

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50107746 (CHEMBL3600828) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Antagonist activity against rat MCHR1 | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM142122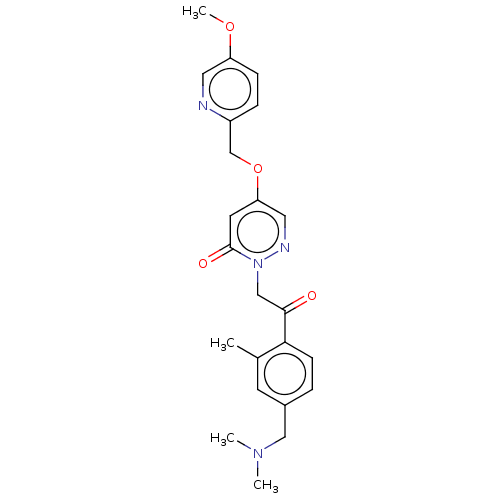 (US8933079, 149 | US8933079, 150 | US8933079, 9.1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Binding affinity to human MCH-R1 expressed in CHO/Galpha16 cells | Bioorg Med Chem Lett 25: 3275-80 (2015) Article DOI: 10.1016/j.bmcl.2015.05.065 BindingDB Entry DOI: 10.7270/Q2T43VV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107817 (CHEMBL3600810) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50329791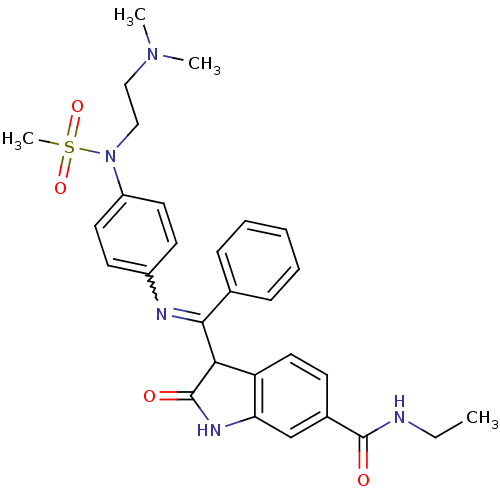 ((Z)-3-((4-(N-(2-(dimethylamino)ethyl)methylsulfona...