

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50367032 (COFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419354 (GR205171A | VOFOPITANT DIHYDROCHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 19: 6430-46 (2011) Article DOI: 10.1016/j.bmc.2011.08.070 BindingDB Entry DOI: 10.7270/Q228081R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365698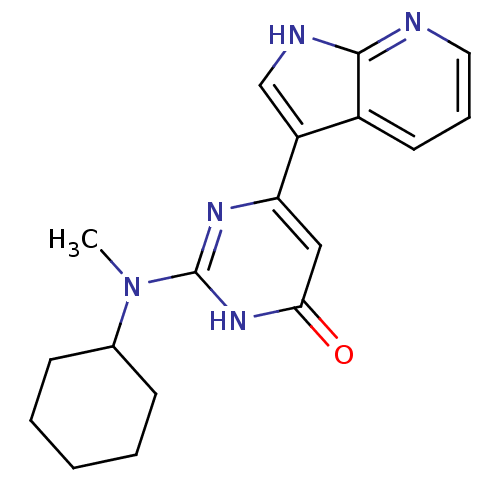 (CHEMBL1958411) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365703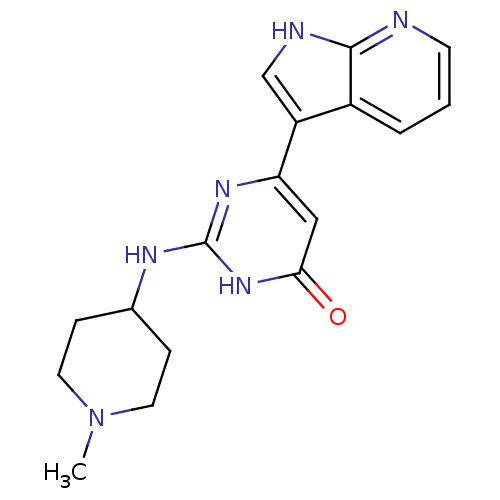 (CHEMBL1958417) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365699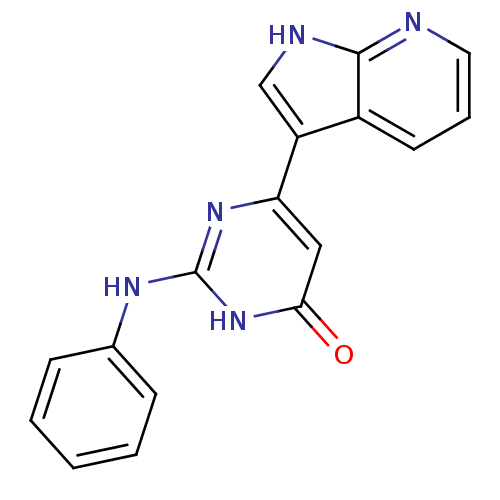 (CHEMBL1958412) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365702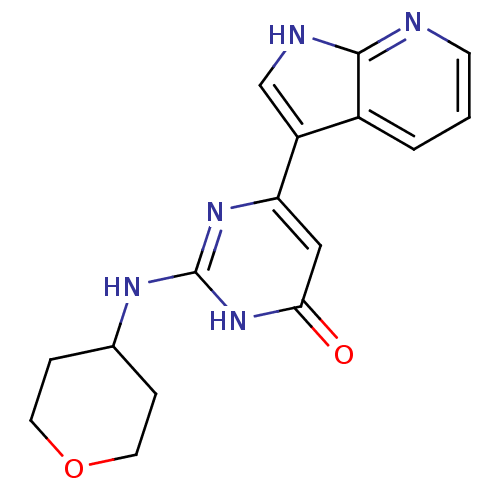 (CHEMBL1958416) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592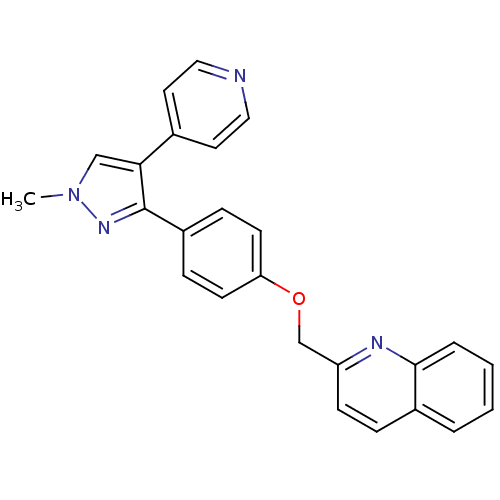 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365701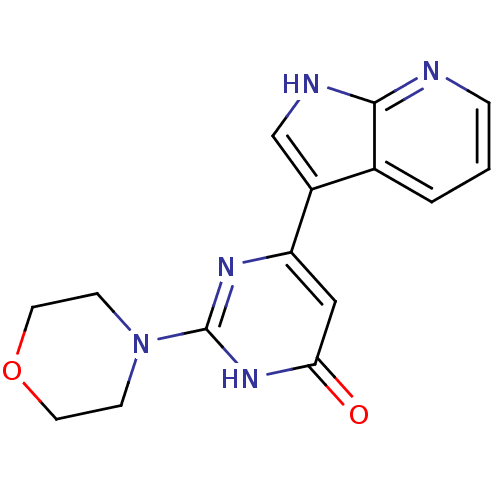 (CHEMBL1958415) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365700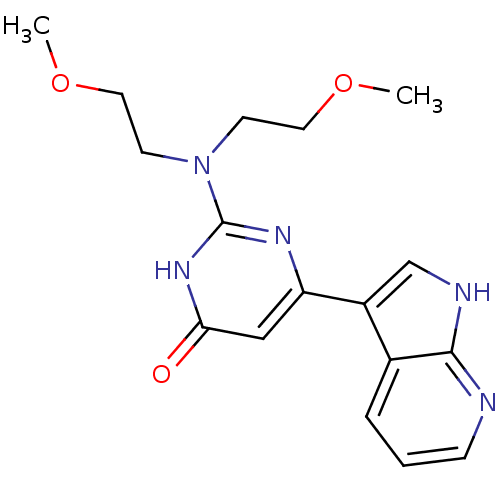 (CHEMBL1958414) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50003019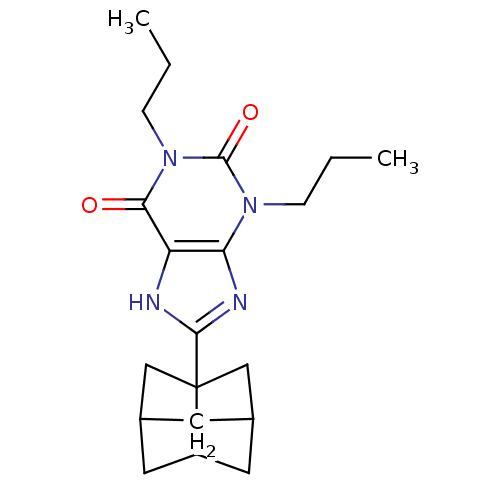 