 Found 472 hits with Last Name = 'santos' and Initial = 'ma'
Found 472 hits with Last Name = 'santos' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297852
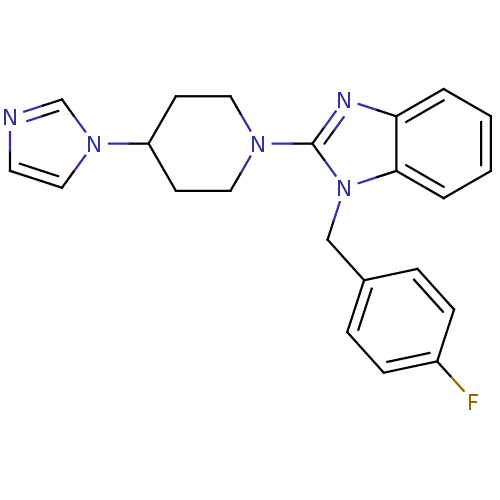
(2-(4-(1H-imidazol-1-yl)piperidin-1-yl)-1-(4-fluoro...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)n2ccnc2)cc1 Show InChI InChI=1S/C22H22FN5/c23-18-7-5-17(6-8-18)15-28-21-4-2-1-3-20(21)25-22(28)26-12-9-19(10-13-26)27-14-11-24-16-27/h1-8,11,14,16,19H,9-10,12-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297863
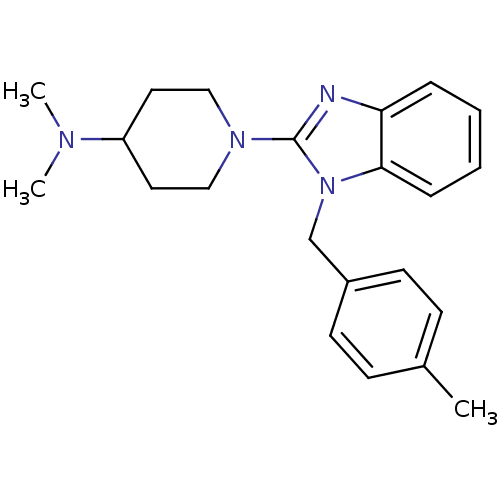
(CHEMBL563451 | N,N-dimethyl-1-(1-(4-methylbenzyl)-...)Show InChI InChI=1S/C22H28N4/c1-17-8-10-18(11-9-17)16-26-21-7-5-4-6-20(21)23-22(26)25-14-12-19(13-15-25)24(2)3/h4-11,19H,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297864
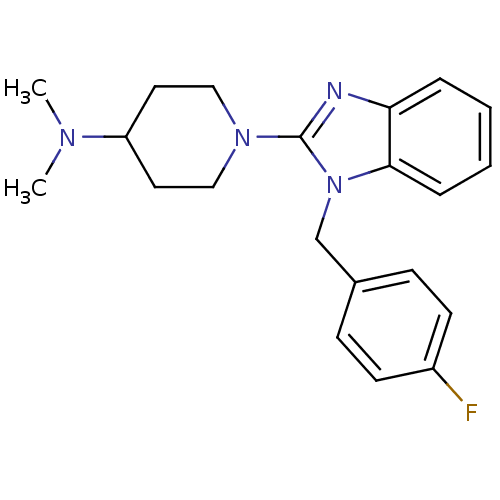
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N,...)Show InChI InChI=1S/C21H25FN4/c1-24(2)18-11-13-25(14-12-18)21-23-19-5-3-4-6-20(19)26(21)15-16-7-9-17(22)10-8-16/h3-10,18H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50326674
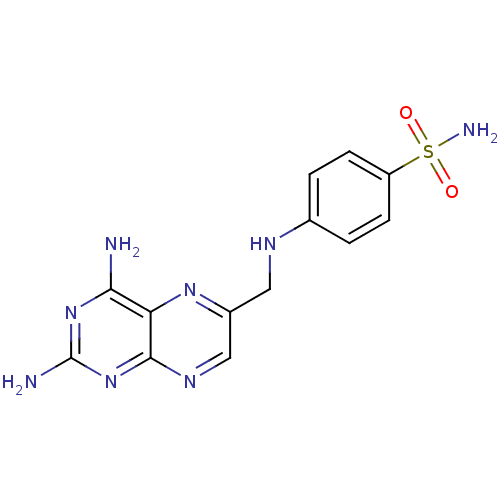
(4-((2,4-Diaminopteridin-6-yl)methylamino)benzenesu...)Show InChI InChI=1S/C13H14N8O2S/c14-11-10-12(21-13(15)20-11)18-6-8(19-10)5-17-7-1-3-9(4-2-7)24(16,22)23/h1-4,6,17H,5H2,(H2,16,22,23)(H4,14,15,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297859
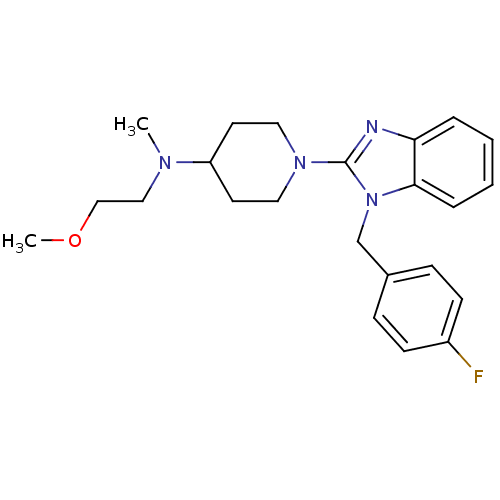
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES COCCN(C)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C23H29FN4O/c1-26(15-16-29-2)20-11-13-27(14-12-20)23-25-21-5-3-4-6-22(21)28(23)17-18-7-9-19(24)10-8-18/h3-10,20H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297866
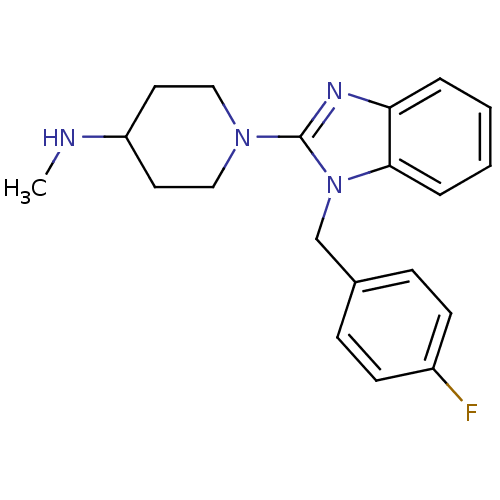