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of TGFbeta receptor | J Med Chem 53: 7287-95 (2010) Article DOI: 10.1021/jm100812a BindingDB Entry DOI: 10.7270/Q2F47PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107818 (CHEMBL3600811) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285776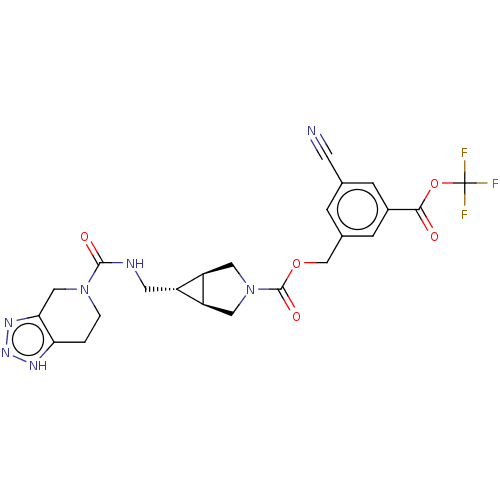 (CHEMBL4172309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285750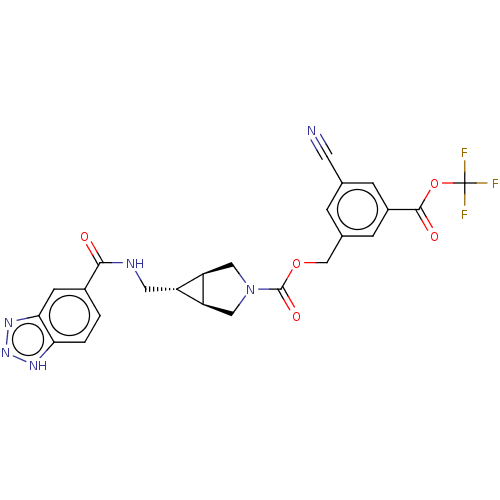 (CHEMBL4173049) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557594 (CHEMBL4788435 | US11485727, Example 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557595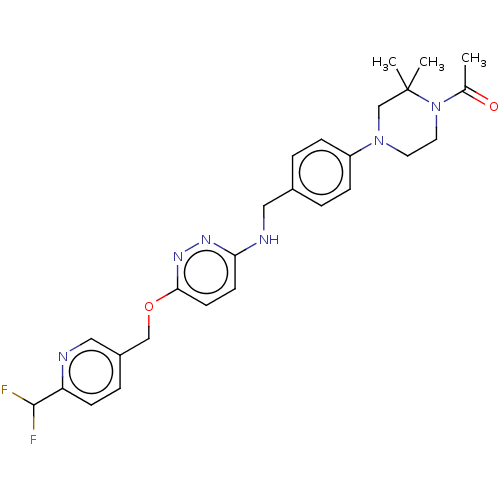 (CHEMBL4745566 | US11485727, Example 1.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285745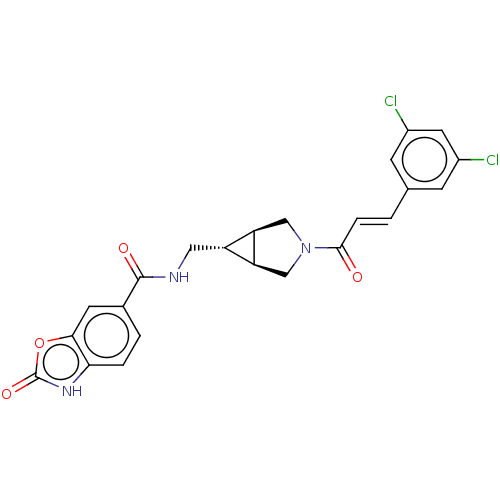 (CHEMBL4162641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557599 (CHEMBL4751395 | US11485727, Example 2.10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557597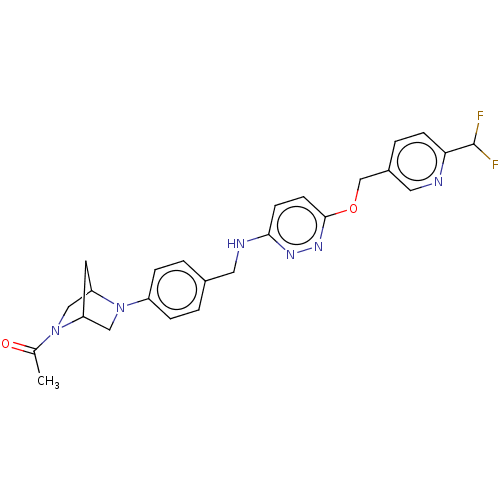 (CHEMBL4758133 | US11485727, Example 2.