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor in rat forebrain membranes using N6-[3H]cyclohexyladenosine | J Med Chem 35: 3066-75 (1992) BindingDB Entry DOI: 10.7270/Q2DN45PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50044431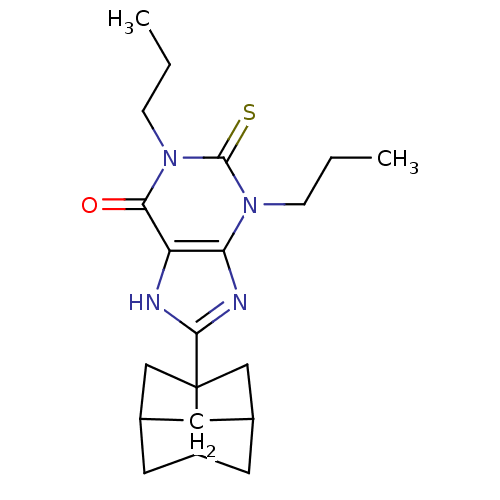 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Tested for binding affinity against Adenosine A1 receptor from rat forebrain membranes, using N6-[3H]- cyclohexyladenosine as radioligand | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50401333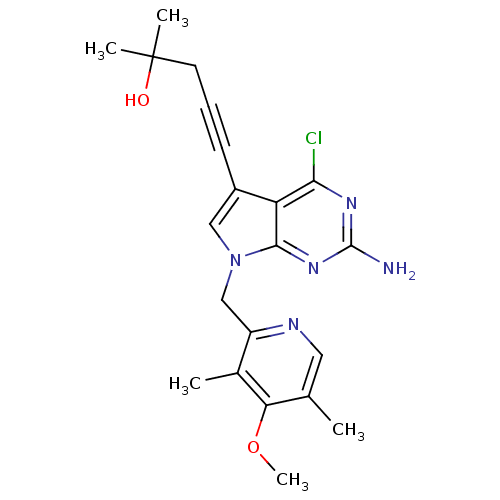 (CHEMBL1230584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assay | J Med Chem 55: 7786-95 (2012) Article DOI: 10.1021/jm300810x BindingDB Entry DOI: 10.7270/Q2V125Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365705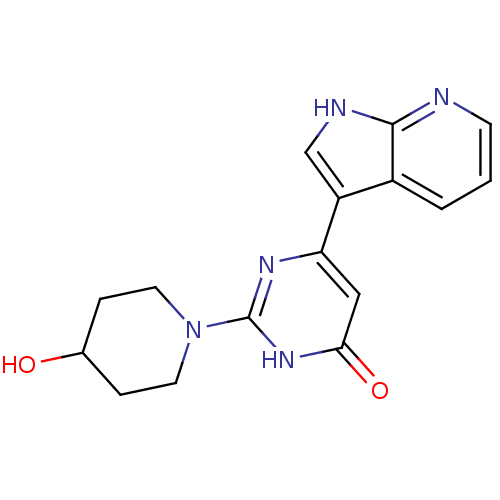 (CHEMBL1958419) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | Neuropsychopharmacology 8: 23-33 (1993) Article DOI: 10.1038/npp.1993.4 BindingDB Entry DOI: 10.7270/Q2XS5SXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546246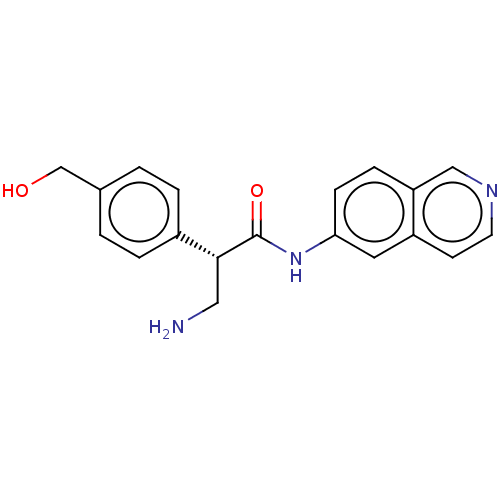 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546246 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365706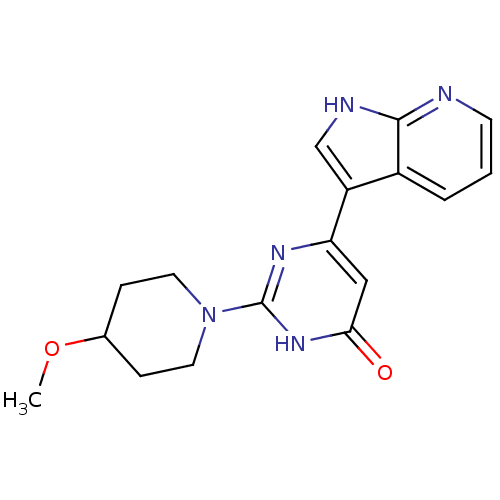 (CHEMBL1958420) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50044429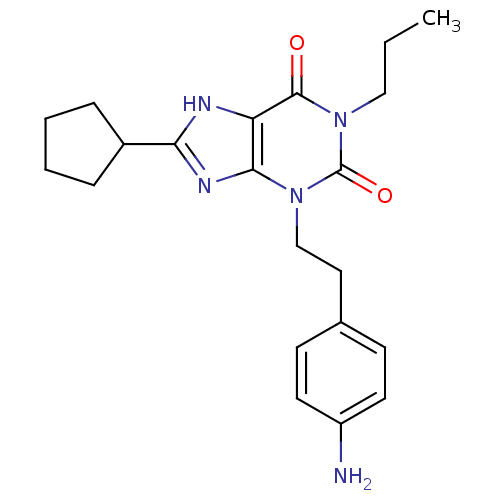 (3-[2-(4-Amino-phenyl)-ethyl]-8-cyclopentyl-1-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor from rat forebrain membranes with N6-[3H]- cyclohexyladenosine | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to adenosine A1 receptor from rat cortical membranes | J Med Chem 35: 2342-5 (1992) BindingDB Entry DOI: 10.7270/Q2T43S20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365704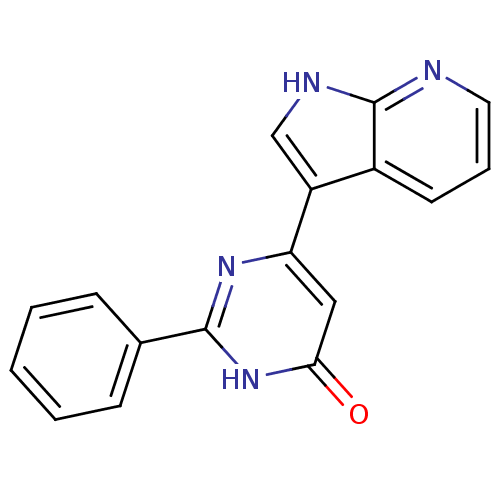 (CHEMBL1958418) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532486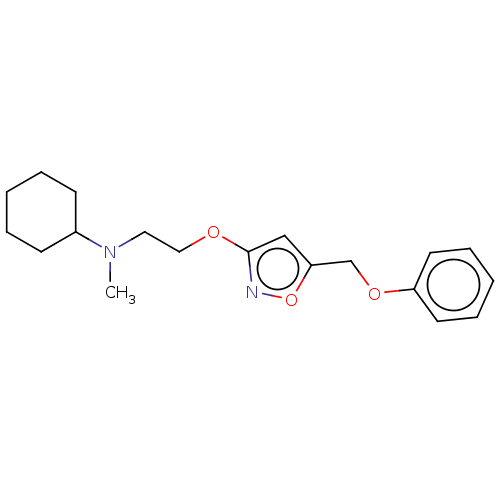 (CHEMBL4564992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532486 (CHEMBL4564992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365693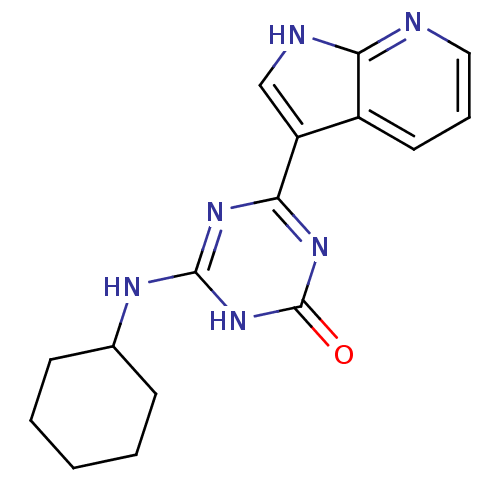 (CHEMBL1958406) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50456840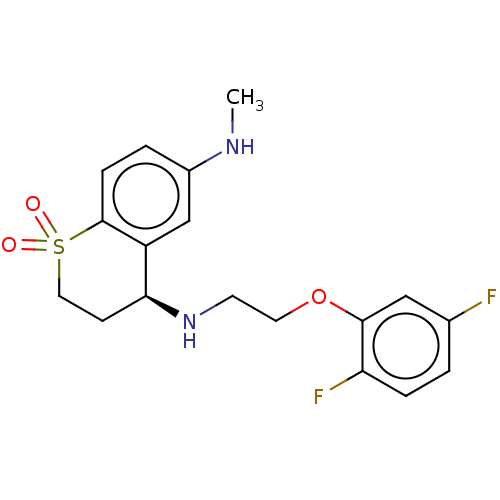 (CHEMBL4207824) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 7-methoxy-[3H]-prazosin from human alpha1D-adrenoreceptor expressed in CHOK1 cell membranes after 60 mins by TopCount liquid scintill... | Eur J Med Chem 139: 114-127 (2017) Article DOI: 10.1016/j.ejmech.2017.07.071 BindingDB Entry DOI: 10.7270/Q2765HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50267577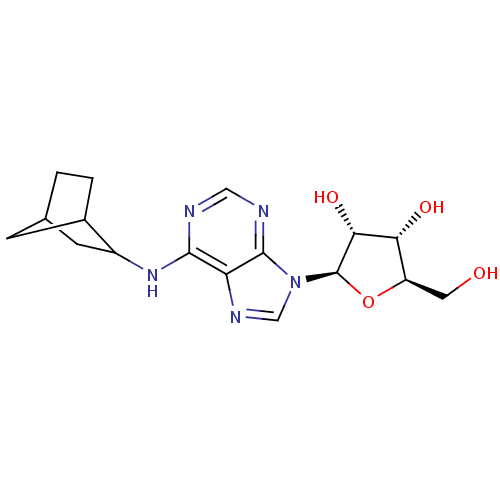 (CHEMBL489640 | N6-((+/-)-endo-norborn-2-yl)adenosi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using N6-[3H]cyclohexyladenosine as radioligand in guinea pig forebrain membranes | J Med Chem 35: 924-30 (1992) BindingDB Entry DOI: 10.7270/Q2NS0VJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to guinea pig forebrain membrane Adenosine A1 receptor | J Med Chem 35: 924-30 (1992) BindingDB Entry DOI: 10.7270/Q2NS0VJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237869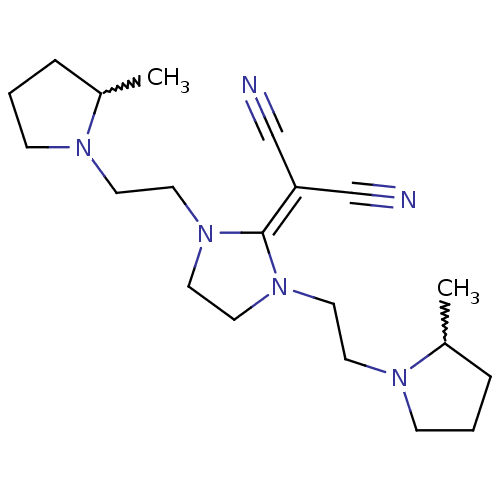 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805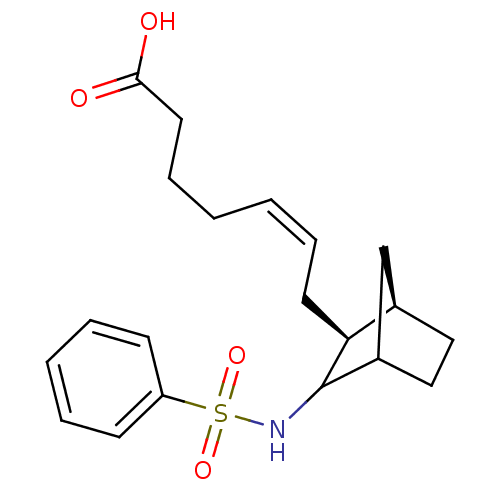 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237874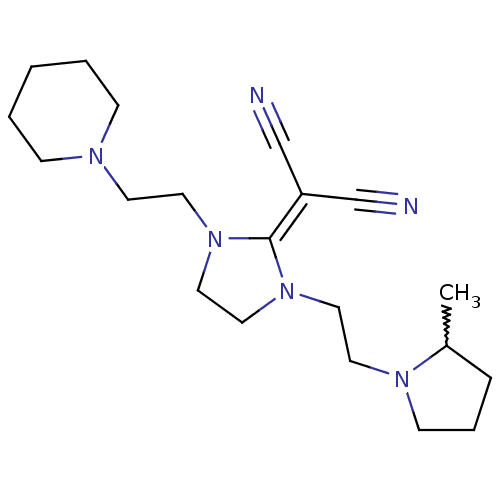 