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show InChI InChI=1S/C20H23FN4/c1-22-17-10-12-24(13-11-17)20-23-18-4-2-3-5-19(18)25(20)14-15-6-8-16(21)9-7-15/h2-9,17,22H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297862
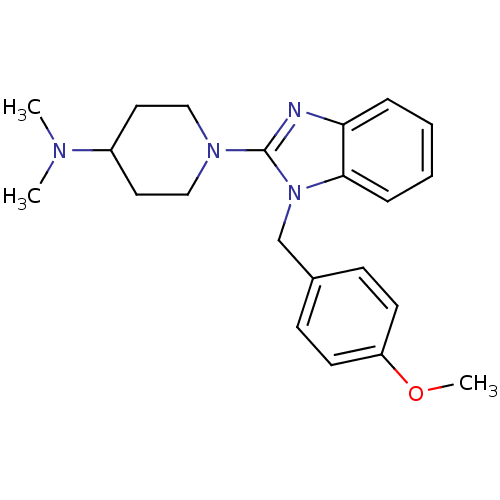
(1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...)Show SMILES COc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)N(C)C)cc1 Show InChI InChI=1S/C22H28N4O/c1-24(2)18-12-14-25(15-13-18)22-23-20-6-4-5-7-21(20)26(22)16-17-8-10-19(27-3)11-9-17/h4-11,18H,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297869
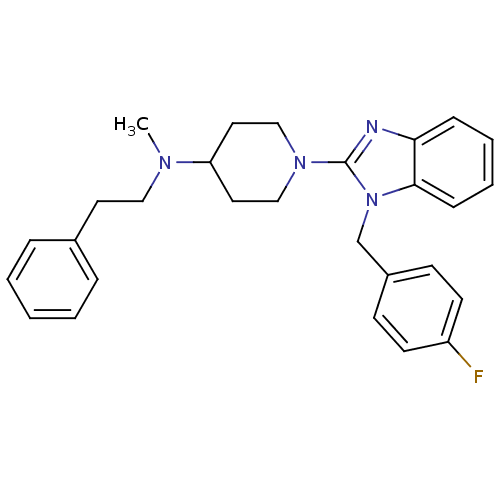
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(CCc1ccccc1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C28H31FN4/c1-31(18-15-22-7-3-2-4-8-22)25-16-19-32(20-17-25)28-30-26-9-5-6-10-27(26)33(28)21-23-11-13-24(29)14-12-23/h2-14,25H,15-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50326678

(4-((2,4-Diaminopteridin-6-yl)methylamino)-N-(4-sul...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NCCc3ccc(cc3)S(N)(=O)=O)cnc2n1 Show InChI InChI=1S/C22H23N9O3S/c23-19-18-20(31-22(24)30-19)28-12-16(29-18)11-27-15-5-3-14(4-6-15)21(32)26-10-9-13-1-7-17(8-2-13)35(25,33)34/h1-8,12,27H,9-11H2,(H,26,32)(H2,25,33,34)(H4,23,24,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22877
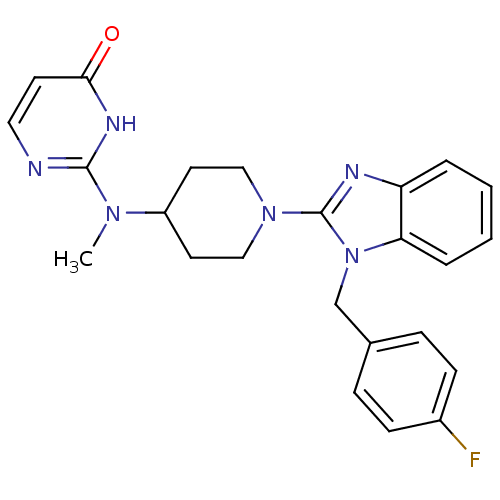
(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297867
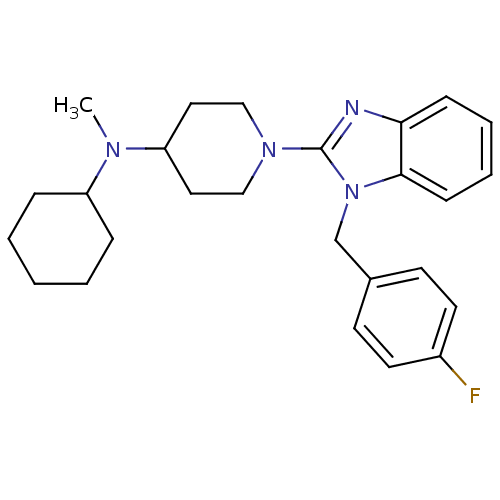
(CHEMBL558933 | N-cyclohexyl-1-(1-(4-fluorobenzyl)-...)Show SMILES CN(C1CCCCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C26H33FN4/c1-29(22-7-3-2-4-8-22)23-15-17-30(18-16-23)26-28-24-9-5-6-10-25(24)31(26)19-20-11-13-21(27)14-12-20/h5-6,9-14,22-23H,2-4,7-8,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297855
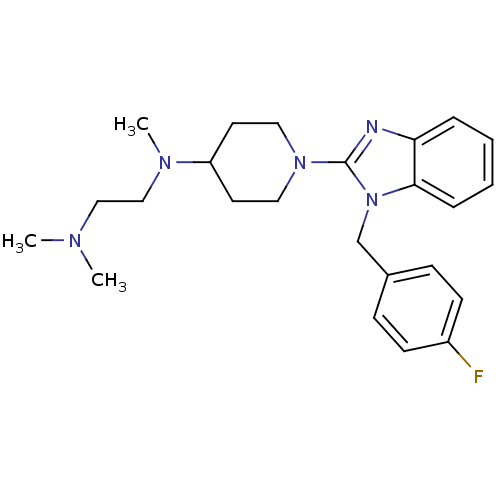
(CHEMBL559061 | N1-(1-(1-(4-fluorobenzyl)-1H-benzo[...)Show SMILES CN(C)CCN(C)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C24H32FN5/c1-27(2)16-17-28(3)21-12-14-29(15-13-21)24-26-22-6-4-5-7-23(22)30(24)18-19-8-10-20(25)11-9-19/h4-11,21H,12-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297858
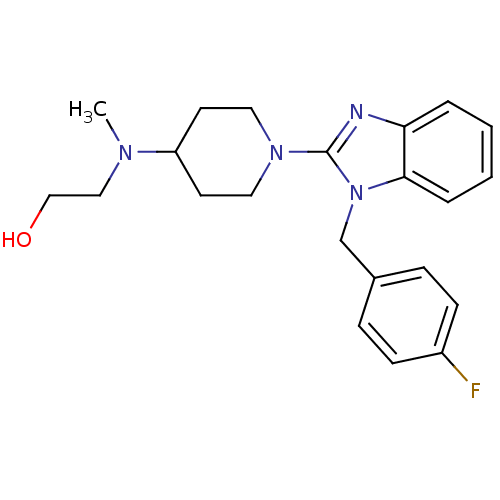
(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show InChI InChI=1S/C22H27FN4O/c1-25(14-15-28)19-10-12-26(13-11-19)22-24-20-4-2-3-5-21(20)27(22)16-17-6-8-18(23)9-7-17/h2-9,19,28H,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50246599

(CHEMBL471537 | N-(4-Phenoxybenzenesulfonyl)-N-{[2-...)Show SMILES CC(C)[C@@H](N(CC(=O)NCCc1ccc(cc1)S(N)(=O)=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H32N4O8S2/c1-19(2)26(27(33)30-34)31(18-25(32)29-17-16-20-8-12-23(13-9-20)40(28,35)36)41(37,38)24-14-10-22(11-15-24)39-21-6-4-3-5-7-21/h3-15,19,26,34H,16-18H2,1-2H3,(H,29,32)(H,30,33)(H2,28,35,36)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50320030
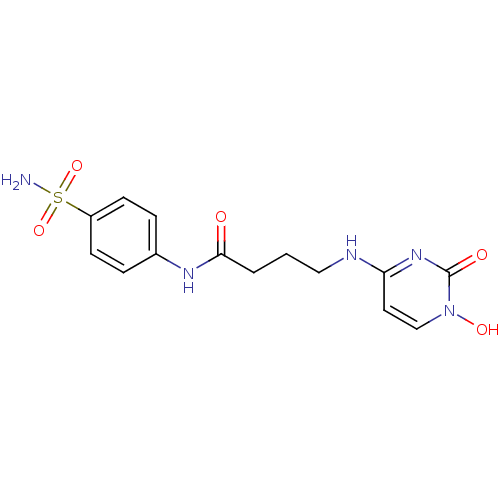
(4-{3-[(4-sulfamoyl-phenyl)carbamoyl]propylamino]-1...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CCCNc2ccn(O)c(=O)n2)cc1 Show InChI InChI=1S/C14H17N5O5S/c15-25(23,24)11-5-3-10(4-6-11)17-13(20)2-1-8-16-12-7-9-19(22)14(21)18-12/h3-7,9,22H,1-2,8H2,(H,17,20)(H2,15,23,24)(H,16,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50320031
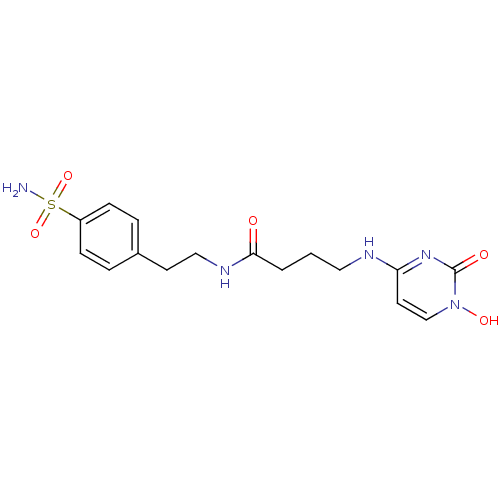
(4-{3-[2-(4-sulfamoyl-phenyl)-ethyl-carbamoyl]propy...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CCCNc2ccn(O)c(=O)n2)cc1 Show InChI InChI=1S/C16H21N5O5S/c17-27(25,26)13-5-3-12(4-6-13)7-10-19-15(22)2-1-9-18-14-8-11-21(24)16(23)20-14/h3-6,8,11,24H,1-2,7,9-10H2,(H,19,22)(H2,17,25,26)(H,18,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297870
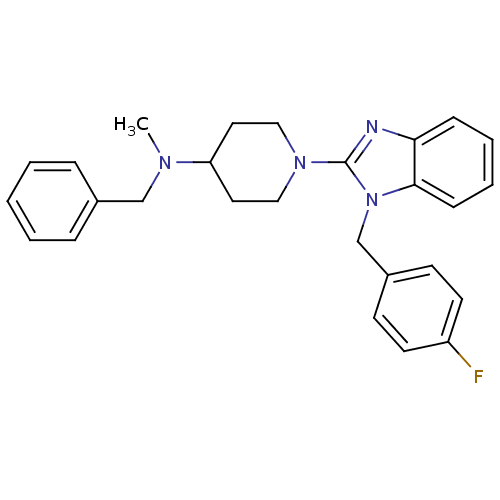
(CHEMBL551888 | N-benzyl-1-(1-(4-fluorobenzyl)-1H-b...)Show SMILES CN(Cc1ccccc1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C27H29FN4/c1-30(19-21-7-3-2-4-8-21)24-15-17-31(18-16-24)27-29-25-9-5-6-10-26(25)32(27)20-22-11-13-23(28)14-12-22/h2-14,24H,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50326680

(4-((2,4-Diaminopteridin-6-yl)methyl)(methyl)amino)...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H25N9O3S/c1-32(13-16-12-28-21-19(29-16)20(24)30-23(25)31-21)17-6-4-15(5-7-17)22(33)27-11-10-14-2-8-18(9-3-14)36(26,34)35/h2-9,12H,10-11,13H2,1H3,(H,27,33)(H2,26,34,35)(H4,24,25,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50241250
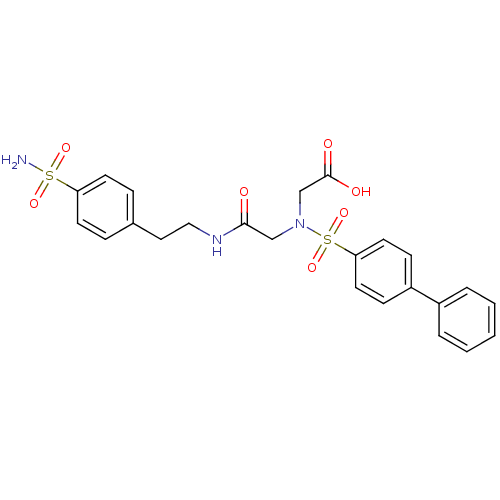
(((bBiphenyl-4-sulfonyl)-{[2-(4-sulfamoyl-phenyl)-e...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(O)=O)S(=O)(=O)c2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H25N3O7S2/c25-35(31,32)21-10-6-18(7-11-21)14-15-26-23(28)16-27(17-24(29)30)36(33,34)22-12-8-20(9-13-22)19-4-2-1-3-5-19/h1-13H,14-17H2,(H,26,28)(H,29,30)(H2,25,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297861
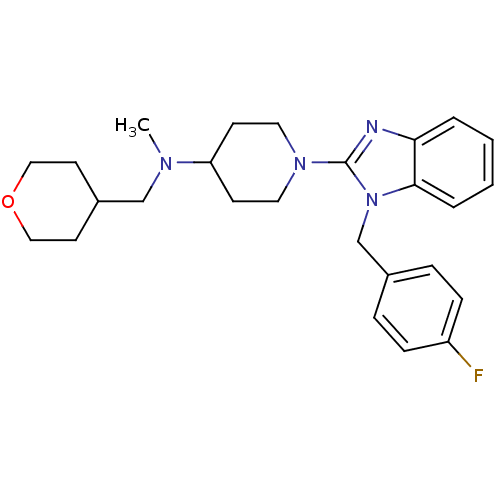
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(CC1CCOCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C26H33FN4O/c1-29(18-21-12-16-32-17-13-21)23-10-14-30(15-11-23)26-28-24-4-2-3-5-25(24)31(26)19-20-6-8-22(27)9-7-20/h2-9,21,23H,10-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50320033
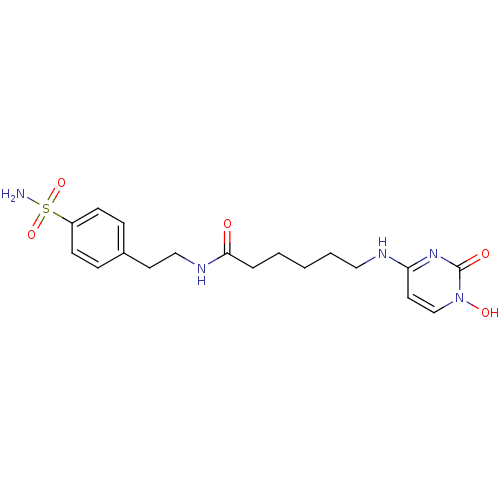
(4-{5-[2-(4-sulfamoyl-phenyl)-ethyl-carbamoyl]penty...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CCCCCNc2ccn(O)c(=O)n2)cc1 Show InChI InChI=1S/C18H25N5O5S/c19-29(27,28)15-7-5-14(6-8-15)9-12-21-17(24)4-2-1-3-11-20-16-10-13-23(26)18(25)22-16/h5-8,10,13,26H,1-4,9,11-12H2,(H,21,24)(H2,19,27,28)(H,20,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50246599

(CHEMBL471537 | N-(4-Phenoxybenzenesulfonyl)-N-{[2-...)Show SMILES CC(C)[C@@H](N(CC(=O)NCCc1ccc(cc1)S(N)(=O)=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H32N4O8S2/c1-19(2)26(27(33)30-34)31(18-25(32)29-17-16-20-8-12-23(13-9-20)40(28,35)36)41(37,38)24-14-10-22(11-15-24)39-21-6-4-3-5-7-21/h3-15,19,26,34H,16-18H2,1-2H3,(H,29,32)(H,30,33)(H2,28,35,36)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50326674

(4-((2,4-Diaminopteridin-6-yl)methylamino)benzenesu...)Show InChI InChI=1S/C13H14N8O2S/c14-11-10-12(21-13(15)20-11)18-6-8(19-10)5-17-7-1-3-9(4-2-7)24(16,22)23/h1-4,6,17H,5H2,(H2,16,22,23)(H4,14,15,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297857
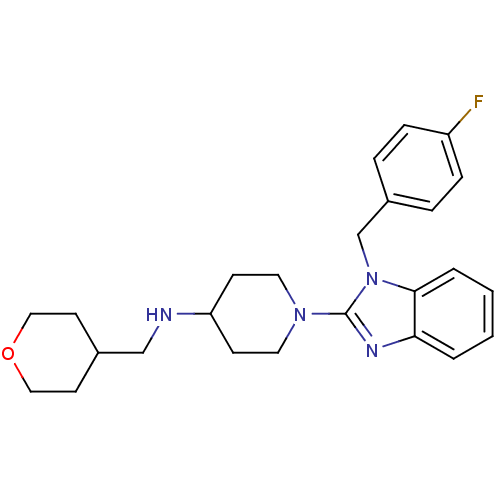
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)NCC2CCOCC2)cc1 Show InChI InChI=1S/C25H31FN4O/c26-21-7-5-20(6-8-21)18-30-24-4-2-1-3-23(24)28-25(30)29-13-9-22(10-14-29)27-17-19-11-15-31-16-12-19/h1-8,19,22,27H,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50326676
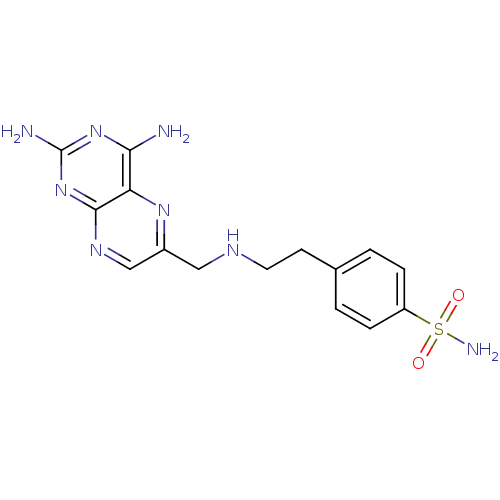
(4-(2-((2,4-Diaminopteridin-6-yl)methylamino)ethyl)...)Show SMILES Nc1nc(N)c2nc(CNCCc3ccc(cc3)S(N)(=O)=O)cnc2n1 Show InChI InChI=1S/C15H18N8O2S/c16-13-12-14(23-15(17)22-13)20-8-10(21-12)7-19-6-5-9-1-3-11(4-2-9)26(18,24)25/h1-4,8,19H,5-7H2,(H2,18,24,25)(H4,16,17,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50241249
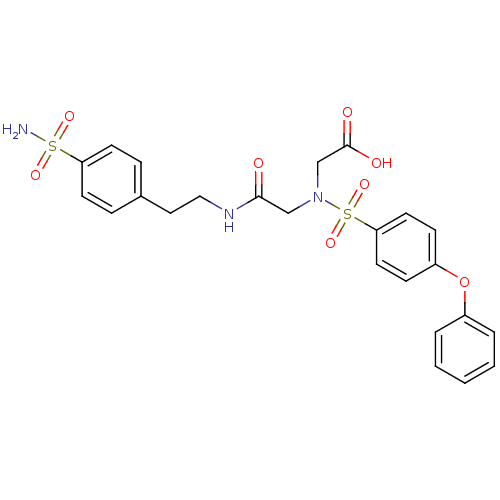
(((4-phenoxy-benzenesulfonyl)-{[2-(4sulfamoyl-pheny...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(O)=O)S(=O)(=O)c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C24H25N3O8S2/c25-36(31,32)21-10-6-18(7-11-21)14-15-26-23(28)16-27(17-24(29)30)37(33,34)22-12-8-20(9-13-22)35-19-4-2-1-3-5-19/h1-13H,14-17H2,(H,26,28)(H,29,30)(H2,25,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297854
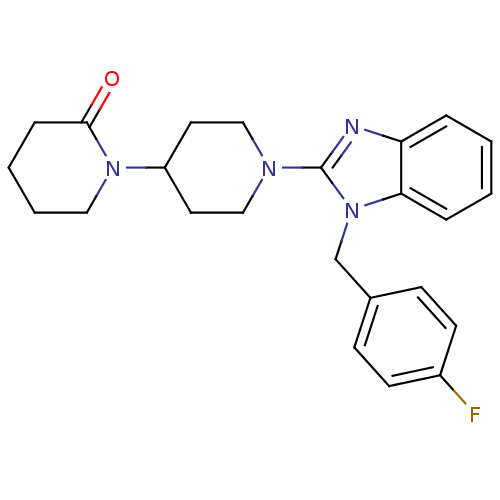
