 Found 66 hits with Last Name = 'rotert' and Initial = 'g'
Found 66 hits with Last Name = 'rotert' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86244
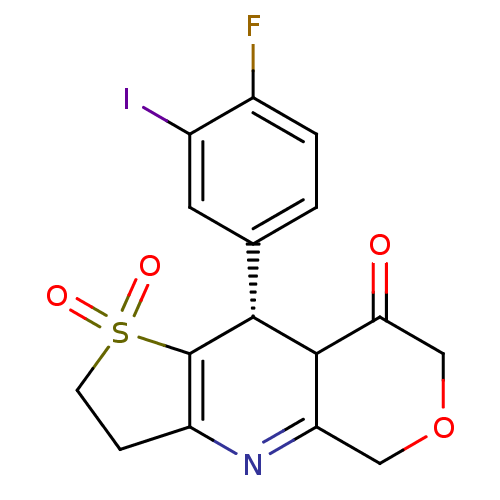
(A-312110)Show SMILES Fc1ccc(cc1I)[C@@H]1C2C(=O)COCC2=NC2=C1S(=O)(=O)CC2 |r,c:17,19| Show InChI InChI=1S/C16H13FINO4S/c17-9-2-1-8(5-10(9)18)14-15-12(6-23-7-13(15)20)19-11-3-4-24(21,22)16(11)14/h1-2,5,14-15H,3-4,6-7H2/t14-,15?/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86250

(CAS_60559-98-0 | NSC_43345 | P1075)Show InChI InChI=1S/C12H17N5/c1-4-12(2,3)17-11(15-9-13)16-10-6-5-7-14-8-10/h5-8H,4H2,1-3H3,(H2,15,16,17) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86247
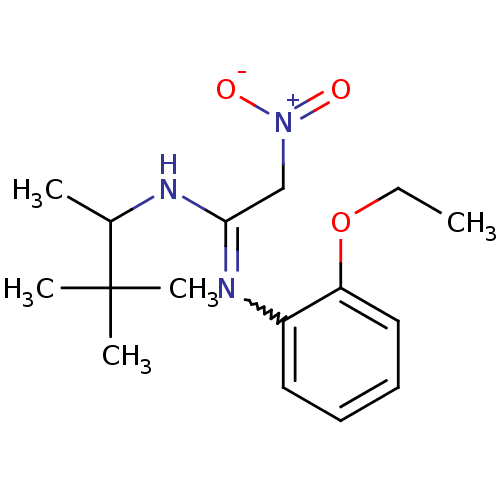
(BAY X 9228 | CAS_3038059 | NSC_3038059)Show SMILES CCOc1ccccc1N=C(C[N+]([O-])=O)NC(C)C(C)(C)C |w:9.9| Show InChI InChI=1S/C16H25N3O3/c1-6-22-14-10-8-7-9-13(14)18-15(11-19(20)21)17-12(2)16(3,4)5/h7-10,12H,6,11H2,1-5H3,(H,17,18) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86250

(CAS_60559-98-0 | NSC_43345 | P1075)Show InChI InChI=1S/C12H17N5/c1-4-12(2,3)17-11(15-9-13)16-10-6-5-7-14-8-10/h5-8H,4H2,1-3H3,(H2,15,16,17) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86247

(BAY X 9228 | CAS_3038059 | NSC_3038059)Show SMILES CCOc1ccccc1N=C(C[N+]([O-])=O)NC(C)C(C)(C)C |w:9.9| Show InChI InChI=1S/C16H25N3O3/c1-6-22-14-10-8-7-9-13(14)18-15(11-19(20)21)17-12(2)16(3,4)5/h7-10,12H,6,11H2,1-5H3,(H,17,18) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86245

(CAS_85371-64-8 | NSC_4826 | PINACIDIL)Show InChI InChI=1S/C13H19N5/c1-10(13(2,3)4)17-12(16-9-14)18-11-5-7-15-8-6-11/h5-8,10H,1-4H3,(H2,15,16,17,18) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86251
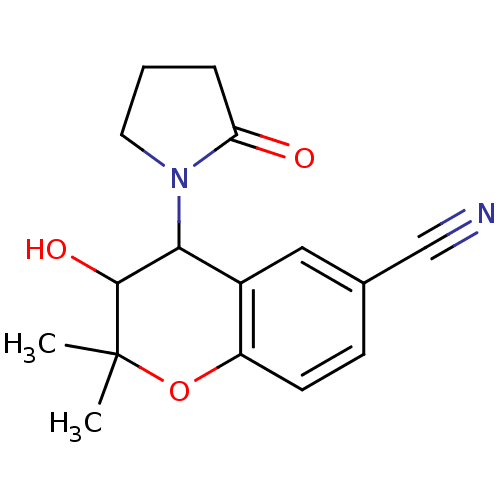
(CAS_94535-50-9 | CROMAKALIM (-) | NSC_93504)Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86245

(CAS_85371-64-8 | NSC_4826 | PINACIDIL)Show InChI InChI=1S/C13H19N5/c1-10(13(2,3)4)17-12(16-9-14)18-11-5-7-15-8-6-11/h5-8,10H,1-4H3,(H2,15,16,17,18) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86249
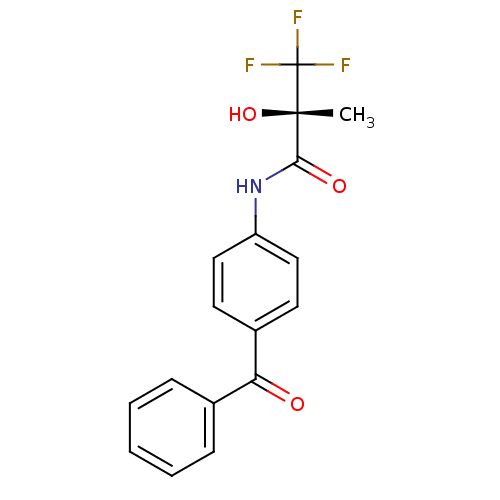
(ZD 6169 | ZD-6169)Show SMILES C[C@](O)(C(=O)Nc1ccc(cc1)C(=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C17H14F3NO3/c1-16(24,17(18,19)20)15(23)21-13-9-7-12(8-10-13)14(22)11-5-3-2-4-6-11/h2-10,24H,1H3,(H,21,23)/t16-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86251

(CAS_94535-50-9 | CROMAKALIM (-) | NSC_93504)Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86249

(ZD 6169 | ZD-6169)Show SMILES C[C@](O)(C(=O)Nc1ccc(cc1)C(=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C17H14F3NO3/c1-16(24,17(18,19)20)15(23)21-13-9-7-12(8-10-13)14(22)11-5-3-2-4-6-11/h2-10,24H,1H3,(H,21,23)/t16-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86246
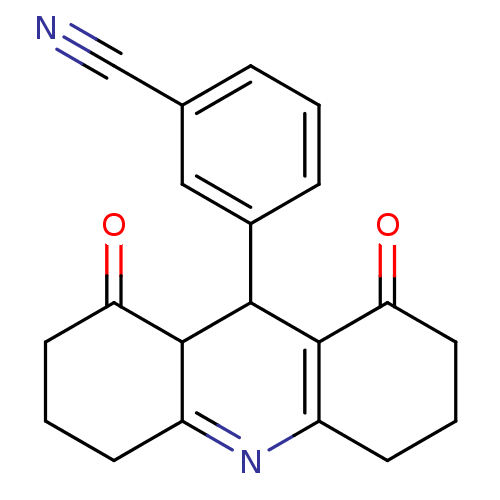
(zm244085)Show SMILES O=C1CCCC2=NC3=C(C(C12)c1cccc(c1)C#N)C(=O)CCC3 |c:7,t:5| Show InChI InChI=1S/C20H18N2O2/c21-11-12-4-1-5-13(10-12)18-19-14(6-2-8-16(19)23)22-15-7-3-9-17(24)20(15)18/h1,4-5,10,18-19H,2-3,6-9H2 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 671 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86246