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50081533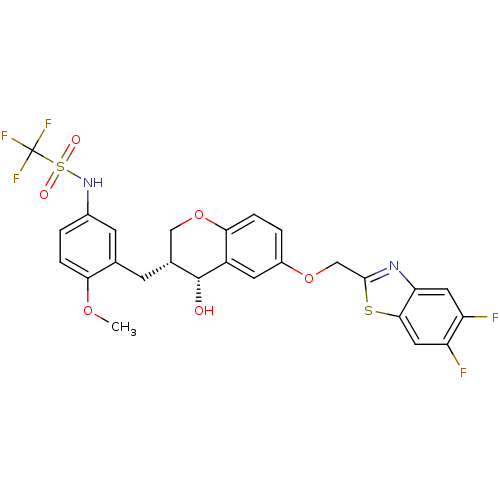 (CHEMBL96224 | CP-199331 | N-{3-[(3R,4R)-6-(5,6-Dif...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the binding of Cysteinyl leukotriene receptor 1 to guinea pig lung membranes | Bioorg Med Chem Lett 9: 2773-8 (1999) BindingDB Entry DOI: 10.7270/Q2MP52G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50019443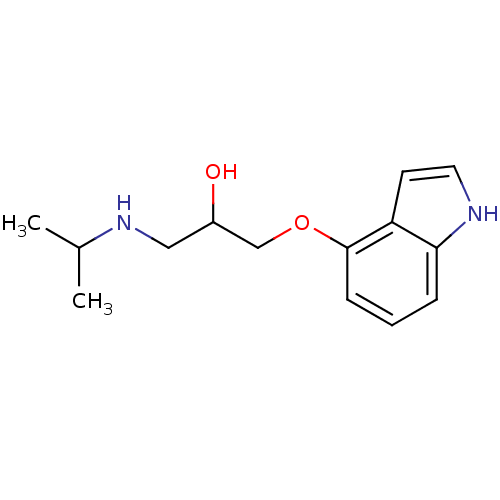 (1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at human adrenergic beta2 receptor | Bioorg Med Chem Lett 17: 5600-4 (2007) Article DOI: 10.1016/j.bmcl.2007.07.086 BindingDB Entry DOI: 10.7270/Q2FJ2GG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185854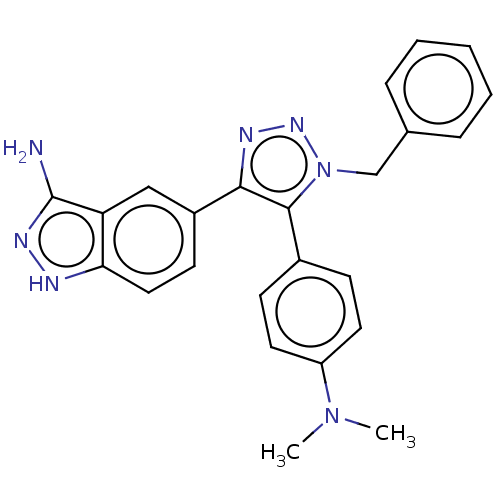 (US9163007, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50388878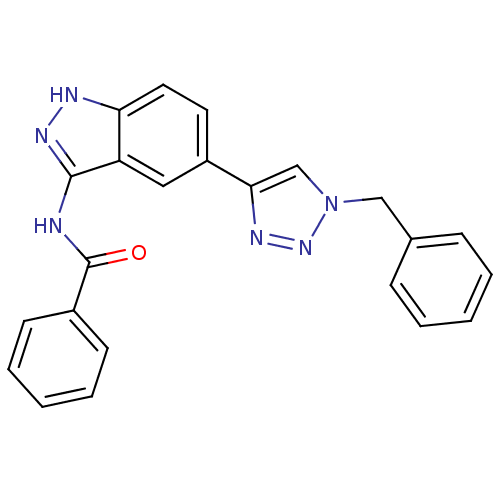 (CHEMBL1999931 | US9163007, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185901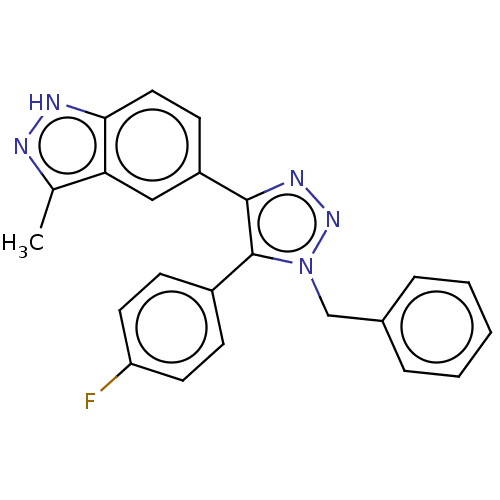 (US9163007, 408) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.428 