(1'-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-1...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)N2CCCCC2=O)cc1 Show InChI InChI=1S/C24H27FN4O/c25-19-10-8-18(9-11-19)17-29-22-6-2-1-5-21(22)26-24(29)27-15-12-20(13-16-27)28-14-4-3-7-23(28)30/h1-2,5-6,8-11,20H,3-4,7,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297850
(1-(4-fluorobenzyl)-2-(4-(4-methyl-1H-pyrazol-1-yl)...)Show SMILES Cc1cnn(c1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C23H24FN5/c1-17-14-25-29(15-17)20-10-12-27(13-11-20)23-26-21-4-2-3-5-22(21)28(23)16-18-6-8-19(24)9-7-18/h2-9,14-15,20H,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
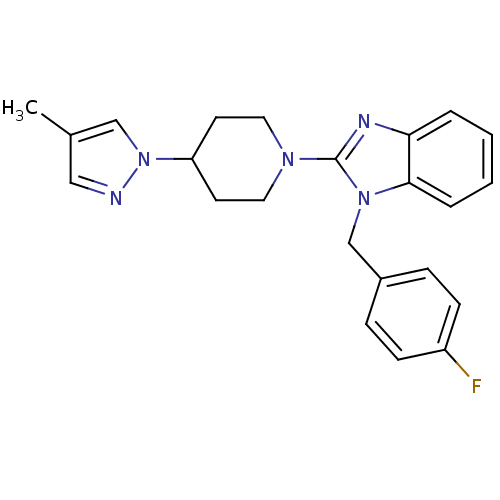
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297849
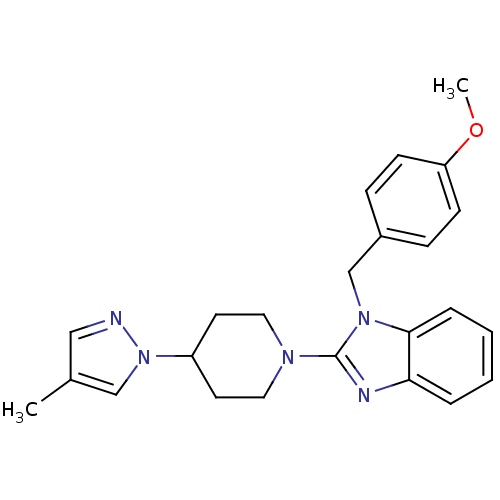
(1-(4-methoxybenzyl)-2-(4-(4-methyl-1H-pyrazol-1-yl...)Show SMILES COc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)n2cc(C)cn2)cc1 Show InChI InChI=1S/C24H27N5O/c1-18-15-25-29(16-18)20-11-13-27(14-12-20)24-26-22-5-3-4-6-23(22)28(24)17-19-7-9-21(30-2)10-8-19/h3-10,15-16,20H,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50241250

(((bBiphenyl-4-sulfonyl)-{[2-(4-sulfamoyl-phenyl)-e...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(O)=O)S(=O)(=O)c2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H25N3O7S2/c25-35(31,32)21-10-6-18(7-11-21)14-15-26-23(28)16-27(17-24(29)30)36(33,34)22-12-8-20(9-13-22)19-4-2-1-3-5-19/h1-13H,14-17H2,(H,26,28)(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50320032
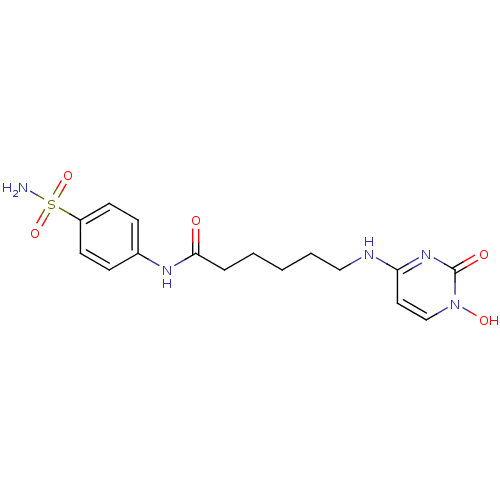
(4-{5-[(4-sulfamoyl-phenyl)carbamoyl]-pentylamino}-...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CCCCCNc2ccn(O)c(=O)n2)cc1 Show InChI InChI=1S/C16H21N5O5S/c17-27(25,26)13-7-5-12(6-8-13)19-15(22)4-2-1-3-10-18-14-9-11-21(24)16(23)20-14/h5-9,11,24H,1-4,10H2,(H,19,22)(H2,17,25,26)(H,18,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50241249