(zm244085)Show SMILES O=C1CCCC2=NC3=C(C(C12)c1cccc(c1)C#N)C(=O)CCC3 |c:7,t:5| Show InChI InChI=1S/C20H18N2O2/c21-11-12-4-1-5-13(10-12)18-19-14(6-2-8-16(19)23)22-15-7-3-9-17(24)20(15)18/h1,4-5,10,18-19H,2-3,6-9H2 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86248
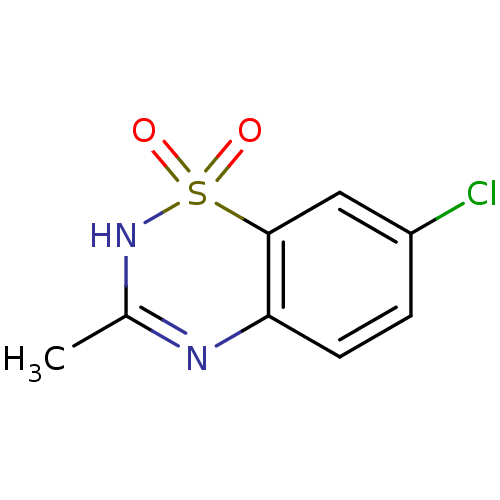
(CAS_364-98-7 | NSC_3019 | diazoxide)Show InChI InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Calcium-activated potassium channel subunit beta
(GUINEA PIG) | BDBM86248

(CAS_364-98-7 | NSC_3019 | diazoxide)Show InChI InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 143-53 (2003)
Article DOI: 10.1124/mol.64.1.143
BindingDB Entry DOI: 10.7270/Q22B8WMJ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM19235

(2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(F)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27F3N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(31)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(32)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19234

(4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2cccc(OCCCC(=O)NCCCC(O)=O)c2)cc1 Show InChI InChI=1S/C36H41N3O7S/c1-27-33(38-47(2,43)44)14-7-15-34(27)39(25-28-10-4-3-5-11-28)26-29-18-20-30(21-19-29)46-32-13-6-12-31(24-32)45-23-9-16-35(40)37-22-8-17-36(41)42/h3-7,10-15,18-21,24,38H,8-9,16-17,22-23,25-26H2,1-2H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | 86 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM19234

(4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2cccc(OCCCC(=O)NCCCC(O)=O)c2)cc1 Show InChI InChI=1S/C36H41N3O7S/c1-27-33(38-47(2,43)44)14-7-15-34(27)39(25-28-10-4-3-5-11-28)26-29-18-20-30(21-19-29)46-32-13-6-12-31(24-32)45-23-9-16-35(40)37-22-8-17-36(41)42/h3-7,10-15,18-21,24,38H,8-9,16-17,22-23,25-26H2,1-2H3,(H,37,40)(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | 4.80 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(RAT) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19236

(2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Br)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27BrF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | 330 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM19238

(2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Cl)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27ClF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM19237
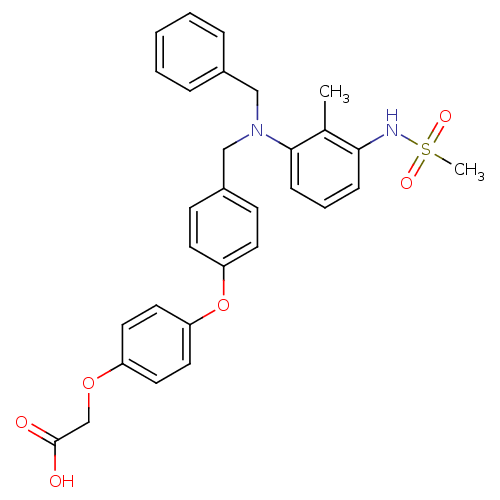
(2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C30H30N2O6S/c1-22-28(31-39(2,35)36)9-6-10-29(22)32(19-23-7-4-3-5-8-23)20-24-11-13-26(14-12-24)38-27-17-15-25(16-18-27)37-21-30(33)34/h3-18,31H,19-21H2,1-2H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM19233