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50073686 (CHEMBL423029 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 526-31 (1999) Article DOI: 10.1021/jm9805945 BindingDB Entry DOI: 10.7270/Q2668CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50073688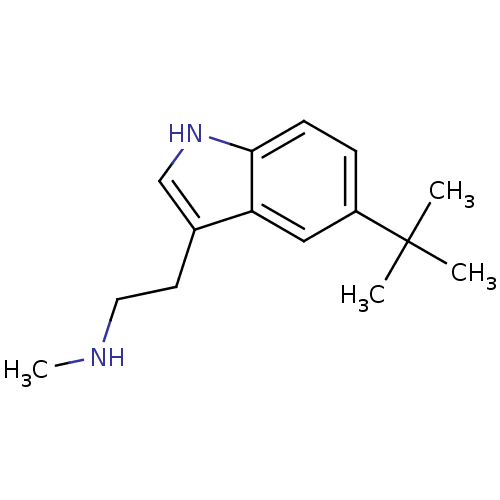 (CHEMBL357034 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 526-31 (1999) Article DOI: 10.1021/jm9805945 BindingDB Entry DOI: 10.7270/Q2668CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Tested for binding affinity against Adenosine A1 receptor from rat forebrain membranes, using N6-[3H]- cyclohexyladenosine as radioligand | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using N6-[3H]-cyclohexyladenosinene in rat whole brain membranes | J Med Chem 35: 924-30 (1992) BindingDB Entry DOI: 10.7270/Q2NS0VJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor in rat forebrain membranes using N6-[3H]cyclohexyladenosine | J Med Chem 35: 3066-75 (1992) BindingDB Entry DOI: 10.7270/Q2DN45PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to adenosine A1 receptor from whole brain membranes | J Med Chem 35: 2342-5 (1992) BindingDB Entry DOI: 10.7270/Q2T43S20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate after 1 hr by liquid scintillation counting | Bioorg Med Chem 20: 4556-63 (2012) Article DOI: 10.1016/j.bmc.2012.05.006 BindingDB Entry DOI: 10.7270/Q2DJ5GPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237872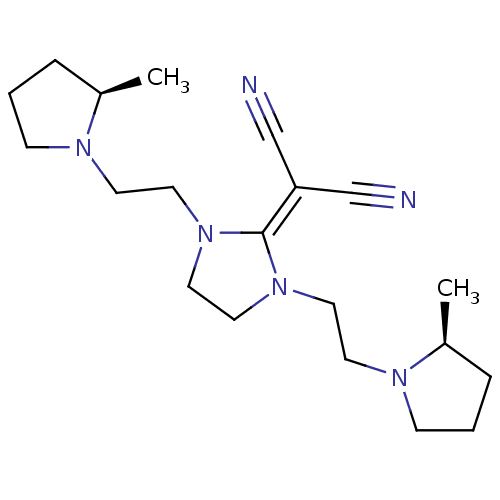 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor in rat forebrain membranes using N6-[3H]cyclohexyladenosine | J Med Chem 35: 3066-75 (1992) BindingDB Entry DOI: 10.7270/Q2DN45PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50292408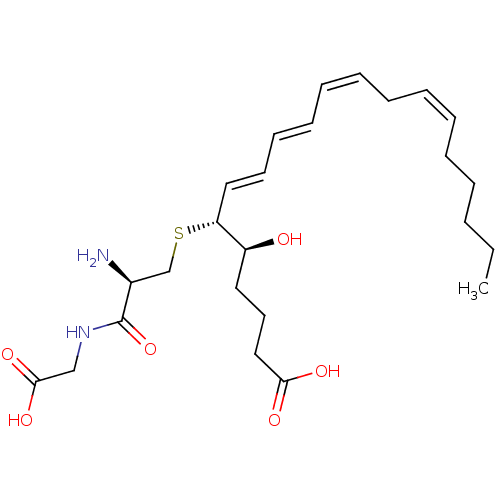 ((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 258: 531-6 (1991) BindingDB Entry DOI: 10.7270/Q2BK19TF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (GUINEA PIG) | BDBM50292408 ((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 258: 531-6 (1991) BindingDB Entry DOI: 10.7270/Q2BK19TF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185888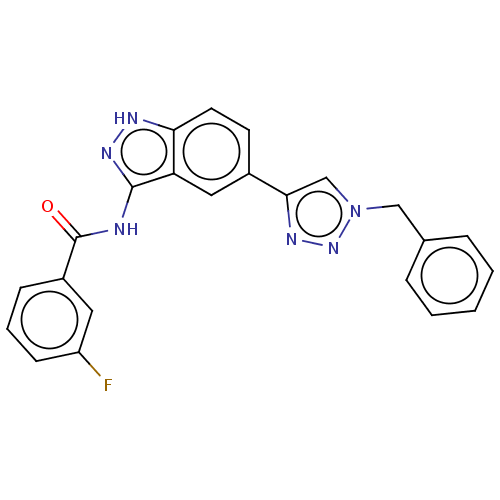 (US9163007, 395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 24440 total ) | Next | Last >> |