 Found 405 hits with Last Name = 'rowley' and Initial = 'j'
Found 405 hits with Last Name = 'rowley' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heparanase
(Homo sapiens (Human)) | BDBM50388329
(CHEMBL2059500)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C54H91N3O60S13/c1-24(2)7-6-8-25(3)30-11-12-31-29-10-9-26-17-28(13-15-53(26,4)32(29)14-16-54(30,31)5)97-19-27-18-57(56-55-27)49-45(114-127(85,86)87)41(110-123(73,74)75)37(33(102-49)20-98-118(58,59)60)106-50-46(115-128(88,89)90)42(111-124(76,77)78)38(34(103-50)21-99-119(61,62)63)107-51-47(116-129(91,92)93)43(112-125(79,80)81)39(35(104-51)22-100-120(64,65)66)108-52-48(117-130(94,95)96)44(113-126(82,83)84)40(109-122(70,71)72)36(105-52)23-101-121(67,68)69/h18,24-26,28-52H,6-17,19-23H2,1-5H3,(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)/p-13/t25-,26+,28+,29+,30-,31+,32+,33-,34-,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45-,46-,47-,48-,49-,50-,51-,52-,53+,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388343
(CHEMBL2059243)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C63H107N3O75S16/c1-28(2)8-6-9-29(3)34-12-13-35-33-11-10-30-20-32(14-16-62(30,4)36(33)15-17-63(34,35)5)116-22-31-21-66(65-64-31)18-7-19-115-57-52(50(136-152(97,98)99)45(134-150(91,92)93)40(122-57)26-120-145(76,77)78)130-60-55(140-156(109,110)111)49(44(133-149(88,89)90)39(125-60)25-119-144(73,74)75)128-58-53(138-154(103,104)105)47(42(131-147(82,83)84)37(123-58)23-117-142(67,68)69)127-59-54(139-155(106,107)108)48(43(132-148(85,86)87)38(124-59)24-118-143(70,71)72)129-61-56(141-157(112,113)114)51(137-153(100,101)102)46(135-151(94,95)96)41(126-61)27-121-146(79,80)81/h21,28-30,32-61H,6-20,22-27H2,1-5H3,(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)(H,97,98,99)(H,100,101,102)(H,103,104,105)(H,106,107,108)(H,109,110,111)(H,112,113,114)/p-16/t29-,30+,32+,33+,34-,35+,36+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58-,59-,60-,61-,62+,63-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388341
(CHEMBL2059241)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C51H88O60S13/c1-22(2)7-6-8-23(3)27-11-12-28-26-10-9-24-17-25(13-15-50(24,4)29(26)14-16-51(27,28)5)95-46-42(40(107-120(76,77)78)36(105-118(70,71)72)32(96-46)20-93-114(58,59)60)102-48-44(110-123(85,86)87)39(35(104-117(67,68)69)31(98-48)19-92-113(55,56)57)100-47-43(109-122(82,83)84)38(34(103-116(64,65)66)30(97-47)18-91-112(52,53)54)101-49-45(111-124(88,89)90)41(108-121(79,80)81)37(106-119(73,74)75)33(99-49)21-94-115(61,62)63/h22-49H,6-21H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-13/t23-,24+,25+,26+,27-,28+,29+,30-,31-,32-,33-,34-,35-,36-,37-,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388342
(CHEMBL2059242)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H83NO61S13/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-29(47)46-19-17-20-87-42-38(36(103-116(72,73)74)32(101-114(66,67)68)27(92-42)23-90-110(54,55)56)98-44-40(106-119(81,82)83)35(31(100-113(63,64)65)26(94-44)22-89-109(51,52)53)96-43-39(105-118(78,79)80)34(30(99-112(60,61)62)25(93-43)21-88-108(48,49)50)97-45-41(107-120(84,85)86)37(104-117(75,76)77)33(102-115(69,70)71)28(95-45)24-91-111(57,58)59/h25-28,30-45H,2-24H2,1H3,(H,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,30-,31-,32-,33-,34+,35+,36+,37+,38+,39+,40+,41+,42+,43-,44-,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388331
(CHEMBL2059499)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C51H88O60S13/c1-22(2)7-6-8-23(3)27-11-12-28-26-10-9-24-17-25(13-15-50(24,4)29(26)14-16-51(27,28)5)95-46-42(108-121(79,80)81)38(104-117(67,68)69)34(30(96-46)18-91-112(52,53)54)100-47-43(109-122(82,83)84)39(105-118(70,71)72)35(31(97-47)19-92-113(55,56)57)101-48-44(110-123(85,86)87)40(106-119(73,74)75)36(32(98-48)20-93-114(58,59)60)102-49-45(111-124(88,89)90)41(107-120(76,77)78)37(103-116(64,65)66)33(99-49)21-94-115(61,62)63/h22-49H,6-21H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-13/t23-,24+,25+,26+,27-,28+,29+,30-,31-,32-,33-,34-,35-,36-,37-,38+,39+,40+,41+,42-,43-,44-,45-,46-,47-,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388345
(CHEMBL2059245)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(36(88-98(64,65)66)33(86-96(58,59)60)30(80-41)19-77-93(49,50)51)84-42-39(90-100(70,71)72)35(32(85-95(55,56)57)29(81-42)18-76-92(46,47)48)83-43-40(91-101(73,74)75)37(89-99(67,68)69)34(87-97(61,62)63)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38+,39+,40+,41+,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50378647
(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388332
(CHEMBL2059498)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C51H87N3O47S10/c1-26(2)8-6-9-27(3)32-12-13-33-31-11-10-28-20-30(14-16-50(28,4)34(31)15-17-51(32,33)5)86-22-29-21-54(53-52-29)18-7-19-85-47-44(99-109(76,77)78)41(38(95-105(64,65)66)35(90-47)23-87-102(55,56)57)93-48-45(100-110(79,80)81)42(39(96-106(67,68)69)36(91-48)24-88-103(58,59)60)94-49-46(101-111(82,83)84)43(98-108(73,74)75)40(97-107(70,71)72)37(92-49)25-89-104(61,62)63/h21,26-28,30-49H,6-20,22-25H2,1-5H3,(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)/p-10/t27-,28+,30+,31+,32-,33+,34+,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45+,46+,47+,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388346
(CHEMBL2059246)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C51H87N3O47S10/c1-26(2)8-6-9-27(3)32-12-13-33-31-11-10-28-20-30(14-16-50(28,4)34(31)15-17-51(32,33)5)86-22-29-21-54(53-52-29)18-7-19-85-47-44(42(98-108(73,74)75)39(96-106(67,68)69)36(90-47)24-88-103(58,59)60)94-48-45(100-110(79,80)81)41(38(95-105(64,65)66)35(91-48)23-87-102(55,56)57)93-49-46(101-111(82,83)84)43(99-109(76,77)78)40(97-107(70,71)72)37(92-49)25-89-104(61,62)63/h21,26-28,30-49H,6-20,22-25H2,1-5H3,(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)/p-10/t27-,28+,30+,31+,32-,33+,34+,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45+,46+,47+,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388335