((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)CC=C1[C@@]2([H])CCC2=CC(=O)CC[C@]12Cc1ccc(C)cc1 |r,c:13,t:20| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24-,25-,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19233

((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)CC=C1[C@@]2([H])CCC2=CC(=O)CC[C@]12Cc1ccc(C)cc1 |r,c:13,t:20| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24-,25-,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | 43 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19238

(2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Cl)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27ClF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | 500 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19235

(2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(F)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27F3N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(31)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(32)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | 440 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19237

(2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C30H30N2O6S/c1-22-28(31-39(2,35)36)9-6-10-29(22)32(19-23-7-4-3-5-8-23)20-24-11-13-26(14-12-24)38-27-17-15-25(16-18-27)37-21-30(33)34/h3-18,31H,19-21H2,1-2H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | 255 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009004
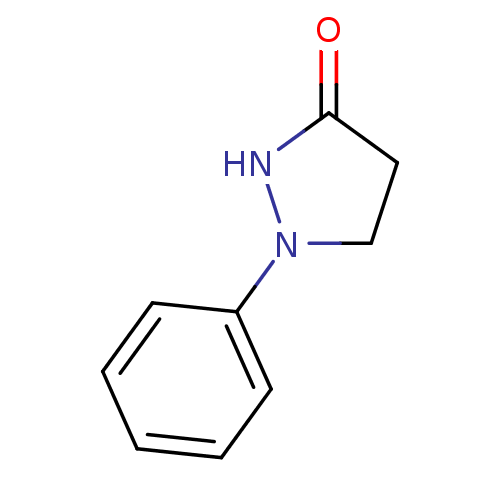
((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human platelet 12-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19234

(4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2cccc(OCCCC(=O)NCCCC(O)=O)c2)cc1 Show InChI InChI=1S/C36H41N3O7S/c1-27-33(38-47(2,43)44)14-7-15-34(27)39(25-28-10-4-3-5-11-28)26-29-18-20-30(21-19-29)46-32-13-6-12-31(24-32)45-23-9-16-35(40)37-22-8-17-36(41)42/h3-7,10-15,18-21,24,38H,8-9,16-17,22-23,25-26H2,1-2H3,(H,37,40)(H,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM19236

(2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Br)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27BrF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM19238

(2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Cl)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27ClF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM19235

(2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(F)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27F3N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(31)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(32)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50009004

((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human whole blood, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50009004

((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against soyabean 15-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50009004

((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against intact rat PMNL, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50009004

((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against intact rat PMNL, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM19234

(4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2cccc(OCCCC(=O)NCCCC(O)=O)c2)cc1 Show InChI InChI=1S/C36H41N3O7S/c1-27-33(38-47(2,43)44)14-7-15-34(27)39(25-28-10-4-3-5-11-28)26-29-18-20-30(21-19-29)46-32-13-6-12-31(24-32)45-23-9-16-35(40)37-22-8-17-36(41)42/h3-7,10-15,18-21,24,38H,8-9,16-17,22-23,25-26H2,1-2H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19237

