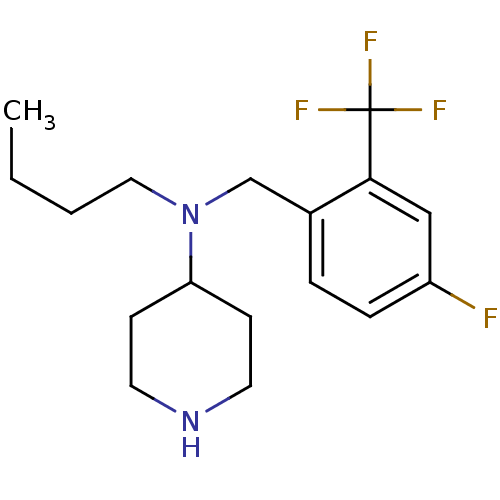
(CHEMBL426316 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-2-3-10-23(15-6-8-22-9-7-15)12-13-4-5-14(18)11-16(13)17(19,20)21/h4-5,11,15,22H,2-3,6-10,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data