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557598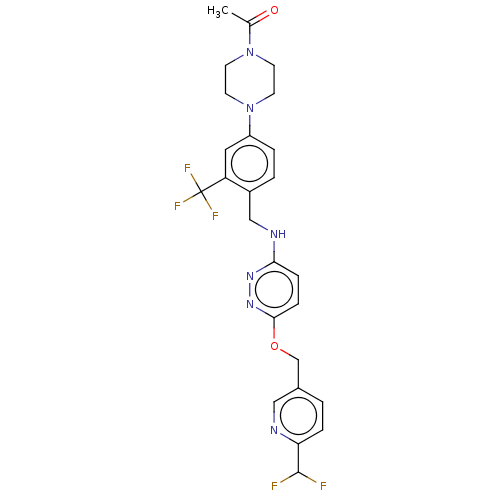 (CHEMBL4784419 | US11485727, Example 2.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285744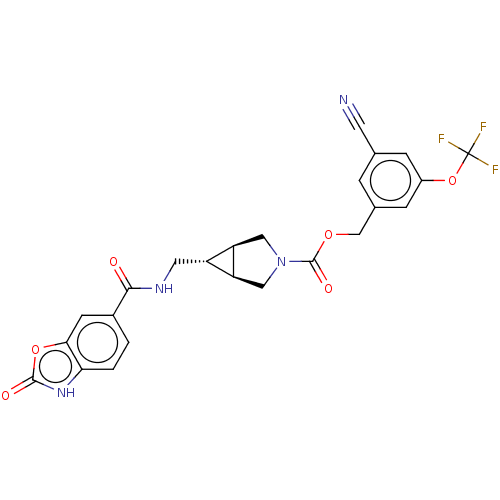 (CHEMBL4168498) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM50557596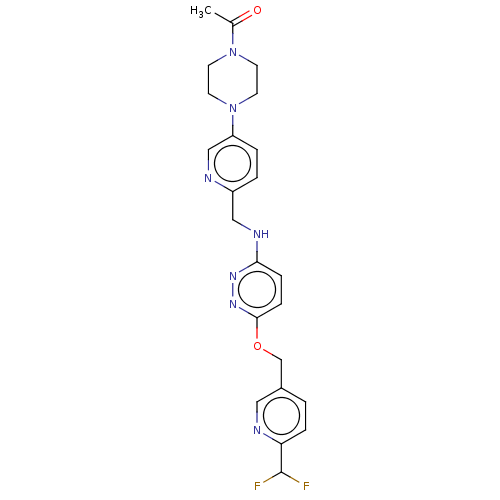 (CHEMBL4743845 | US11485727, Example 2.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575715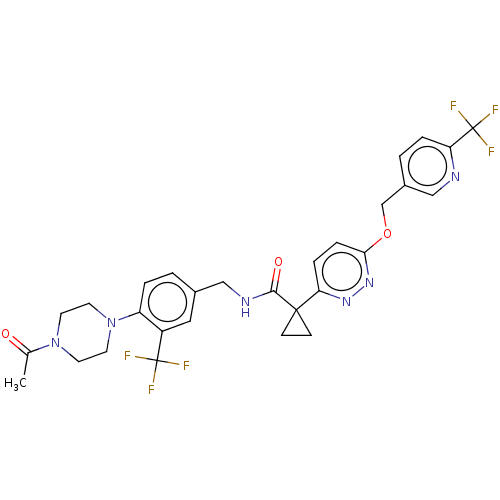 (US11465982, Example 1.15) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107760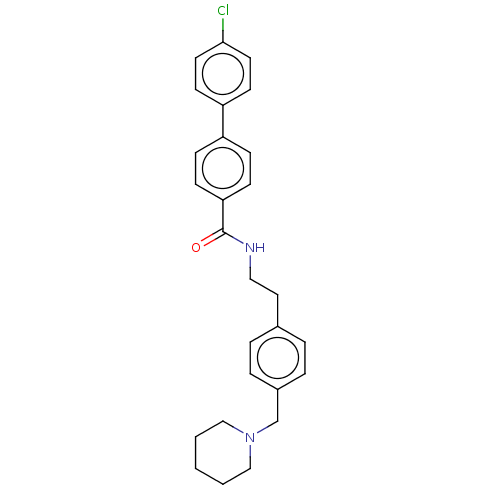 (CHEMBL3600803) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107761 (CHEMBL3600804) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107764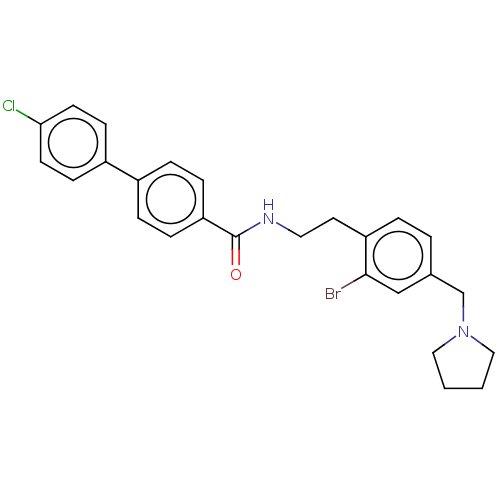 (CHEMBL3600807) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579837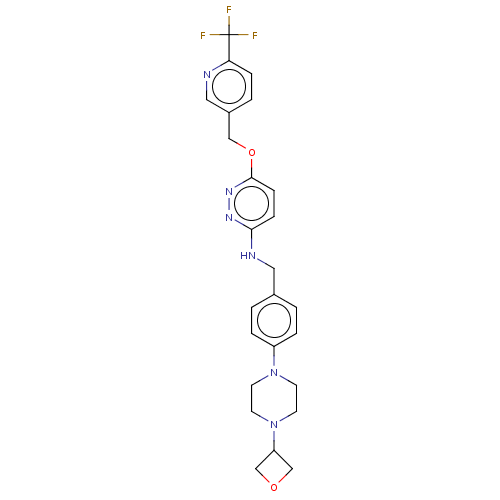 (US11485727, Example 2.8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107733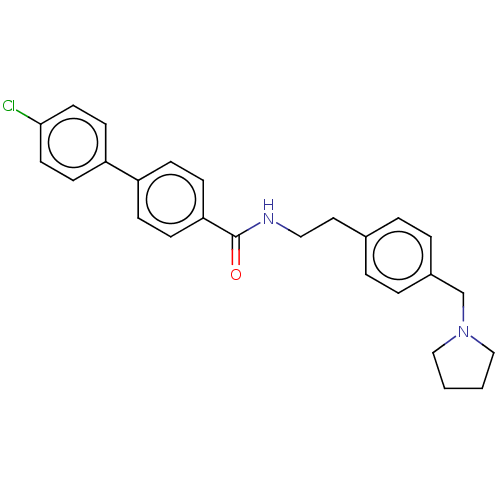 (CHEMBL3600801) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM516881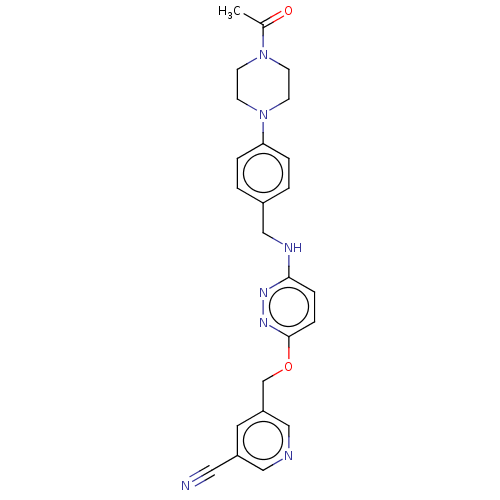 (US11104665, Example 1.2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM191570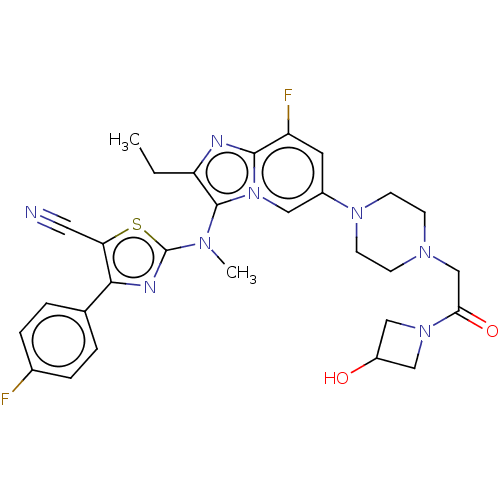 (US10526329, Compound 12 | US11072611, Compound 12 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM191570 (US10526329, Compound 12 | US11072611, Compound 12 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM516893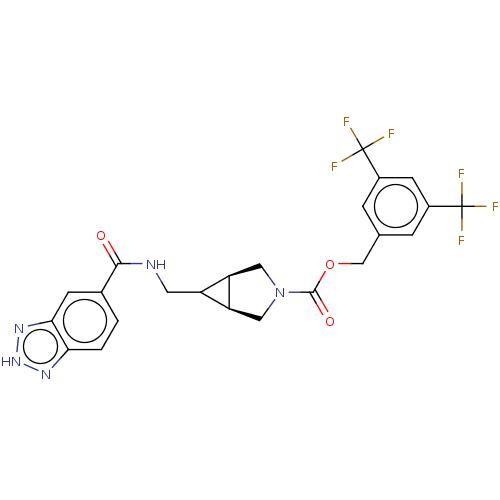 (US11104665, ACS Med. Chem. Lett. 2017, 8, 1252-125...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50285777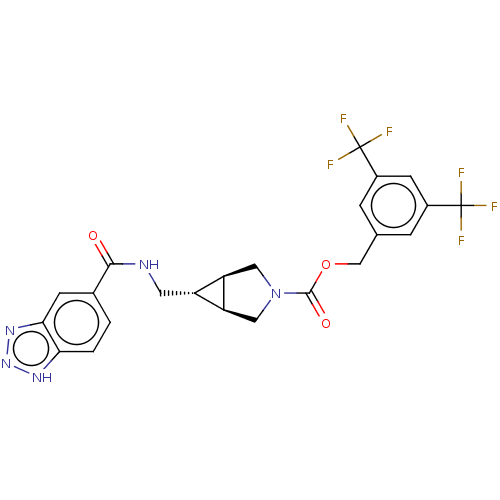 (CHEMBL4165749) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of