(CHEMBL2059503)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(89-99(67,68)69)35(86-96(58,59)60)32(29(80-41)18-76-92(46,47)48)83-42-39(90-100(70,71)72)36(87-97(61,62)63)33(30(81-42)19-77-93(49,50)51)84-43-40(91-101(73,74)75)37(88-98(64,65)66)34(85-95(55,56)57)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38-,39-,40-,41-,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388336
(CHEMBL2059504)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H83NO61S13/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-29(47)46-19-17-20-87-42-38(104-117(75,76)77)34(100-113(63,64)65)30(25(92-42)21-88-108(48,49)50)96-43-39(105-118(78,79)80)35(101-114(66,67)68)31(26(93-43)22-89-109(51,52)53)97-44-40(106-119(81,82)83)36(102-115(69,70)71)32(27(94-44)23-90-110(54,55)56)98-45-41(107-120(84,85)86)37(103-116(72,73)74)33(99-112(60,61)62)28(95-45)24-91-111(57,58)59/h25-28,30-45H,2-24H2,1H3,(H,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,30-,31-,32-,33-,34+,35+,36+,37+,38-,39-,40-,41-,42-,43-,44-,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388330
(CHEMBL2059247)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(89-99(67,68)69)35(32(85-95(55,56)57)29(80-41)18-76-92(46,47)48)83-42-39(90-100(70,71)72)36(33(86-96(58,59)60)30(81-42)19-77-93(49,50)51)84-43-40(91-101(73,74)75)37(88-98(64,65)66)34(87-97(61,62)63)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38+,39+,40+,41+,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388328
(CHEMBL2059505)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C39H73NO47S10/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-27(41)40-19-17-20-72-37-34(85-95(63,64)65)31(82-92(54,55)56)28(24(76-37)21-73-88(42,43)44)79-38-35(86-96(66,67)68)32(83-93(57,58)59)29(25(77-38)22-74-89(45,46)47)80-39-36(87-97(69,70)71)33(84-94(60,61)62)30(81-91(51,52)53)26(78-39)23-75-90(48,49)50/h24-26,28-39H,2-23H2,1H3,(H,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)/p-10/t24-,25-,26-,28-,29-,30-,31+,32+,33+,34-,35-,36-,37-,38-,39-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388334
(CHEMBL2059501)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C48H83N3O46S10/c1-23(2)7-6-8-24(3)29-11-12-30-28-10-9-25-17-27(13-15-47(25,4)31(28)14-16-48(29,30)5)82-19-26-18-51(50-49-26)44-41(95-105(73,74)75)38(92-102(64,65)66)35(32(86-44)20-83-98(52,53)54)89-45-42(96-106(76,77)78)39(93-103(67,68)69)36(33(87-45)21-84-99(55,56)57)90-46-43(97-107(79,80)81)40(94-104(70,71)72)37(91-101(61,62)63)34(88-46)22-85-100(58,59)60/h18,23-25,27-46,101H,6-17,19-22H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H2,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-10/t24-,25+,27+,28+,29-,30+,31+,32-,33-,34-,35-,36-,37-,38+,39+,40+,41-,42-,43-,44-,45-,46-,47+,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388333
(CHEMBL2059502)Show SMILES C[C@H](CCC(=O)N[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](OC(C)=O)[C@]12C)OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H83NO66S14/c1-19(24-8-9-25-23-7-6-21-13-22(108-122(66,67)68)11-12-49(21,3)26(23)14-31(50(24,25)4)100-20(2)52)5-10-32(53)51-45-41(114-128(84,85)86)37(110-124(72,73)74)33(27(101-45)15-96-118(54,55)56)105-46-42(115-129(87,88)89)38(111-125(75,76)77)34(28(102-46)16-97-119(57,58)59)106-47-43(116-130(90,91)92)39(112-126(78,79)80)35(29(103-47)17-98-120(60,61)62)107-48-44(117-131(93,94)95)40(113-127(81,82)83)36(109-123(69,70)71)30(104-48)18-99-121(63,64)65/h19,21-31,33-48H,5-18H2,1-4H3,(H,51,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)/p-14/t19-,21+,22+,23+,24-,25+,26+,27-,28-,29-,30-,31+,33-,34-,35-,36-,37+,38+,39+,40+,41-,42-,43-,44-,45-,46-,47-,48-,49+,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388344
(CHEMBL2059244)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C39H68O32S7/c1-20(2)7-6-8-21(3)25-11-12-26-24-10-9-22-17-23(13-15-38(22,4)27(24)14-16-39(25,26)5)63-36-34(32(69-76(52,53)54)30(67-74(46,47)48)28(64-36)18-61-72(40,41)42)66-37-35(71-78(58,59)60)33(70-77(55,56)57)31(68-75(49,50)51)29(65-37)19-62-73(43,44)45/h20-37H,6-19H2,1-5H3,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)/p-7/t21-,22+,23+,24+,25-,26+,27+,28-,29-,30-,31-,32+,33+,34+,35+,36+,37-,38+,39-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388340
(CHEMBL2059510)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@@H]2O[C@H](COS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C42H71N3O32S7/c1-22(2)7-6-8-23(3)28-11-12-29-27-10-9-24-17-26(13-15-41(24,4)30(27)14-16-42(28,29)5)67-19-25-18-45(44-43-25)39-37(76-83(61,62)63)35(74-81(55,56)57)33(31(70-39)20-68-78(46,47)48)72-40-38(77-84(64,65)66)36(75-82(58,59)60)34(73-80(52,53)54)32(71-40)21-69-79(49,50)51/h18,22-24,26-40H,6-17,19-21H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)/p-7/t23-,24+,26+,27+,28-,29+,30+,31-,32-,33-,34+,35+,36+,37-,38-,39-,40+,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388338
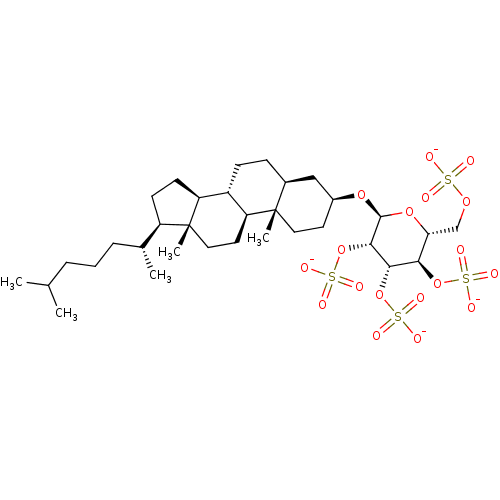
(CHEMBL2059508)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C33H58O18S4/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(13-15-32(21,4)26(23)14-16-33(24,25)5)47-31-30(51-55(43,44)45)29(50-54(40,41)42)28(49-53(37,38)39)27(48-31)18-46-52(34,35)36/h19-31H,6-18H2,1-5H3,(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)/p-4/t20-,21+,22+,23+,24-,25+,26+,27-,28-,29+,30+,31+,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520310
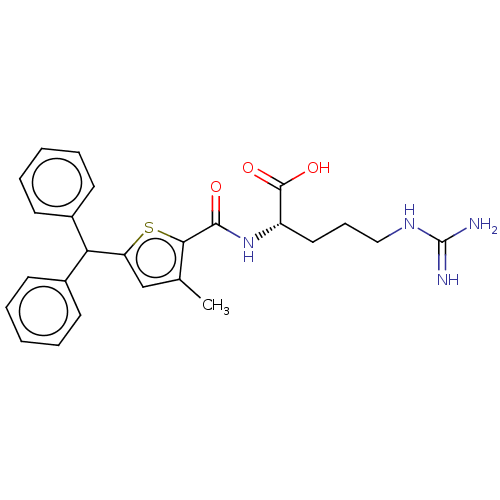
(CHEMBL4470864)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O3S/c1-16-15-20(21(17-9-4-2-5-10-17)18-11-6-3-7-12-18)33-22(16)23(30)29-19(24(31)32)13-8-14-28-25(26)27/h2-7,9-12,15,19,21H,8,13-14H2,1H3,(H,29,30)(H,31,32)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520310

(CHEMBL4470864)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O3S/c1-16-15-20(21(17-9-4-2-5-10-17)18-11-6-3-7-12-18)33-22(16)23(30)29-19(24(31)32)13-8-14-28-25(26)27/h2-7,9-12,15,19,21H,8,13-14H2,1H3,(H,29,30)(H,31,32)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520316
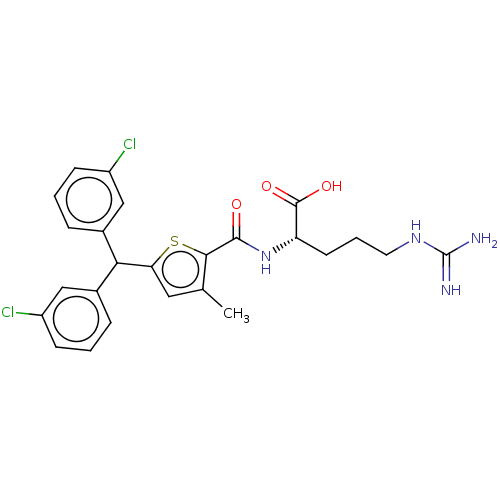
(CHEMBL4445758)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1cccc(Cl)c1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-11-20(21(15-5-2-7-17(26)12-15)16-6-3-8-18(27)13-16)35-22(14)23(32)31-19(24(33)34)9-4-10-30-25(28)29/h2-3,5-8,11-13,19,21H,4,9-10H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520316

(CHEMBL4445758)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1cccc(Cl)c1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-11-20(21(15-5-2-7-17(26)12-15)16-6-3-8-18(27)13-16)35-22(14)23(32)31-19(24(33)34)9-4-10-30-25(28)29/h2-3,5-8,11-13,19,21H,4,9-10H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520305

(CHEMBL4559976)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(C1CCCCC1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H32N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1,3-4,8-9,13-14,17-18,21H,2,5-7,10-12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-,21?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520309
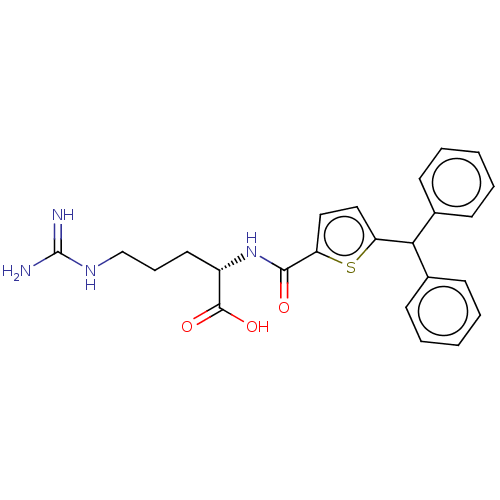
(CHEMBL4576800)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H26N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1-6,8-11,13-14,18,21H,7,12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520305

(CHEMBL4559976)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(C1CCCCC1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H32N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1,3-4,8-9,13-14,17-18,21H,2,5-7,10-12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-,21?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520309

(CHEMBL4576800)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H26N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1-6,8-11,13-14,18,21H,7,12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520303
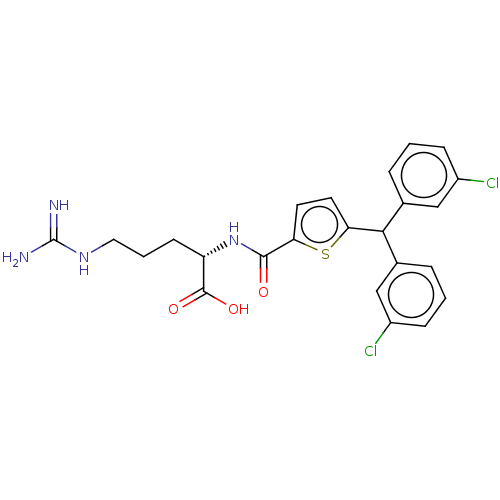
(CHEMBL4459830)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1cccc(Cl)c1)c1cccc(Cl)c1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-6-1-4-14(12-16)21(15-5-2-7-17(26)13-15)19-9-10-20(34-19)22(31)30-18(23(32)33)8-3-11-29-24(27)28/h1-2,4-7,9-10,12-13,18,21H,3,8,11H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520303

(CHEMBL4459830)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1cccc(Cl)c1)c1cccc(Cl)c1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-6-1-4-14(12-16)21(15-5-2-7-17(26)13-15)19-9-10-20(34-19)22(31)30-18(23(32)33)8-3-11-29-24(27)28/h1-2,4-7,9-10,12-13,18,21H,3,8,11H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520310

(CHEMBL4470864)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O3S/c1-16-15-20(21(17-9-4-2-5-10-17)18-11-6-3-7-12-18)33-22(16)23(30)29-19(24(31)32)13-8-14-28-25(26)27/h2-7,9-12,15,19,21H,8,13-14H2,1H3,(H,29,30)(H,31,32)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591317
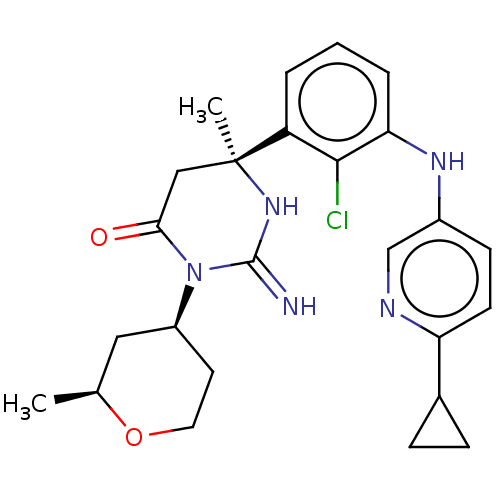
(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520310

(CHEMBL4470864)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O3S/c1-16-15-20(21(17-9-4-2-5-10-17)18-11-6-3-7-12-18)33-22(16)23(30)29-19(24(31)32)13-8-14-28-25(26)27/h2-7,9-12,15,19,21H,8,13-14H2,1H3,(H,29,30)(H,31,32)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520304
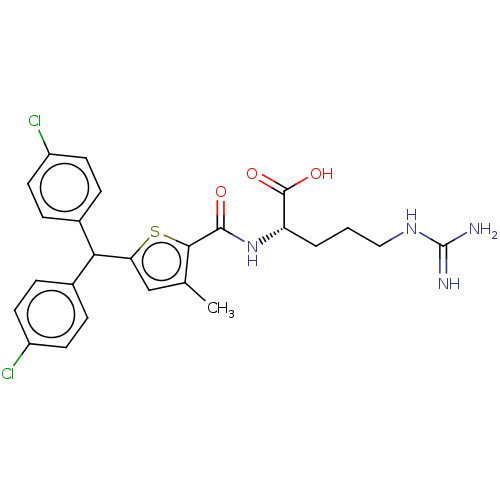
(CHEMBL4459627)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520304

(CHEMBL4459627)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist at C3a receptor in human LAD2 cells assessed as inhibition of C3a-induced beta-hexosaminidase release preincubated for 30 mins followed by... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591318

(CHEMBL5204196)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2cnc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520304

(CHEMBL4459627)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520304

(CHEMBL4459627)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591315
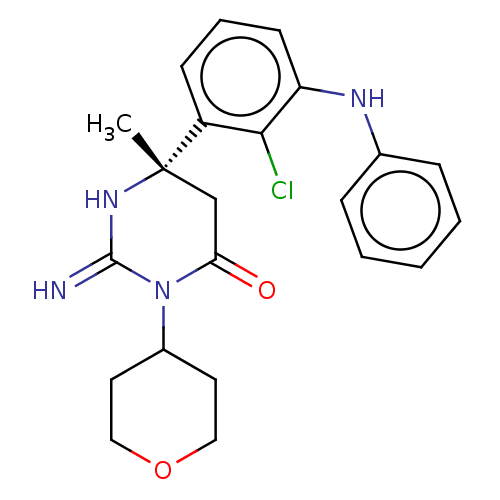
(CHEMBL5172999)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50019689
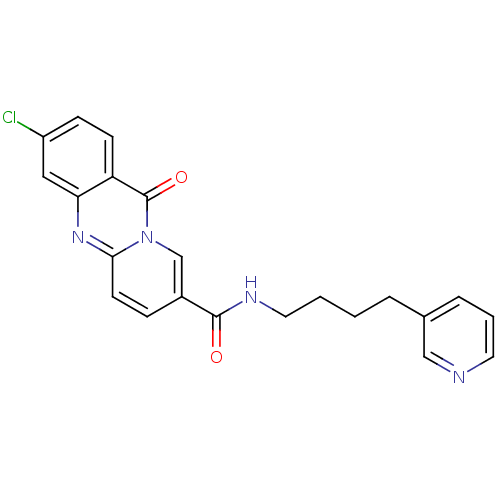
(3-Chloro-11-oxo-11H-pyrido[2,1-b]quinazoline-8-car...)Show SMILES Clc1ccc2c(c1)nc1ccc(cn1c2=O)C(=O)NCCCCc1cccnc1 Show InChI InChI=1S/C22H19ClN4O2/c23-17-7-8-18-19(12-17)26-20-9-6-16(14-27(20)22(18)29)21(28)25-11-2-1-4-15-5-3-10-24-13-15/h3,5-10,12-14H,1-2,4,11H2,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet thromboxane synthase (TXA2) was determined in human platelets |
J Med Chem 30: 185-93 (1987)
BindingDB Entry DOI: 10.7270/Q2222VBH |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50019683
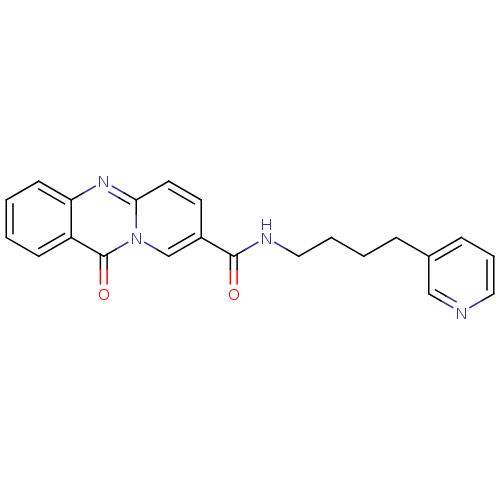
(11-Oxo-11H-pyrido[2,1-b]quinazoline-8-carboxylic a...)Show InChI InChI=1S/C22H20N4O2/c27-21(24-13-4-3-6-16-7-5-12-23-14-16)17-10-11-20-25-19-9-2-1-8-18(19)22(28)26(20)15-17/h1-2,5,7-12,14-15H,3-4,6,13H2,(H,24,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet thromboxane synthase (TXA2) was determined in human platelets |
J Med Chem 30: 185-93 (1987)
BindingDB Entry DOI: 10.7270/Q2222VBH |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50019678
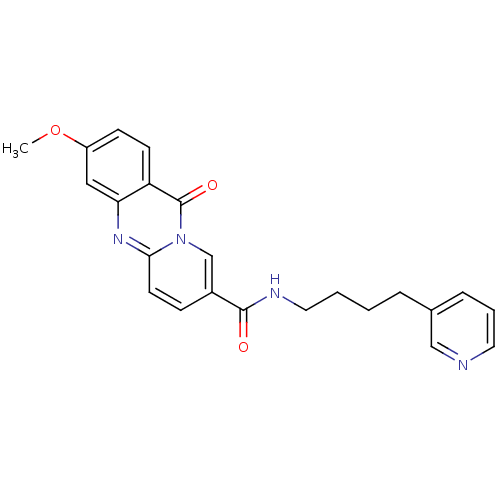
(3-Methoxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...)Show SMILES COc1ccc2c(c1)nc1ccc(cn1c2=O)C(=O)NCCCCc1cccnc1 Show InChI InChI=1S/C23H22N4O3/c1-30-18-8-9-19-20(13-18)26-21-10-7-17(15-27(21)23(19)29)22(28)25-12-3-2-5-16-6-4-11-24-14-16/h4,6-11,13-15H,2-3,5,12H2,1H3,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet thromboxane synthase (TXA2) was determined in human platelets |
J Med Chem 30: 185-93 (1987)
BindingDB Entry DOI: 10.7270/Q2222VBH |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520301
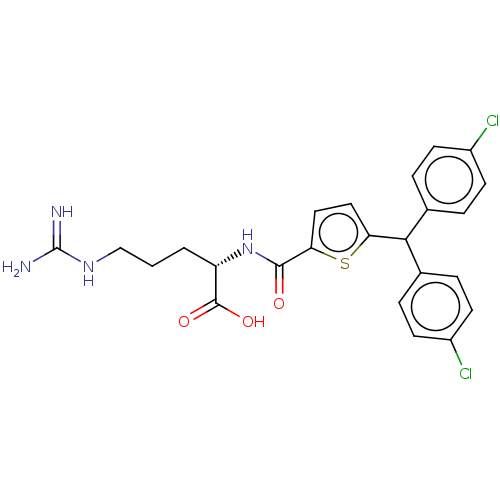
(CHEMBL4558167)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-7-3-14(4-8-16)21(15-5-9-17(26)10-6-15)19-11-12-20(34-19)22(31)30-18(23(32)33)2-1-13-29-24(27)28/h3-12,18,21H,1-2,13H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Type 1 fimbiral adhesin FimH
(Escherichia coli (strain UTI89 / UPEC)) | BDBM50197181
(CHEMBL3899291)Show SMILES [H][C@@]1(O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O)[C@H](O)c1ccc(cc1C)-c1ccc2cc[nH]c(=O)c2c1 |r| Show InChI InChI=1S/C23H25NO7/c1-11-8-13(14-3-2-12-6-7-24-23(30)16(12)9-14)4-5-15(11)18(26)22-21(29)20(28)19(27)17(10-25)31-22/h2-9,17-22,25-29H,10H2,1H3,(H,24,30)/t17-,18-,19-,20+,21+,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fimbrion Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli UTI89 FimH-mediated biofilm formation after 48 hrs by crystal violet staining-based assay |
J Med Chem 59: 9390-9408 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00948
BindingDB Entry DOI: 10.7270/Q2NG4SK0 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520321
(CHEMBL4473192)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H26F2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591319

(CHEMBL5204856)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(NC(=O)c2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520301

(CHEMBL4558167)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-7-3-14(4-8-16)21(15-5-9-17(26)10-6-15)19-11-12-20(34-19)22(31)30-18(23(32)33)2-1-13-29-24(27)28/h3-12,18,21H,1-2,13H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50006812
(7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...)Show SMILES CCCc1c(OCC(O)COc2ccc3c(oc(cc3=O)C(O)=O)c2CCC)ccc(C(C)=O)c1O Show InChI InChI=1S/C27H30O9/c1-4-6-19-22(10-8-17(15(3)28)25(19)31)34-13-16(29)14-35-23-11-9-18-21(30)12-24(27(32)33)36-26(18)20(23)7-5-2/h8-12,16,29,31H,4-7,13-14H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of SRS-A-induced contractions in guinea pig ileum |
J Med Chem 30: 173-8 (1987)
BindingDB Entry DOI: 10.7270/Q2D220TJ |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520321

(CHEMBL4473192)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H26F2N4O3S/c1-14-13-20(21(15-4-8-17(26)9-5-15)16-6-10-18(27)11-7-16)35-22(14)23(32)31-19(24(33)34)3-2-12-30-25(28)29/h4-11,13,19,21H,2-3,12H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in human MDM cells assessed as inhibition of C3a-induced intracellular calcium release preincubated for 30 mins f... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591316

(CHEMBL5208335)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data