(2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C30H30N2O6S/c1-22-28(31-39(2,35)36)9-6-10-29(22)32(19-23-7-4-3-5-8-23)20-24-11-13-26(14-12-24)38-27-17-15-25(16-18-27)37-21-30(33)34/h3-18,31H,19-21H2,1-2H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50000540
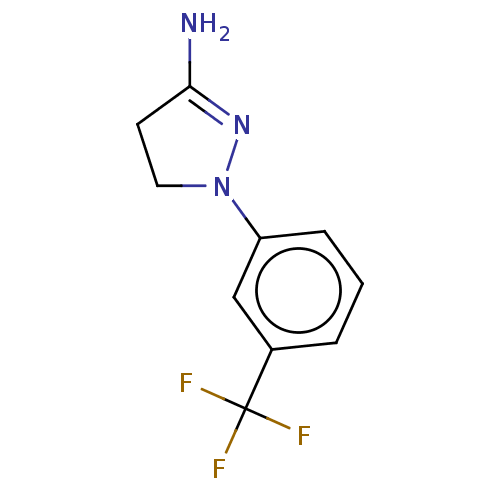
(1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazo...)Show InChI InChI=1S/C10H10F3N3/c11-10(12,13)7-2-1-3-8(6-7)16-5-4-9(14)15-16/h1-3,6H,4-5H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against intact rat PMNL, PGE-2 cyclooxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19238

(2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccc(Oc2ccc(Cl)c(OCC(O)=O)c2)cc1)Cc1ccc(F)cc1F Show InChI InChI=1S/C30H27ClF2N2O6S/c1-19-27(34-42(2,38)39)4-3-5-28(19)35(17-21-8-9-22(32)14-26(21)33)16-20-6-10-23(11-7-20)41-24-12-13-25(31)29(15-24)40-18-30(36)37/h3-15,34H,16-18H2,1-2H3,(H,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50009941

(1-Phenyl-tetrahydro-pyridazin-3-one | CHEMBL30110)Show InChI InChI=1S/C10H12N2O/c13-10-7-4-8-12(11-10)9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against intact human PMNL, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50009941

(1-Phenyl-tetrahydro-pyridazin-3-one | CHEMBL30110)Show InChI InChI=1S/C10H12N2O/c13-10-7-4-8-12(11-10)9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against intact human PMNL, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50009941

(1-Phenyl-tetrahydro-pyridazin-3-one | CHEMBL30110)Show InChI InChI=1S/C10H12N2O/c13-10-7-4-8-12(11-10)9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against intact rat PMNL, LTB4 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50009004

((phenidone)1-Phenyl-pyrazolidin-3-one | 1-Phenyl-p...)Show InChI InChI=1S/C9H10N2O/c12-9-6-7-11(10-9)8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against RBL broken cell-supematant 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM19234

(4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2cccc(OCCCC(=O)NCCCC(O)=O)c2)cc1 Show InChI InChI=1S/C36H41N3O7S/c1-27-33(38-47(2,43)44)14-7-15-34(27)39(25-28-10-4-3-5-11-28)26-29-18-20-30(21-19-29)46-32-13-6-12-31(24-32)45-23-9-16-35(40)37-22-8-17-36(41)42/h3-7,10-15,18-21,24,38H,8-9,16-17,22-23,25-26H2,1-2H3,(H,37,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM19237

(2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...)Show SMILES Cc1c(NS(C)(=O)=O)cccc1N(Cc1ccccc1)Cc1ccc(Oc2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C30H30N2O6S/c1-22-28(31-39(2,35)36)9-6-10-29(22)32(19-23-7-4-3-5-8-23)20-24-11-13-26(14-12-24)38-27-17-15-25(16-18-27)37-21-30(33)34/h3-18,31H,19-21H2,1-2H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories
| Assay Description
For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... |
J Med Chem 48: 5295-304 (2005)
Article DOI: 10.1021/jm050205o
BindingDB Entry DOI: 10.7270/Q21C1V5P |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50000540

(1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazo...)Show InChI InChI=1S/C10H10F3N3/c11-10(12,13)7-2-1-3-8(6-7)16-5-4-9(14)15-16/h1-3,6H,4-5H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against RBL broken cell-supematant 5-lipoxygenase was evaluated |
Bioorg Med Chem Lett 2: 1353-1356 (1992)
Article DOI: 10.1016/S0960-894X(00)80511-9
BindingDB Entry DOI: 10.7270/Q2H41RC2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data