(((4-phenoxy-benzenesulfonyl)-{[2-(4sulfamoyl-pheny...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(O)=O)S(=O)(=O)c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C24H25N3O8S2/c25-36(31,32)21-10-6-18(7-11-21)14-15-26-23(28)16-27(17-24(29)30)37(33,34)22-12-8-20(9-13-22)35-19-4-2-1-3-5-19/h1-13H,14-17H2,(H,26,28)(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50241252
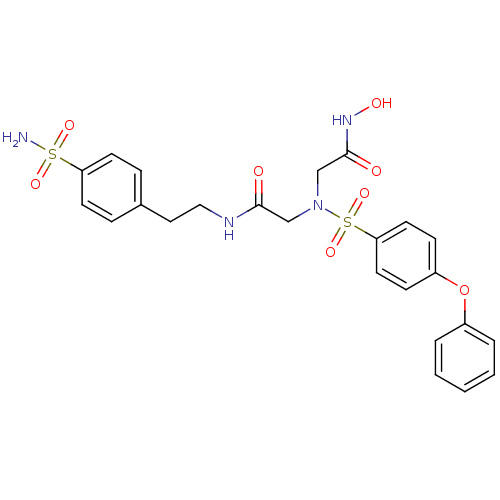
(2-[hydroxycarbamoylmethyl-(4-phenoxy-benzenesulfon...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(=O)NO)S(=O)(=O)c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C24H26N4O8S2/c25-37(32,33)21-10-6-18(7-11-21)14-15-26-23(29)16-28(17-24(30)27-31)38(34,35)22-12-8-20(9-13-22)36-19-4-2-1-3-5-19/h1-13,31H,14-17H2,(H,26,29)(H,27,30)(H2,25,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50320032

(4-{5-[(4-sulfamoyl-phenyl)carbamoyl]-pentylamino}-...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)CCCCCNc2ccn(O)c(=O)n2)cc1 Show InChI InChI=1S/C16H21N5O5S/c17-27(25,26)13-7-5-12(6-8-13)19-15(22)4-2-1-3-10-18-14-9-11-21(24)16(23)20-14/h5-9,11,24H,1-4,10H2,(H,19,22)(H2,17,25,26)(H,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50241253
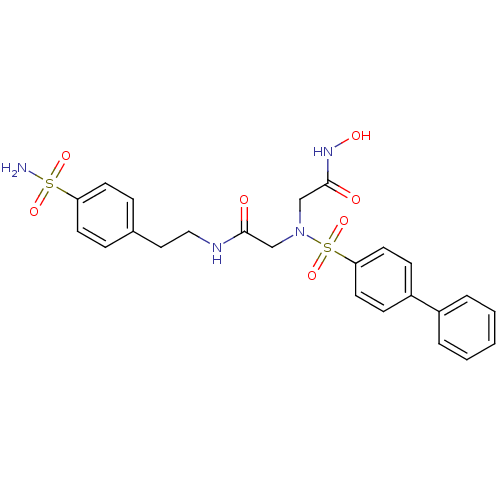
(2-[(biphenyl-4-sulfonyl)-hydroxycarbamoylmethyl-am...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CC(=O)NO)S(=O)(=O)c2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H26N4O7S2/c25-36(32,33)21-10-6-18(7-11-21)14-15-26-23(29)16-28(17-24(30)27-31)37(34,35)22-12-8-20(9-13-22)19-4-2-1-3-5-19/h1-13,31H,14-17H2,(H,26,29)(H,27,30)(H2,25,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50241251
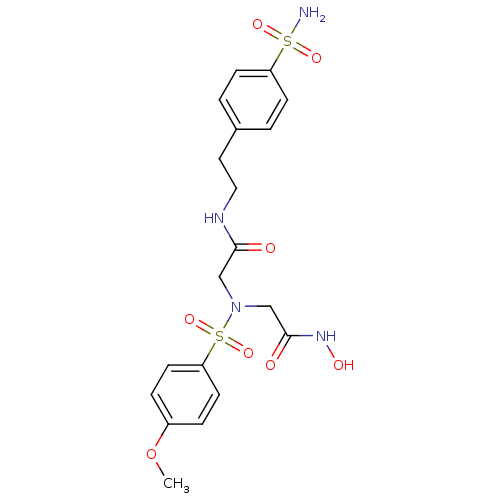
