

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motilin receptor (Homo sapiens (Human)) | BDBM50143028 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143032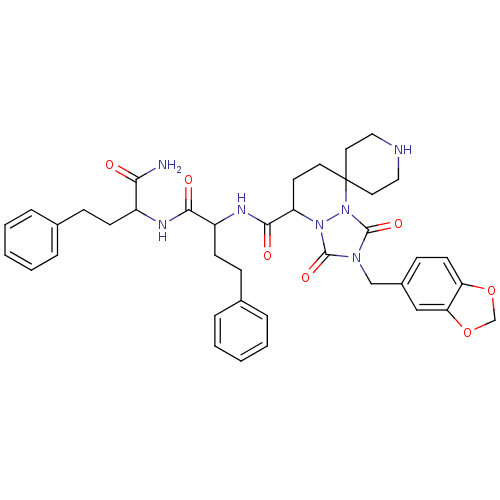 (8'N-[1-(1-carbamoyl-3-phenylpropylcarbamoyl)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143038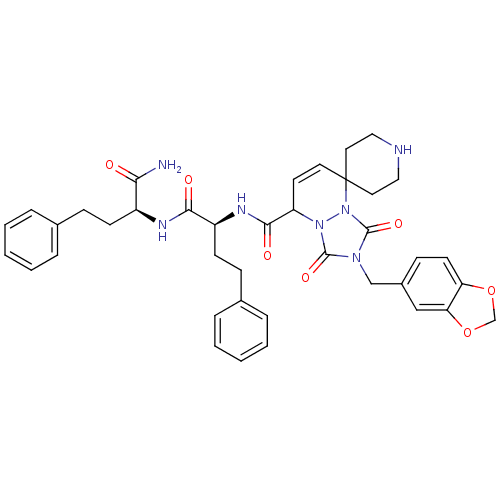 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143039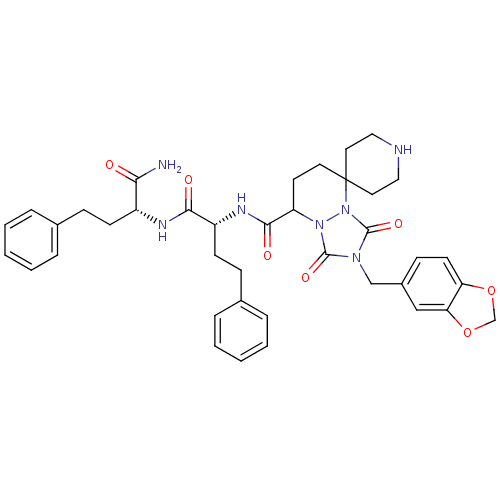 (8'N-[1-[1-carbamoyl-3-phenyl-(1R)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143037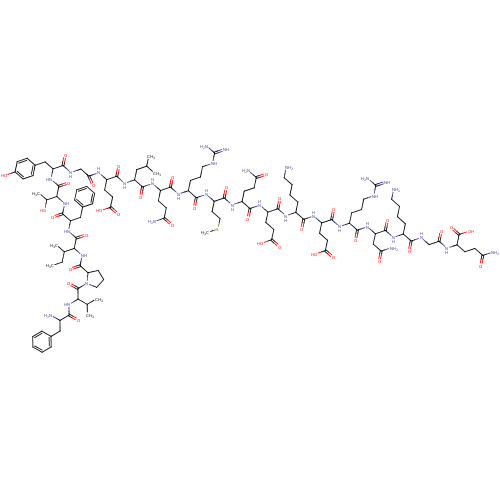 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50344952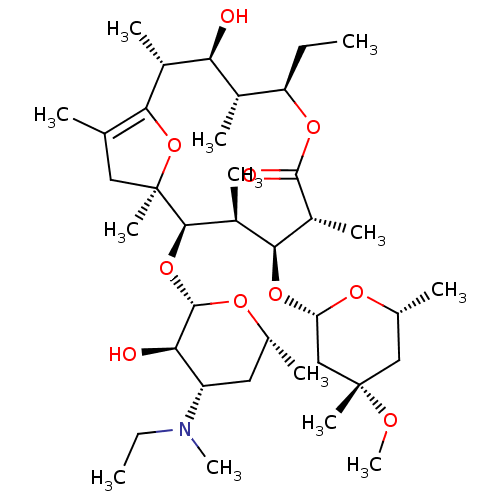 ((2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143034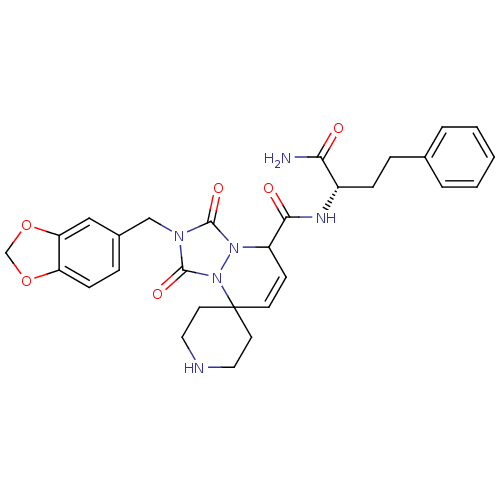 (8'N-[1-carbamoyl-3-phenyl-(1S)-propyl]-2'-benzo[d]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143033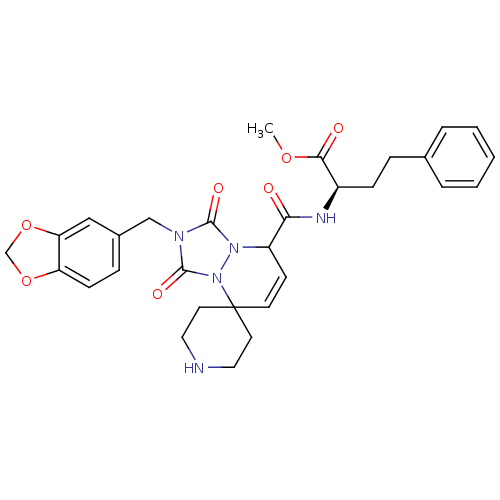 (CHEMBL46272 | methyl 2-[2'-benzo[d][1,3]dioxol-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143031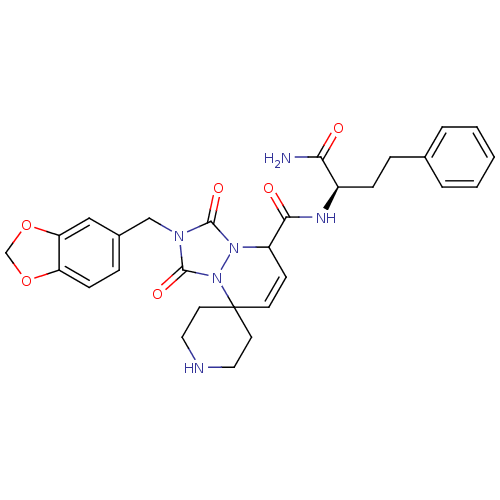 (8'N-[1-carbamoyl-3-phenyl-(1R)-propyl]-2'-benzo[d]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143034 (8'N-[1-carbamoyl-3-phenyl-(1S)-propyl]-2'-benzo[d]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50344942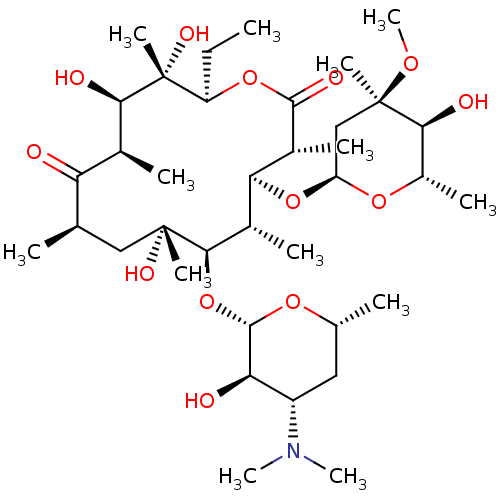 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32522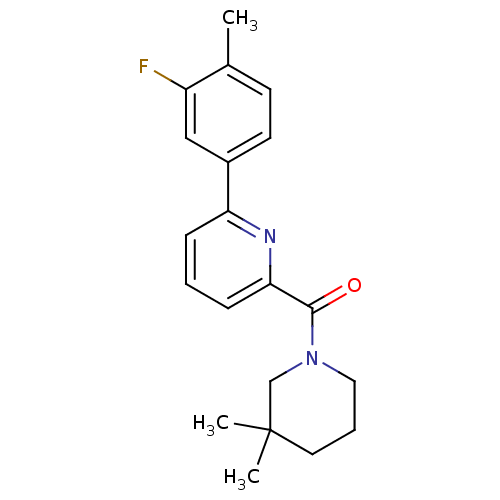 (pyridine amide, 30) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32521 (pyridine amide, 29) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32520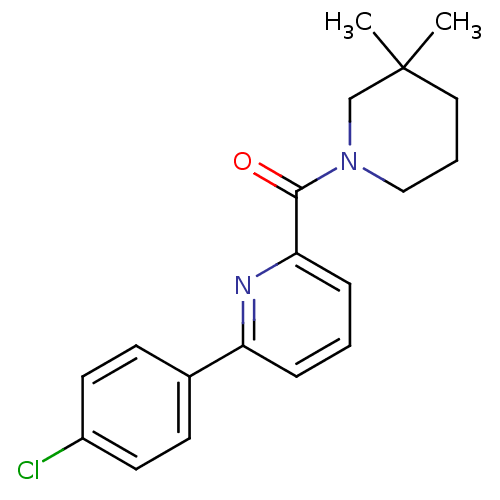 (pyridine amide, 28) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32527 (pyridine amide, 35) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32523 (pyridine amide, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32526 (pyridine amide, 34) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32508 (pyridine amide, 23) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32519 (pyridine amide, 27) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32524 (pyridine amide, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32529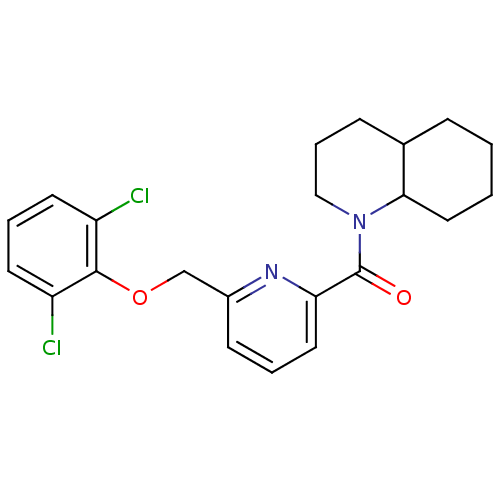 (pyridine amide, 37) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32525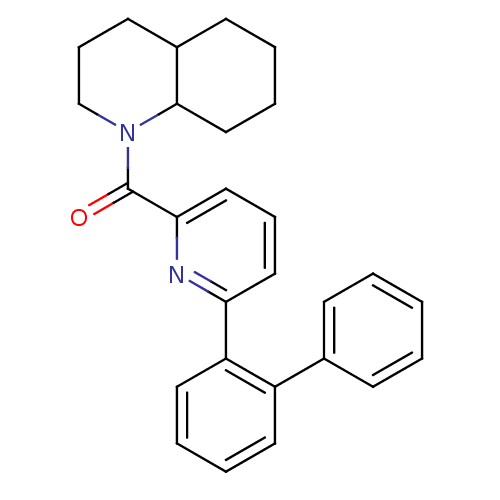 (pyridine amide, 33) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32528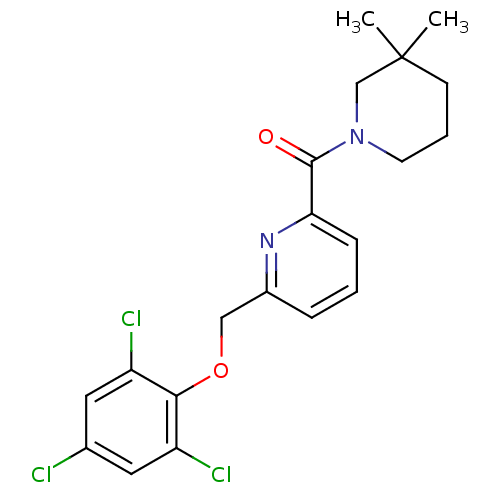 (pyridine amide, 36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32512 (pyridine amide, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32507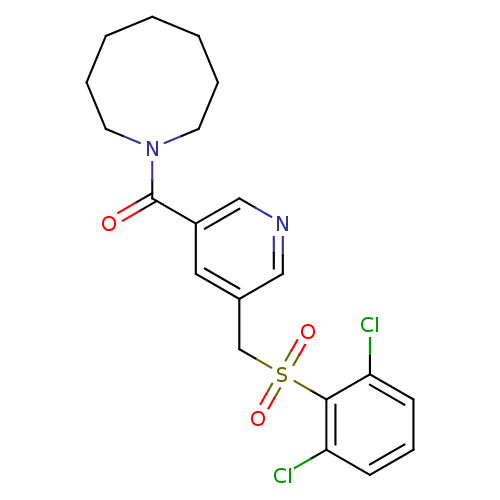 (pyridine amide, 22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32518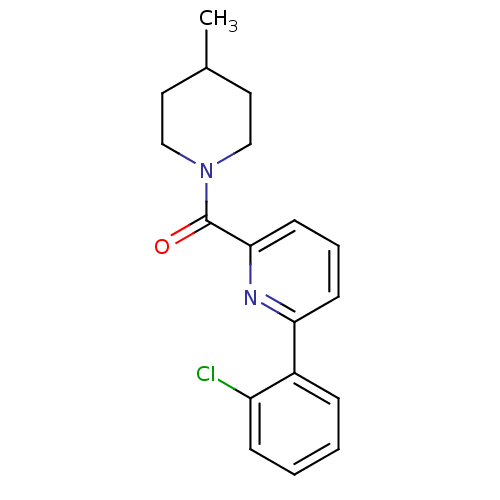 (pyridine amide, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32506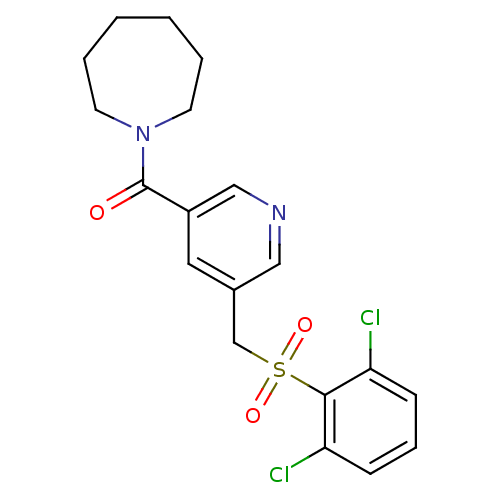 (pyridine amide, 21) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32500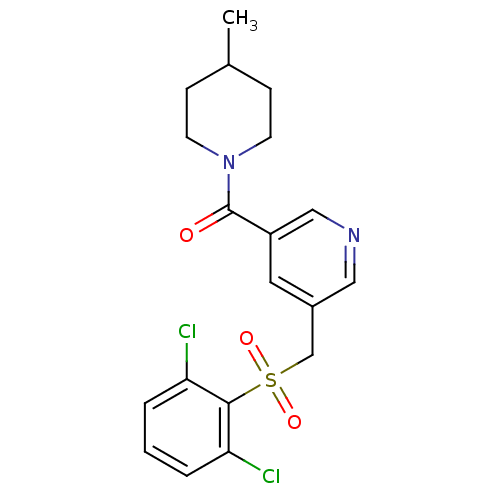 (pyridine amide, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32499 (pyridine amide, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32513 (pyridine amide, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32501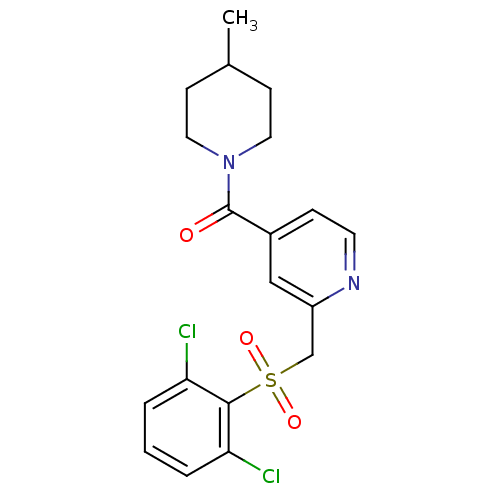 (pyridine amide, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32514 (pyridine amide, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32509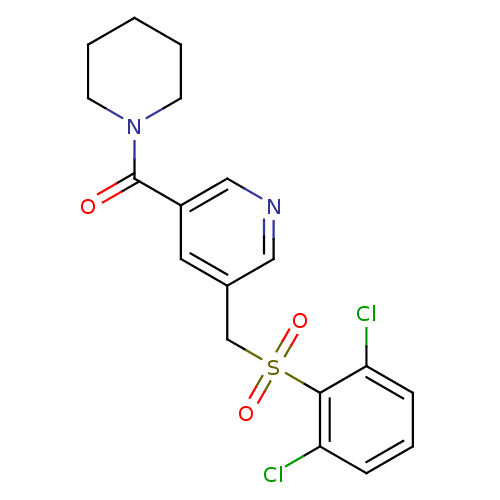 (pyridine amide, 24) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32515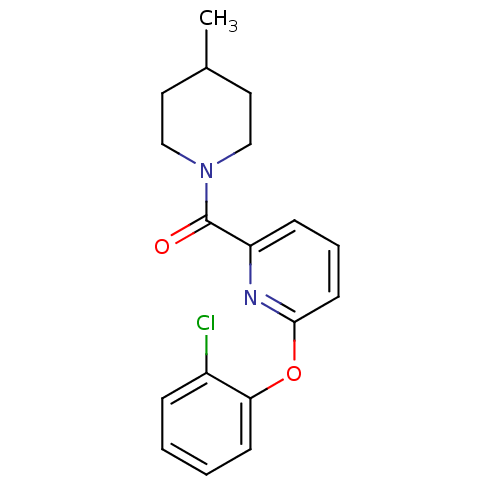 (pyridine amide, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32502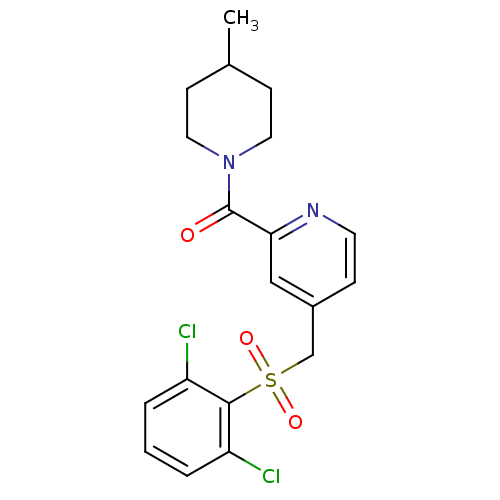 (pyridine amide, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32516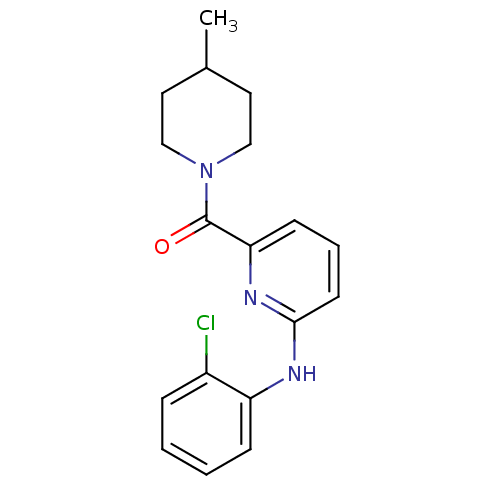 (pyridine amide, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 381 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32503 (pyridine amide, 18) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32504 (pyridine amide, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32505 (pyridine amide, 20) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32510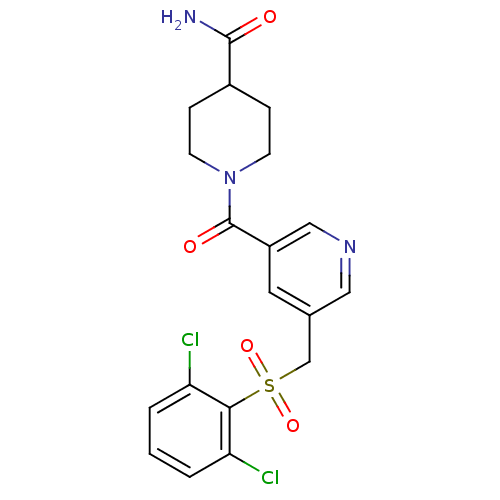 (pyridine amide, 25) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32517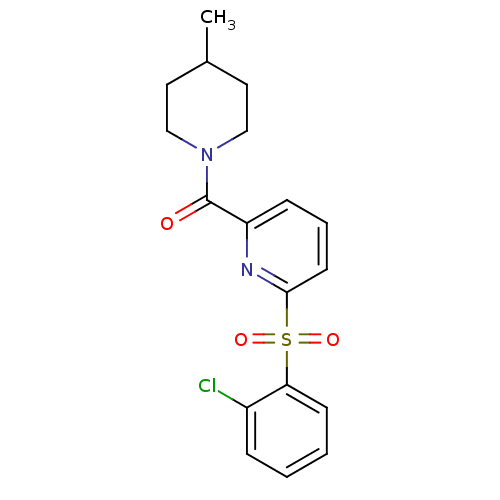 (pyridine amide, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.67E+3 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32511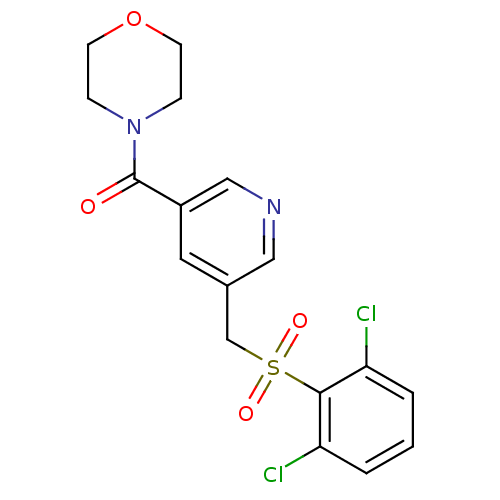 (pyridine amide, 26) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143031 (8'N-[1-carbamoyl-3-phenyl-(1R)-propyl]-2'-benzo[d]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50344952 ((2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143037 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143033 (CHEMBL46272 | methyl 2-[2'-benzo[d][1,3]dioxol-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143032 (8'N-[1-(1-carbamoyl-3-phenylpropylcarbamoyl)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143028 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143038 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143040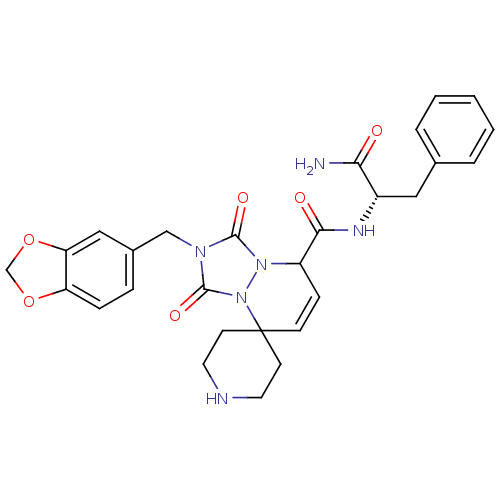 (8'N-[1-carbamoyl-2-phenyl-(1S)-ethyl]-2'-benzo[d][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro effective concentration towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |