

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485444 (CHEMBL2059813) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50458169 (Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485438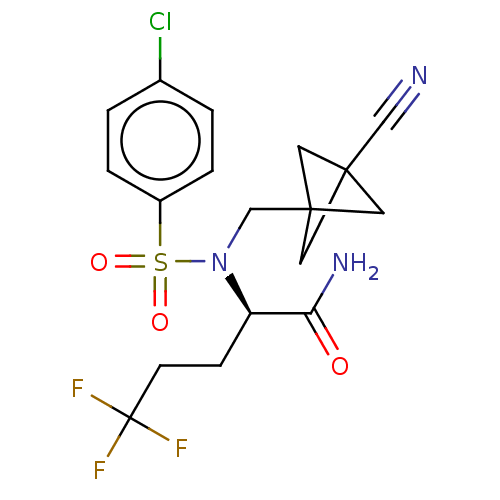 (CHEMBL2059021) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485443 (CHEMBL2059814) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485431 (CHEMBL2059815) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485434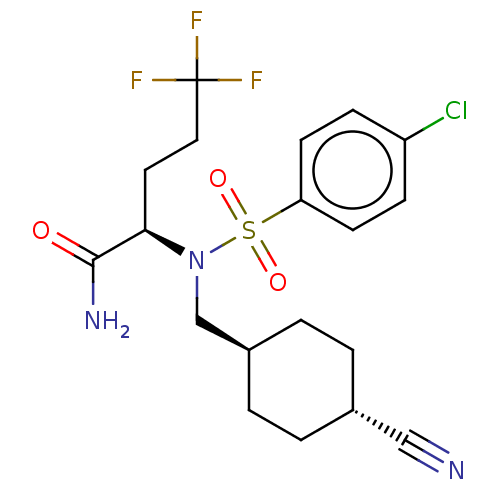 (CHEMBL2059016) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485434 (CHEMBL2059016) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485436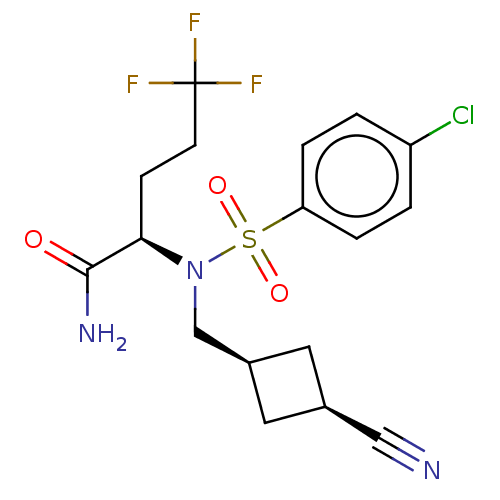 (CHEMBL2059011) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485447 (CHEMBL2059819) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485435 (CHEMBL2059014) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485446 (CHEMBL2059015) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485445 (CHEMBL2059818) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485440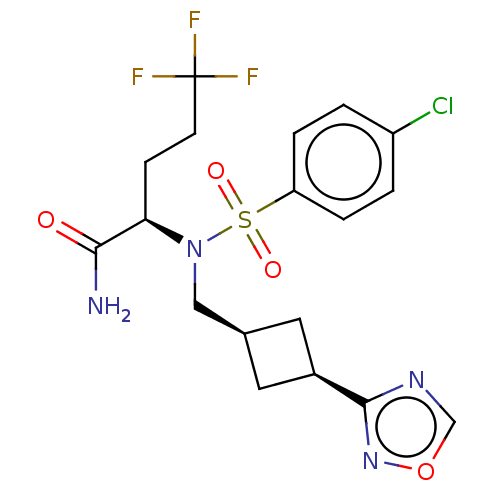 (CHEMBL2059020) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485433 (CHEMBL2059018) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485437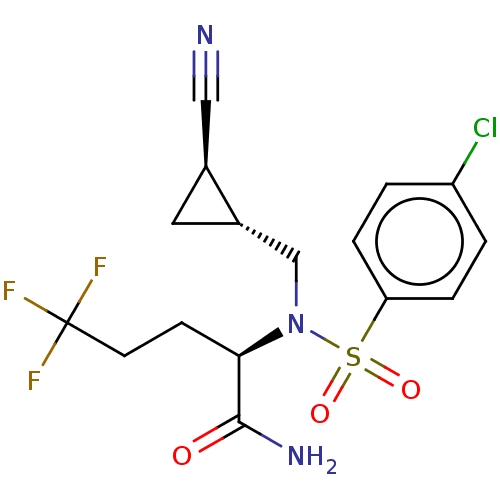 (CHEMBL2059820) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485439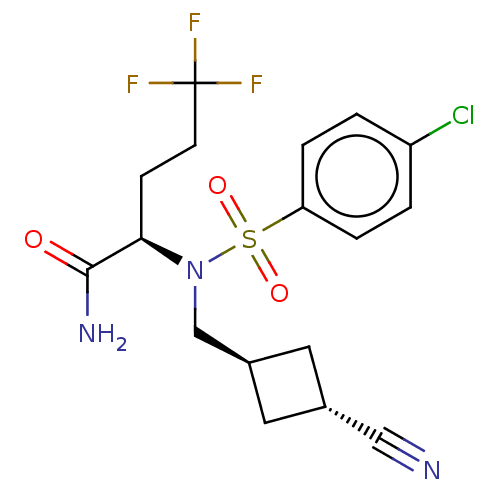 (CHEMBL2059012) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485430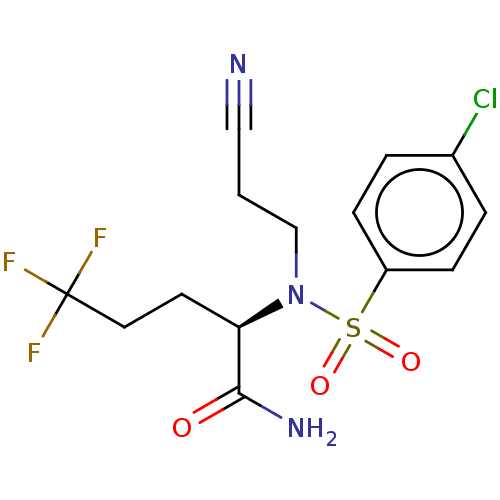 (CHEMBL2059816) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485441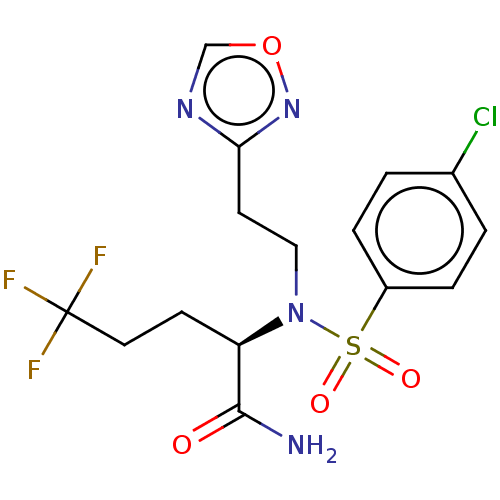 (CHEMBL2059017) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322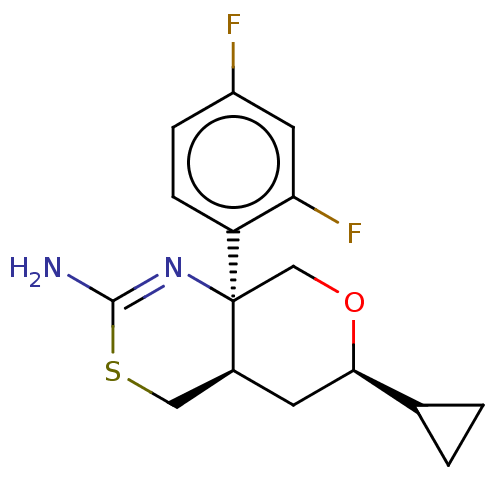 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078321 (CHEMBL3414711 | US9260455, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324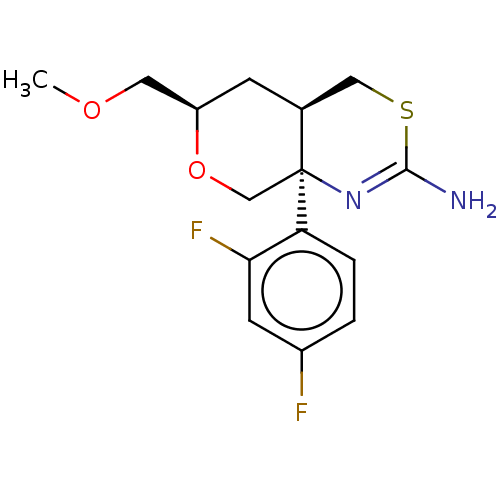 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078349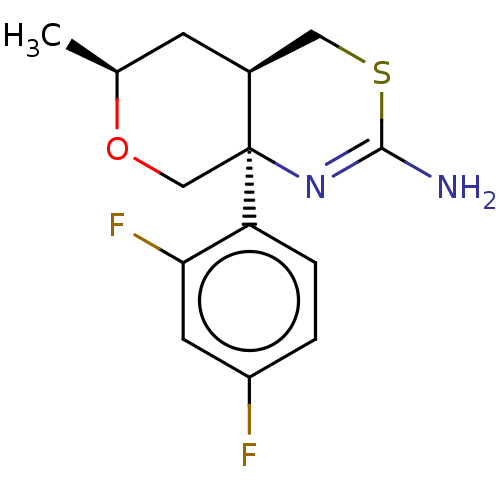 (CHEMBL3414707 | US9260455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485432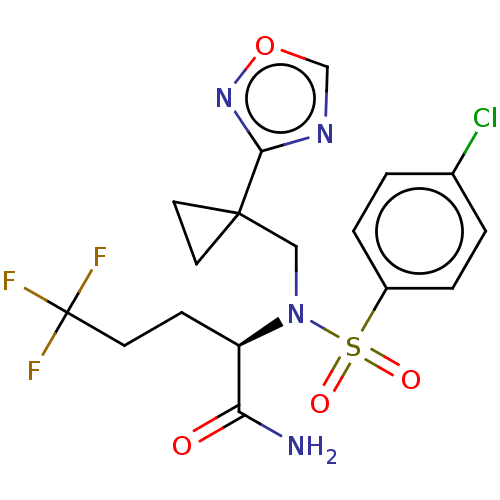 (CHEMBL2059019) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078323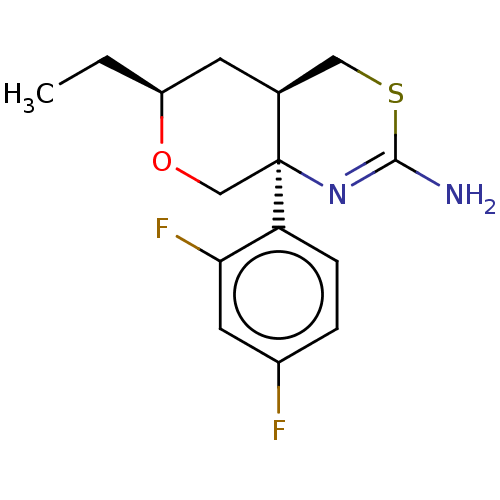 (CHEMBL3414709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078320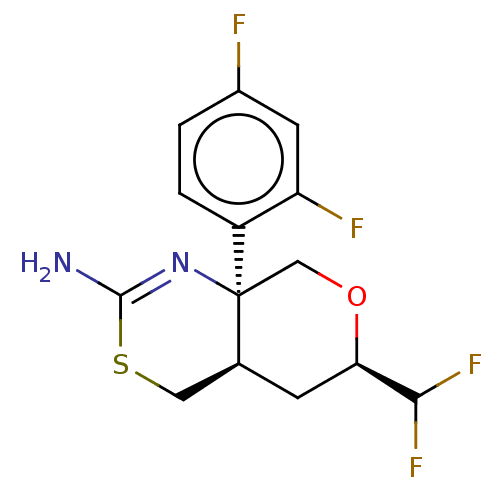 (CHEMBL3414700 | US9260455, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485442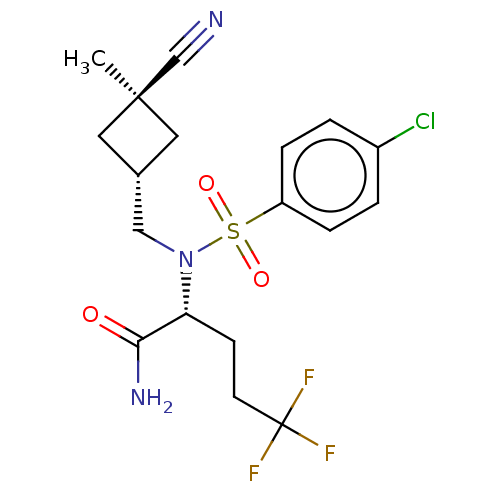 (CHEMBL2059013) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142394 (US8933221, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485448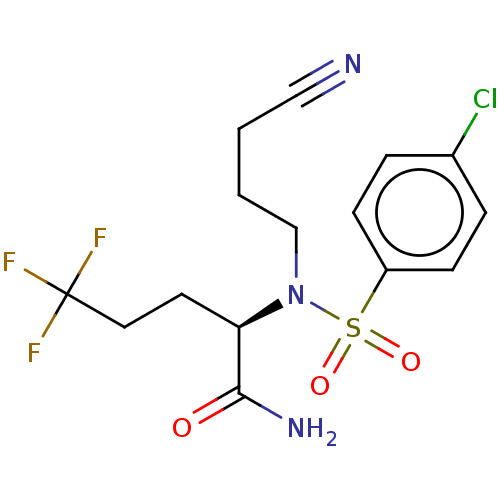 (CHEMBL2059817) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078321 (CHEMBL3414711 | US9260455, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480807 (CHEMBL576553) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](S)-5-chloro-N-(3-ethyl-1-hydroxypentan-2-yl)thiophene-2-sulfonamide from gamma secretase in human SH-SY5Y cells after 1 hr | Bioorg Med Chem 17: 4708-17 (2009) Article DOI: 10.1016/j.bmc.2009.04.052 BindingDB Entry DOI: 10.7270/Q2VX0KB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142369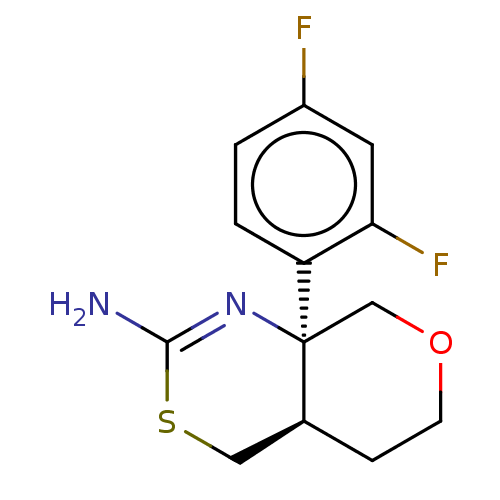 (US8933221, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078352 (CHEMBL3414705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078323 (CHEMBL3414709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078320 (CHEMBL3414700 | US9260455, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078349 (CHEMBL3414707 | US9260455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078351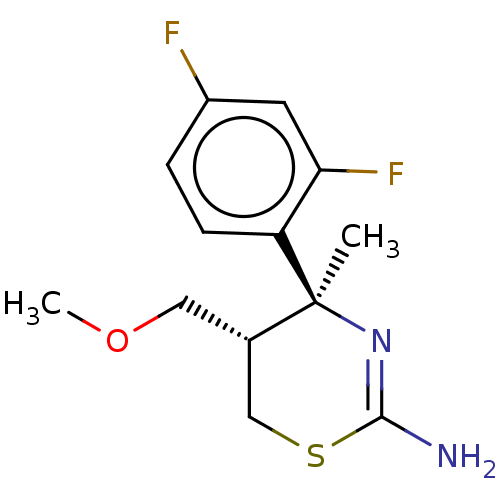 (CHEMBL3414703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142394 (US8933221, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078350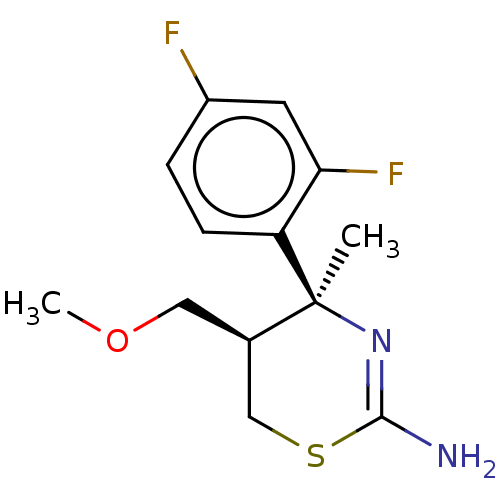 (CHEMBL3414704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM142369 (US8933221, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078352 (CHEMBL3414705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078351 (CHEMBL3414703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078321 (CHEMBL3414711 | US9260455, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078353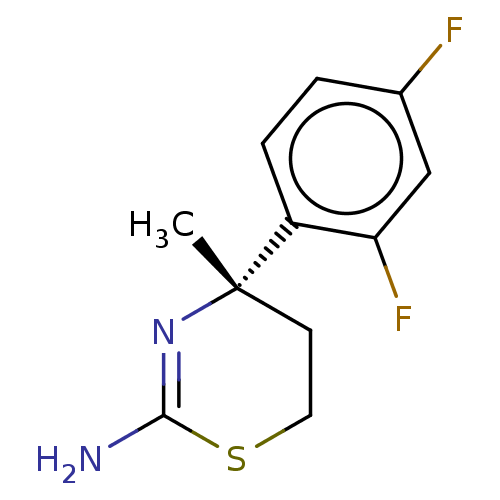 (CHEMBL3414702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078323 (CHEMBL3414709) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078349 (CHEMBL3414707 | US9260455, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM142394 (US8933221, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |