

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213578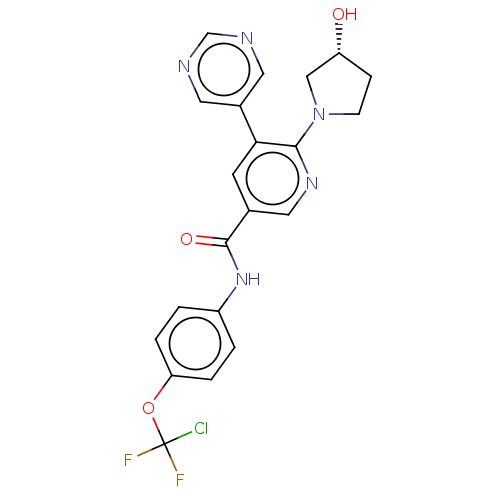 (US9278981, 170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213594 (US9278981, 186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50459091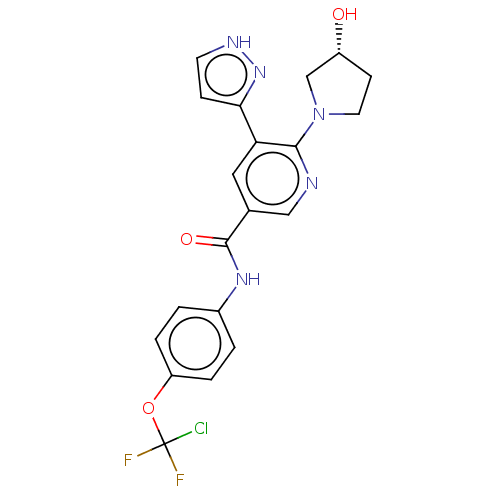 (ABL-001 | ABL001 | ABL001-NX | Asciminib | NVP-ABL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213656 (US9278981, 248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50459090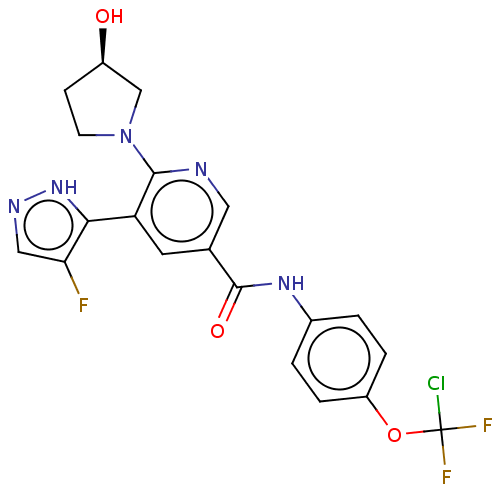 (CHEMBL4213152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM33970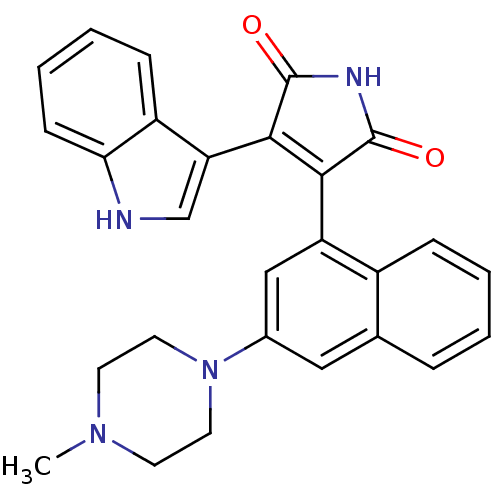 (maleimide derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50112349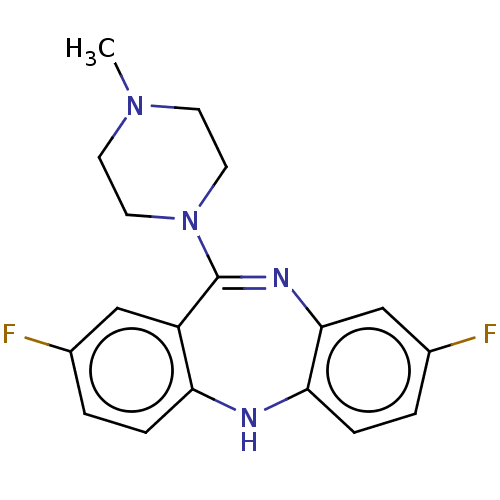 (CHEMBL3609328) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human histamine H1 receptor | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50459089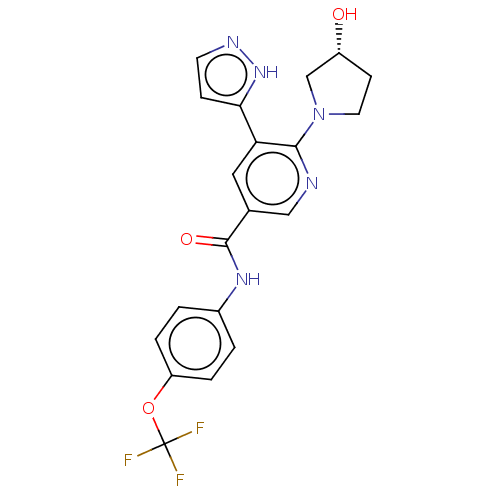 (CHEMBL4217559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM230760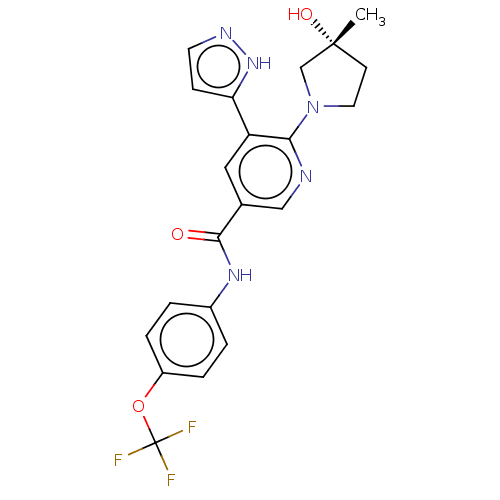 (US9340537, 14 | US9896444, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM2683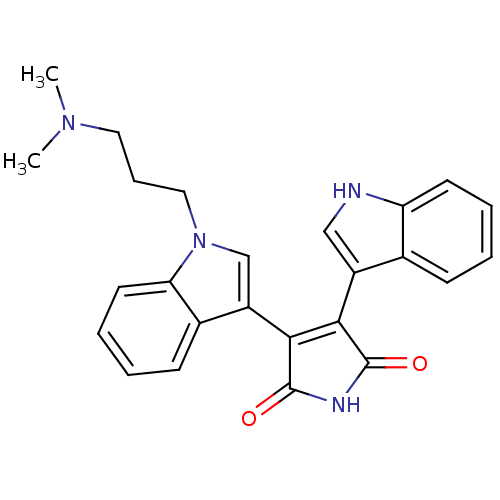 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM33970 (maleimide derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50391897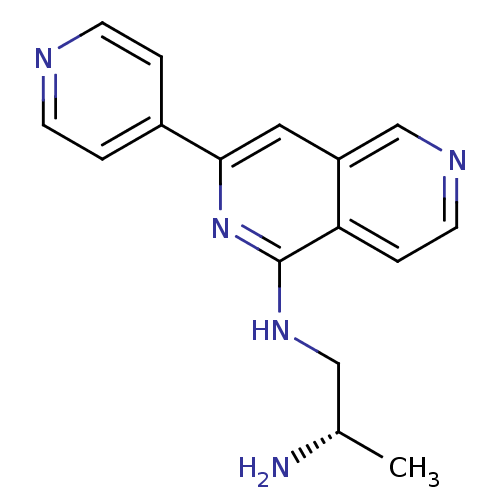 (CHEMBL2147537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM33968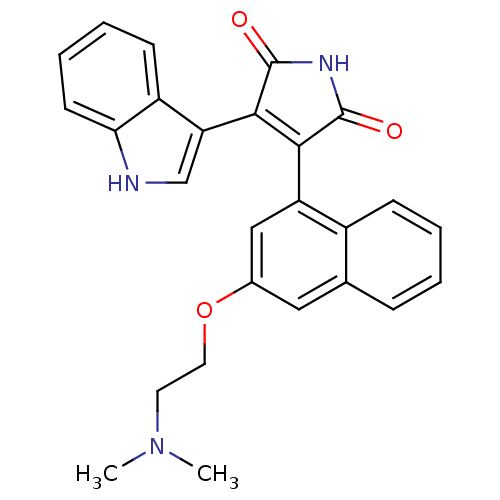 (maleimide derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM33968 (maleimide derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213441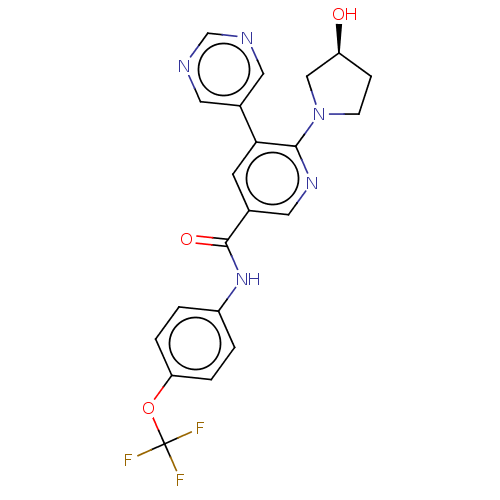 (US9278981, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM2683 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50533774 (CHEMBL4469006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... | ACS Med Chem Lett 7: 762-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00119 BindingDB Entry DOI: 10.7270/Q2V98CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM33968 (maleimide derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213443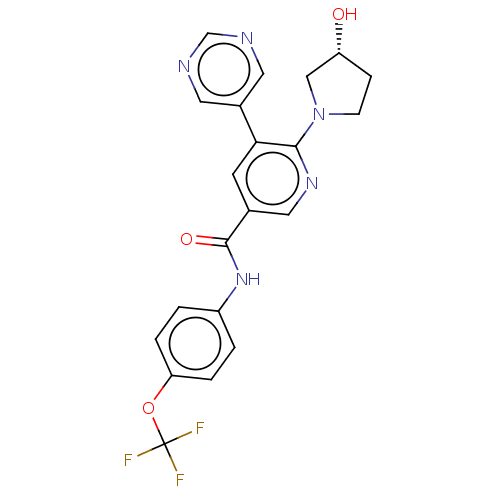 (US9278981, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM118300 (US8653092, 68) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... | ACS Med Chem Lett 8: 975-980 (2017) Article DOI: 10.1021/acsmedchemlett.7b00293 BindingDB Entry DOI: 10.7270/Q2SX6GR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50391902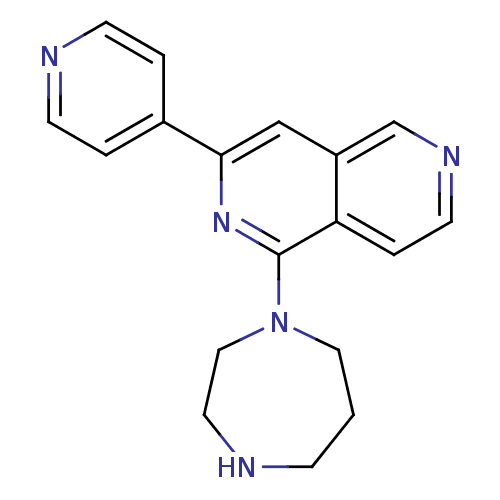 (CHEMBL2147542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM33968 (maleimide derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM33970 (maleimide derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50533772 (CHEMBL4521888) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... | ACS Med Chem Lett 7: 762-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00119 BindingDB Entry DOI: 10.7270/Q2V98CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50324316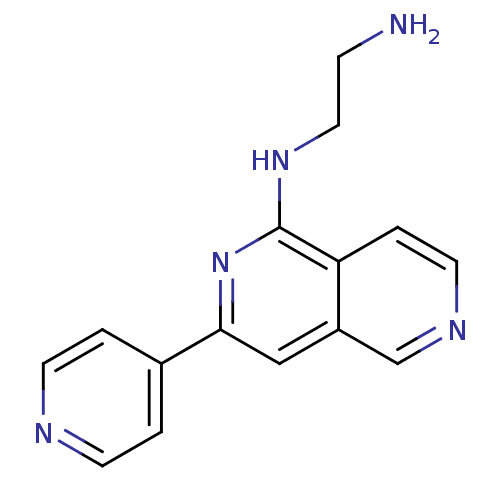 (CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50391897 (CHEMBL2147537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50324316 (CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112348 (CHEMBL3609372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM33968 (maleimide derivative, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human mTOR after 60 mins in presence of [gamma-33P]-ATP by microplate scintillation counting | ACS Med Chem Lett 7: 762-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00119 BindingDB Entry DOI: 10.7270/Q2V98CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50324319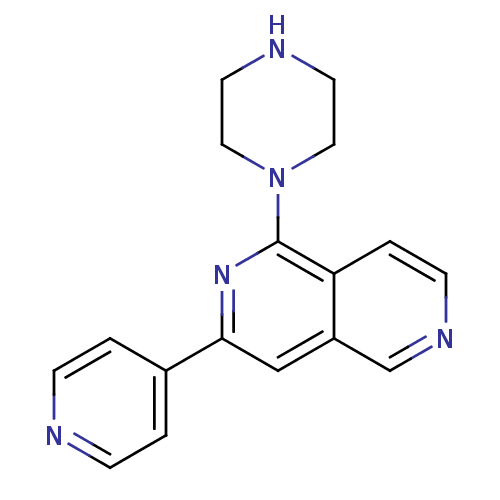 (1-Piperazin-1-yl-3-pyridin-4-yl[2,6]naphthyridine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50112349 (CHEMBL3609328) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human muscarinic M1 receptor | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM2683 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM2683 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213434 (US9278981, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50391902 (CHEMBL2147542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM33969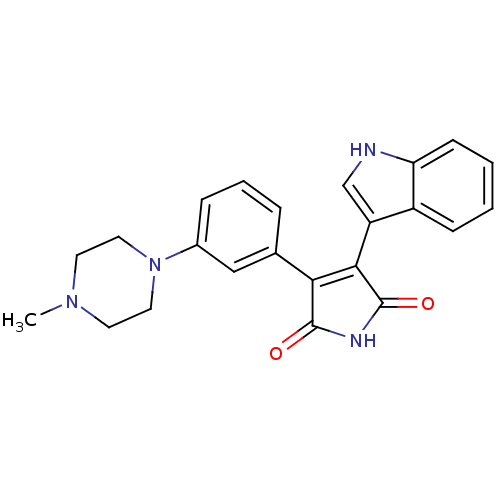 (maleimide derivative, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM33970 (maleimide derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112347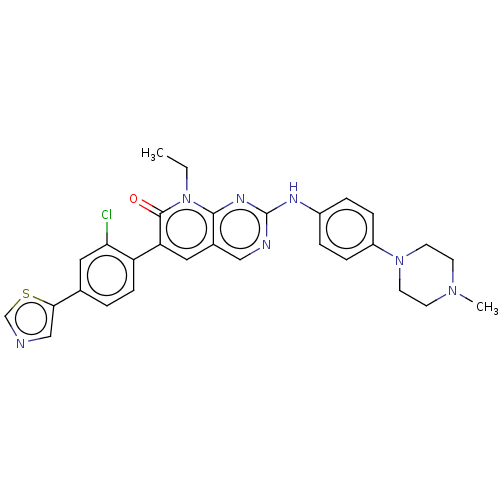 (CHEMBL3609327 | FRAX597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PAK1 by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50324319 (1-Piperazin-1-yl-3-pyridin-4-yl[2,6]naphthyridine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50391903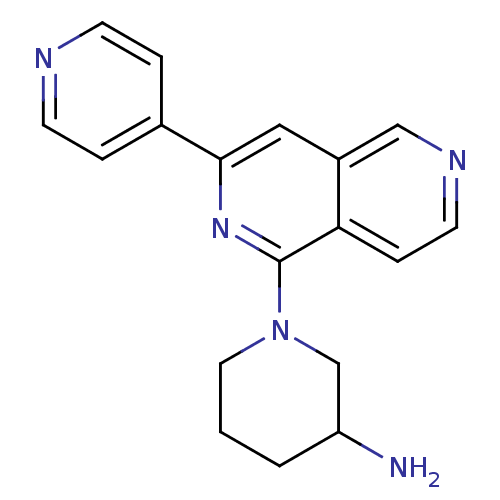 (CHEMBL2147543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50391897 (CHEMBL2147537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCdelta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity a... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112355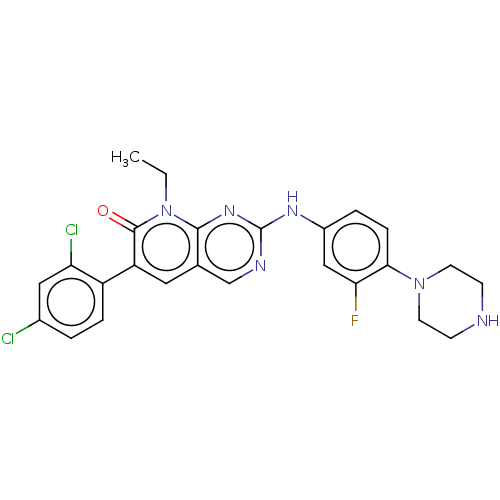 (CHEMBL3609326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length PAK1 (unknown origin) by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM2683 (2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis | Assay Description Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... | J Med Chem 52: 6193-6 (2009) Article DOI: 10.1021/jm901108b BindingDB Entry DOI: 10.7270/Q25X278Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 668 total ) | Next | Last >> |