human ATX | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579844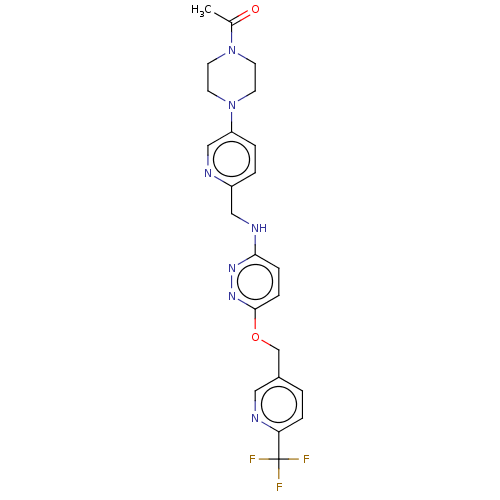 (US11485727, Example 2.15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579832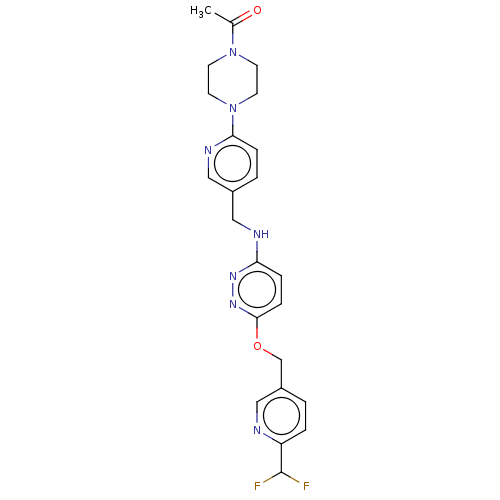 (US11485727, Example 2.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575719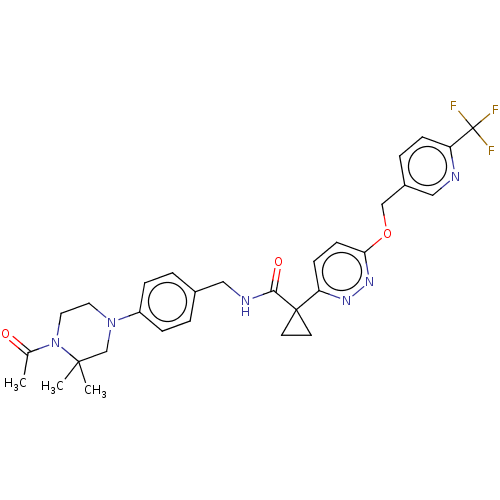 (US11465982, Example 1.19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575717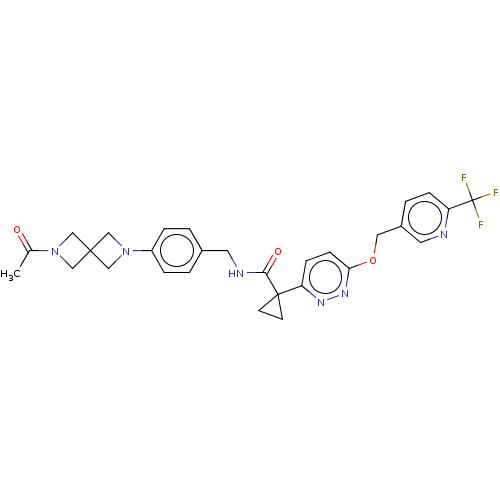 (US11465982, Example 1.17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285748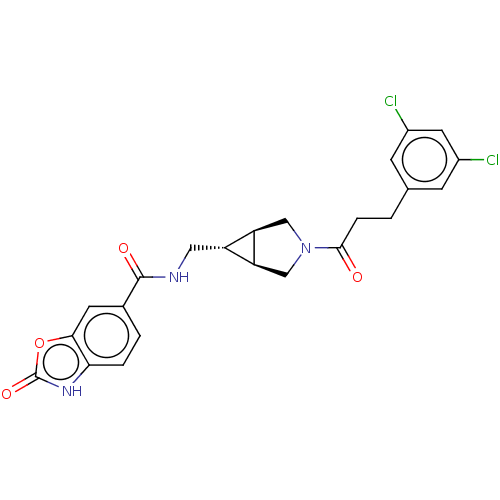 (CHEMBL4173341) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579830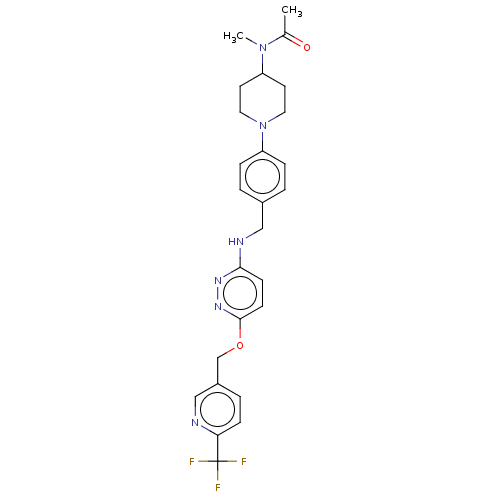 (N-methyl-N-[1-(4-{[(6-{[6-(trifluoromethyl)pyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285774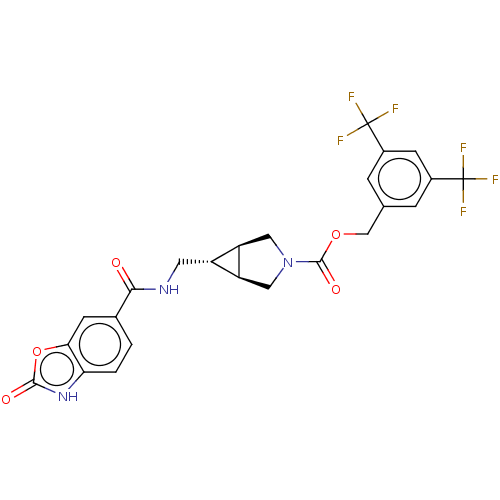 (CHEMBL4169550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575706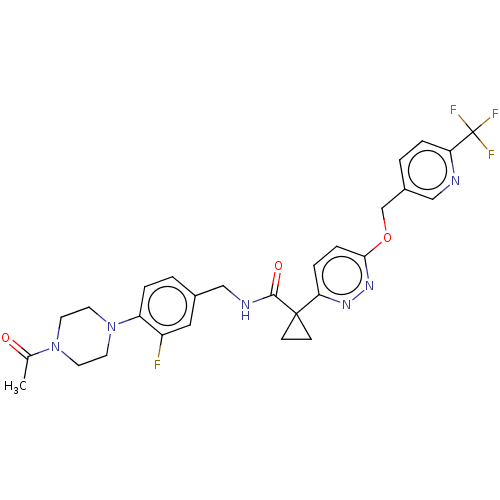 (US11465982, Example 1.6) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575710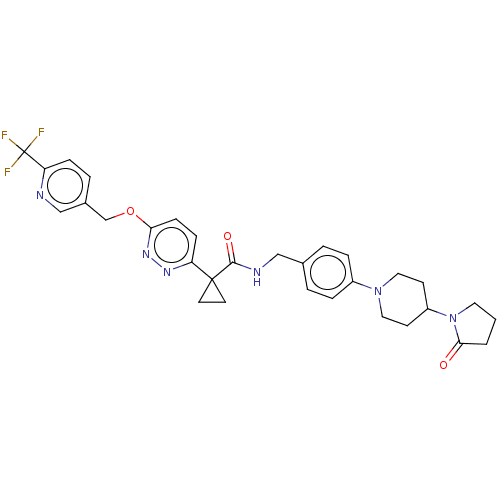 (US11465982, Example 1.10) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579848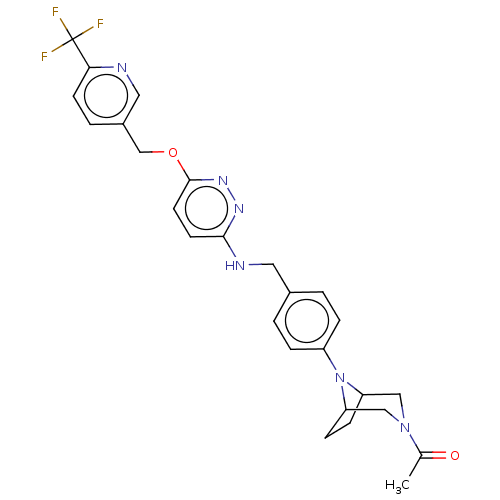 (US11485727, Example 2.19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579850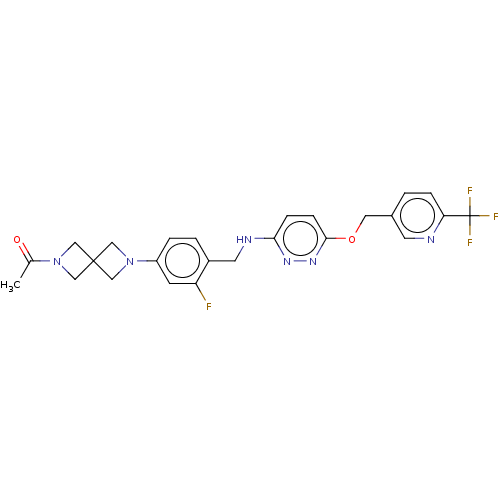 (US11485727, Example 2.21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579834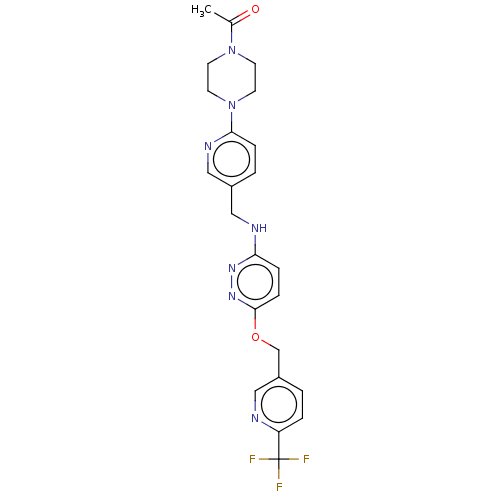 (US11485727, Example 2.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575722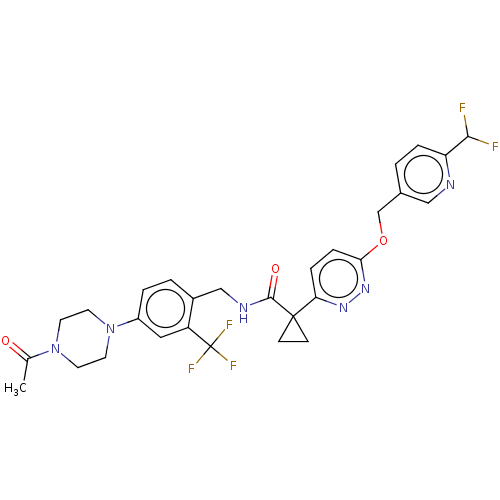 (US11465982, Example 1.22) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575711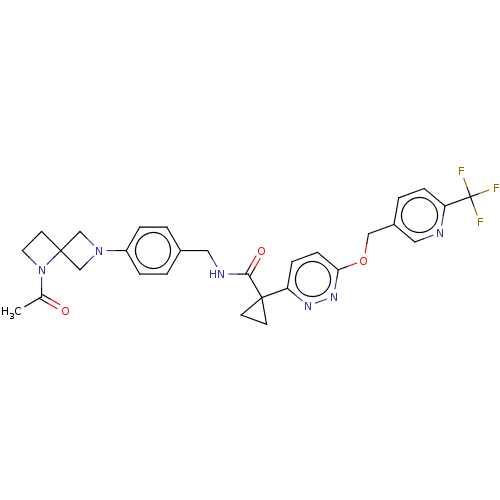 (US11465982, Example 1.11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM575713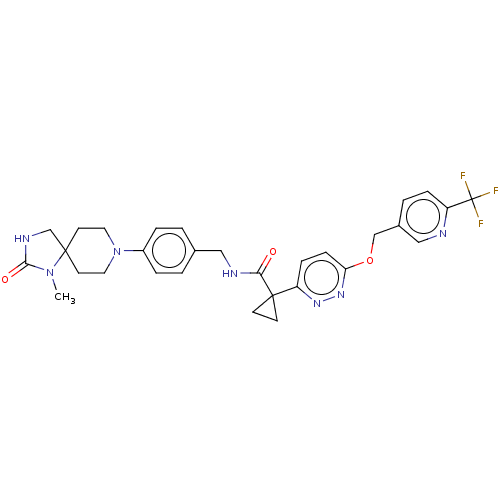 (US11465982, Example 1.13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579824 (US11485727, Example 1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579853 (1-[4-(4-{[(6-{[6-(Difluoromethyl)pyridin-3-yl]meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-1 (Homo sapiens (Human)) | BDBM579849 (US11485727, Example 2.20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 5 nM recombinant ATX (Cayman Chemicals) was supplemented to 50 mM Tris buffer (pH 8.0) containing 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2 0.14 mM NaCl, and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107820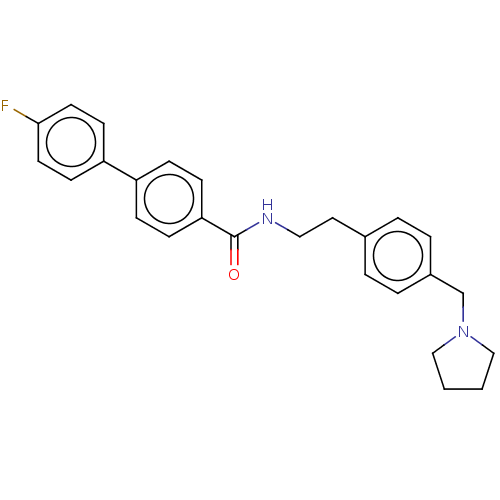 (CHEMBL3600812) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107735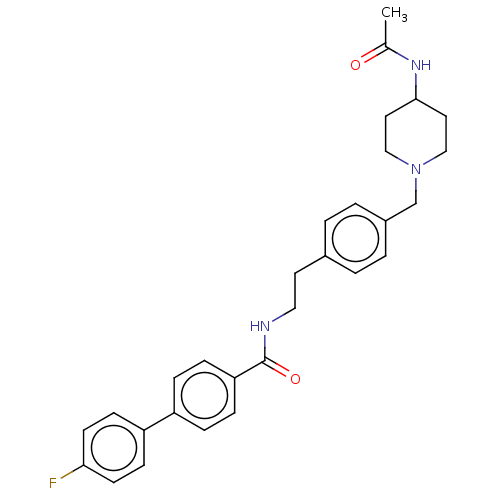 (CHEMBL3600815) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM230371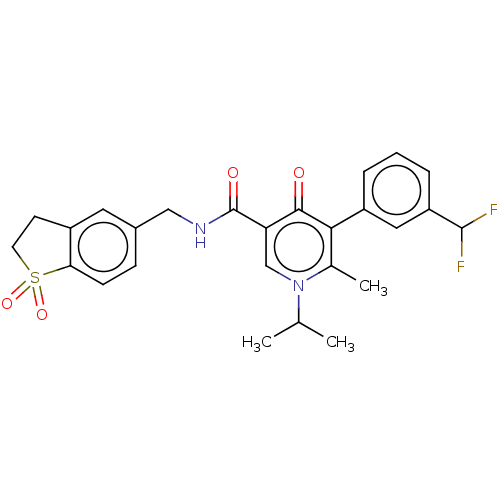 (US9340507, 29.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compound buffer: 100 mM Tris, 500 mM NaCl, adjusted to pH 7.5; Assay buffer: 100 mM Tris, 500 mM NaCl, adjusted to pH 7.5, containing 0.01% BSA. Test... | US Patent US9340507 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106487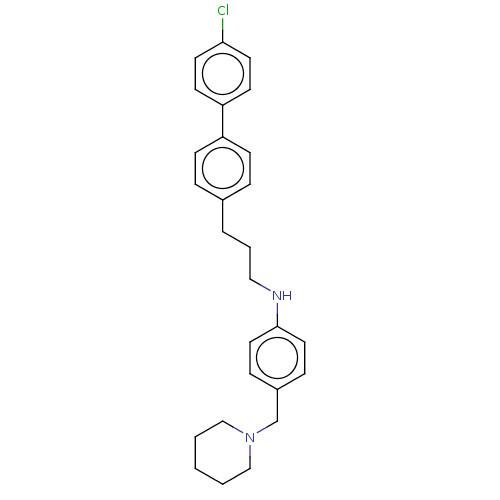 (CHEMBL3600970) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106579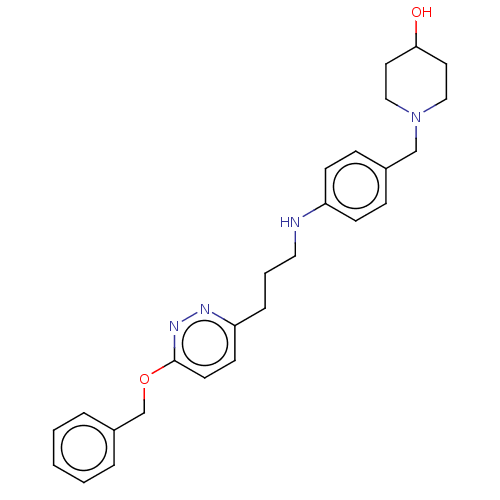 (CHEMBL3601036) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50329793 ((Z)-3-((4-(N-(2-(dimethylamino)ethyl)acetamido)phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of TGFbeta receptor | J Med Chem 53: 7287-95 (2010) Article DOI: 10.1021/jm100812a BindingDB Entry DOI: 10.7270/Q2F47PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3292 total ) | Next | Last >> |