(2-[hydroxycarbamoylmethyl-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NO)CC(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H24N4O8S2/c1-31-15-4-8-17(9-5-15)33(29,30)23(13-19(25)22-26)12-18(24)21-11-10-14-2-6-16(7-3-14)32(20,27)28/h2-9,26H,10-13H2,1H3,(H,21,24)(H,22,25)(H2,20,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297860
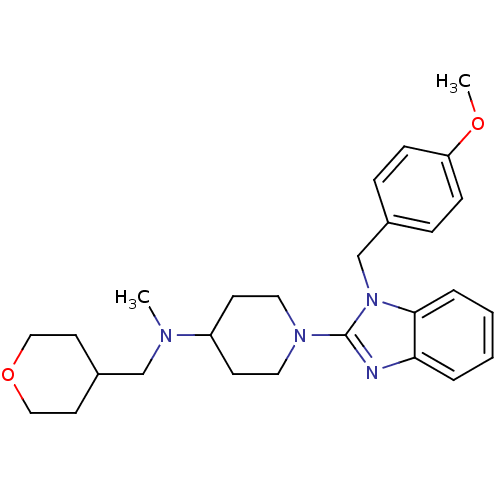
(1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...)Show SMILES COc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)N(C)CC2CCOCC2)cc1 Show InChI InChI=1S/C27H36N4O2/c1-29(19-22-13-17-33-18-14-22)23-11-15-30(16-12-23)27-28-25-5-3-4-6-26(25)31(27)20-21-7-9-24(32-2)10-8-21/h3-10,22-23H,11-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297856
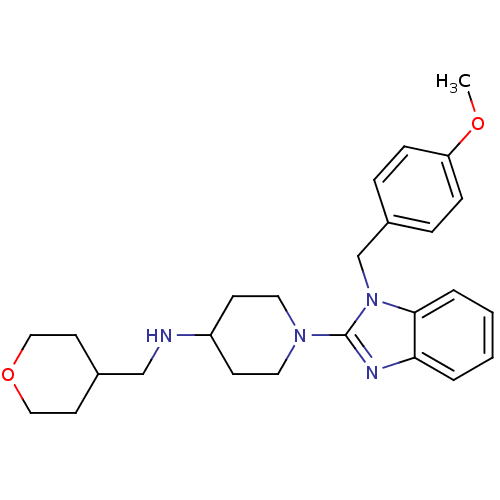
(1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...)Show SMILES COc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)NCC2CCOCC2)cc1 Show InChI InChI=1S/C26H34N4O2/c1-31-23-8-6-21(7-9-23)19-30-25-5-3-2-4-24(25)28-26(30)29-14-10-22(11-15-29)27-18-20-12-16-32-17-13-20/h2-9,20,22,27H,10-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 20: 3623-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.109
BindingDB Entry DOI: 10.7270/Q2J38TJR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain by stopped-flow CO2 hydrase assay |
J Med Chem 51: 7968-79 (2008)
Article DOI: 10.1021/jm800964f
BindingDB Entry DOI: 10.7270/Q2XK8FDN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 18: 5081-9 (2010)
Article DOI: 10.1016/j.bmc.2010.05.072
BindingDB Entry DOI: 10.7270/Q2T154M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297861

(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(CC1CCOCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C26H33FN4O/c1-29(18-21-12-16-32-17-13-21)23-10-14-30(15-11-23)26-28-24-4-2-3-5-25(24)31(26)19-20-6-8-22(27)9-7-20/h2-9,21,23H,10-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data