

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16485 (2-{6-methoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16469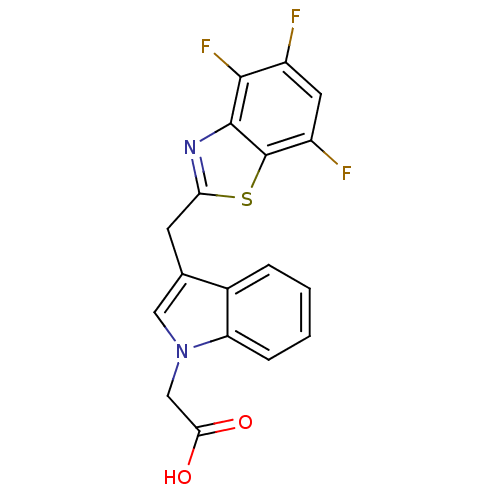 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16491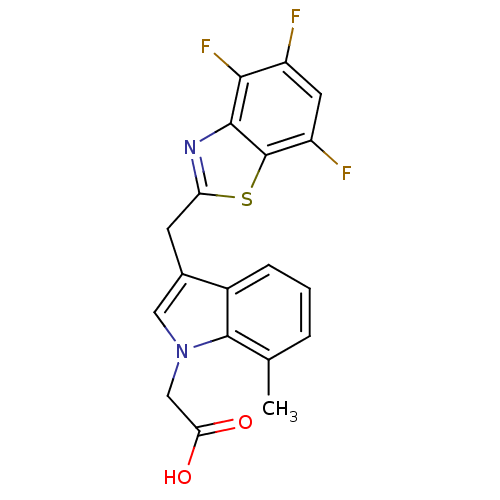 (2-{7-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16488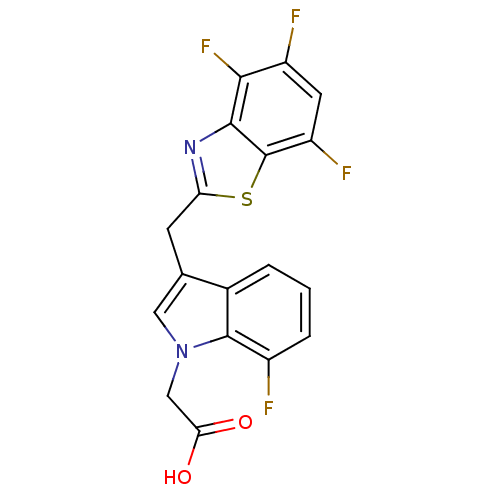 (2-{7-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16482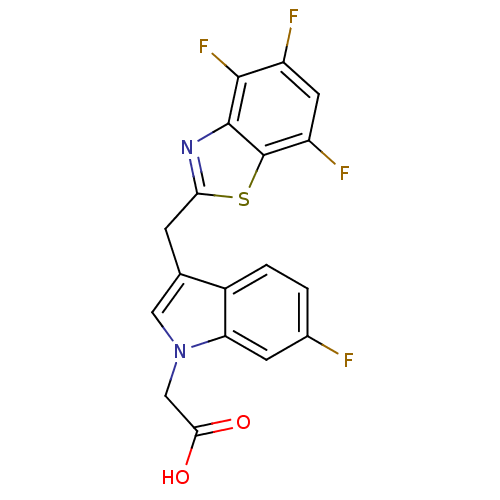 (2-{6-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16481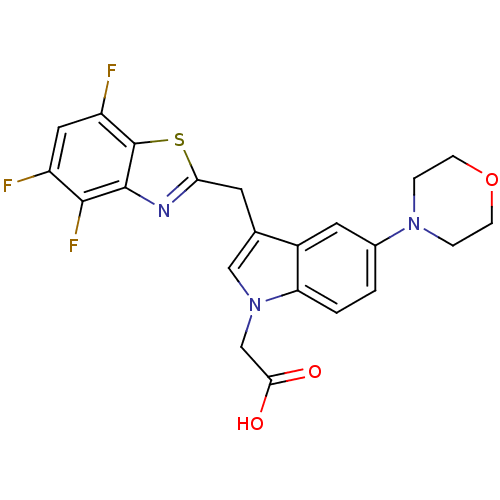 (2-[5-(morpholin-4-yl)-3-[(4,5,7-trifluoro-1,3-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16496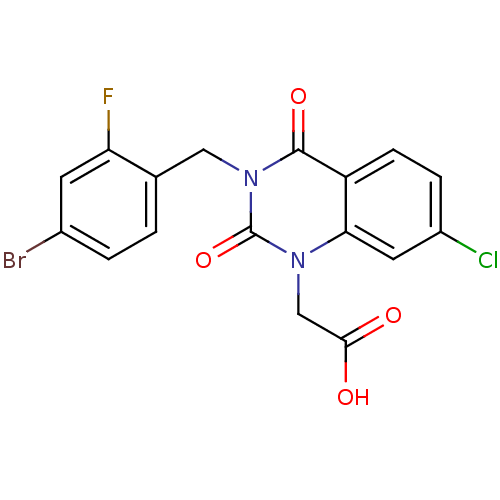 (2-{3-[(4-bromo-2-fluorophenyl)methyl]-7-chloro-2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16483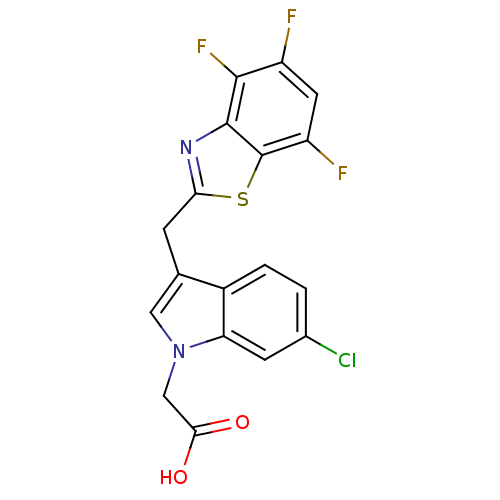 (2-{6-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16476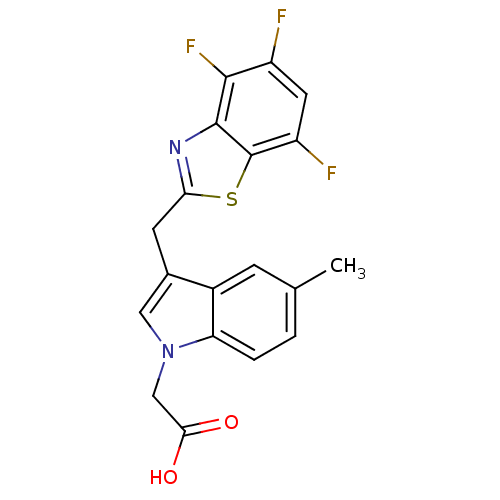 (2-{5-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16477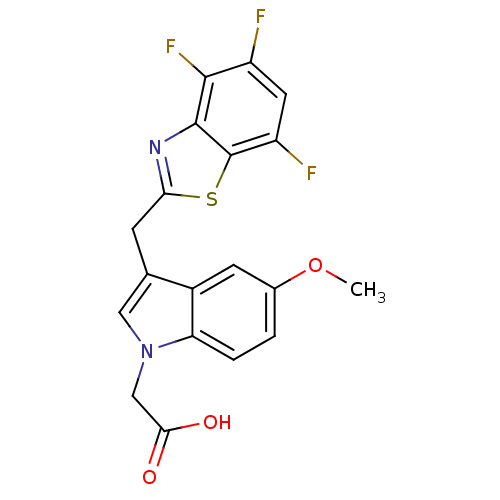 (2-{5-methoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16493 (2-{3-[2-(4,5,7-trifluoro-1,3-benzothiazol-2-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16470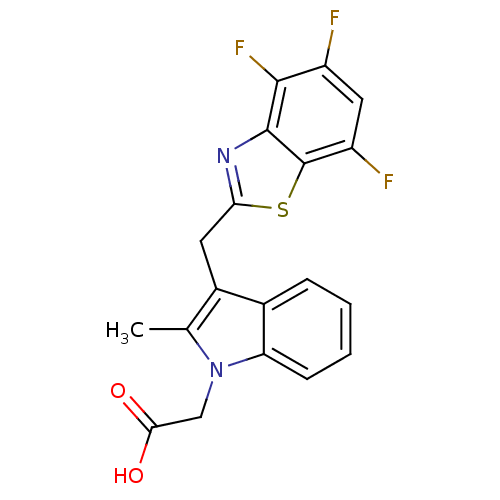 (2-{2-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16464 (2-{3-[(5-fluoro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16489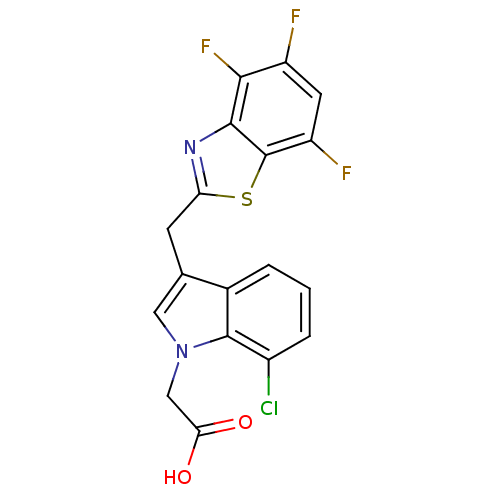 (2-{7-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16473 (2-{5-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16484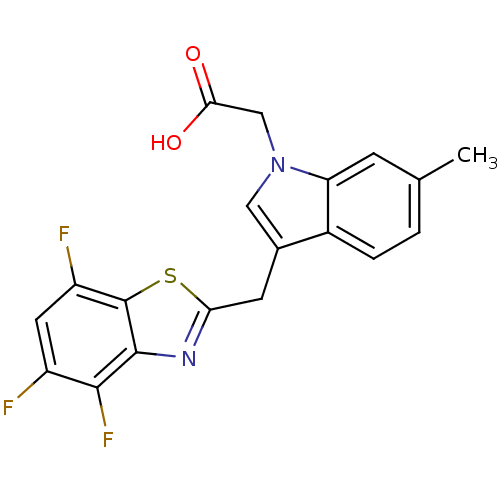 (2-{6-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16474 (2-{5-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50079859 ((R)-2-[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16472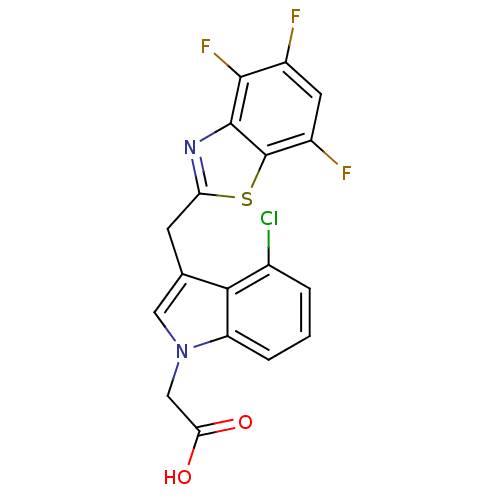 (2-{4-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16478 (2-[5-(benzyloxy)-3-[(4,5,7-trifluoro-1,3-benzothia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16475 (2-{5-bromo-3-[(4,5,7-trifluoro-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16314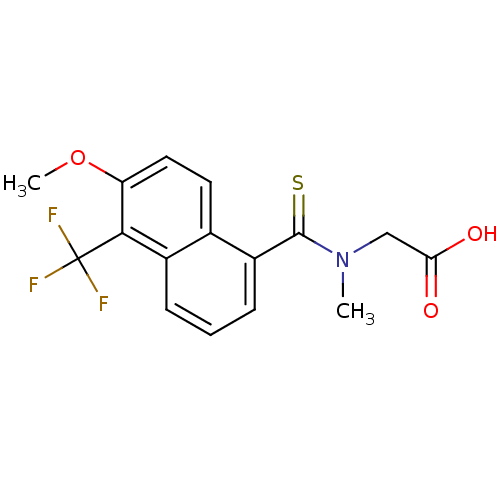 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16490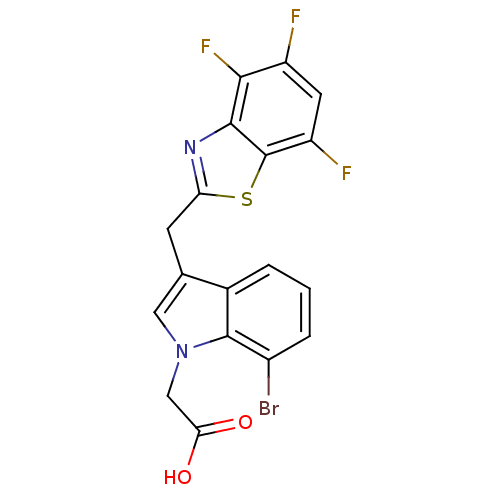 (2-{7-bromo-3-[(4,5,7-trifluoro-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16487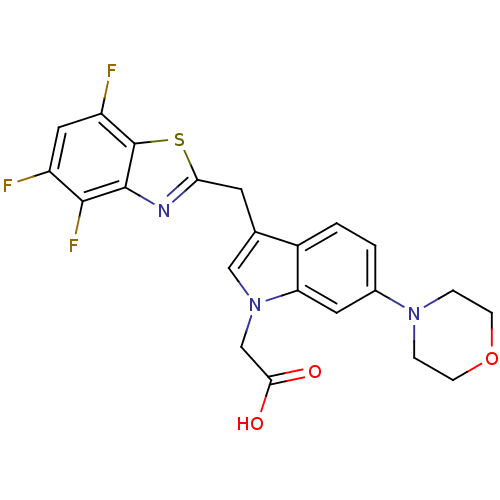 (2-[6-(morpholin-4-yl)-3-[(4,5,7-trifluoro-1,3-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16494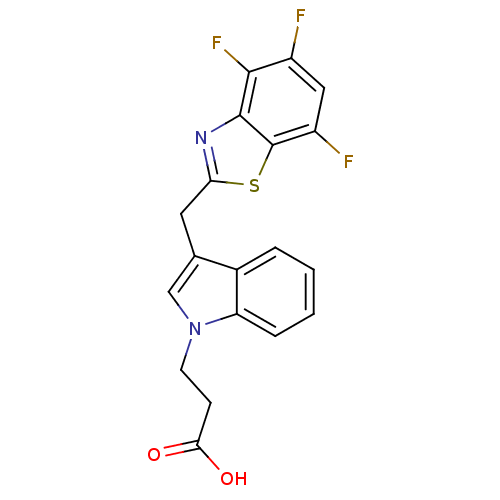 (3-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086986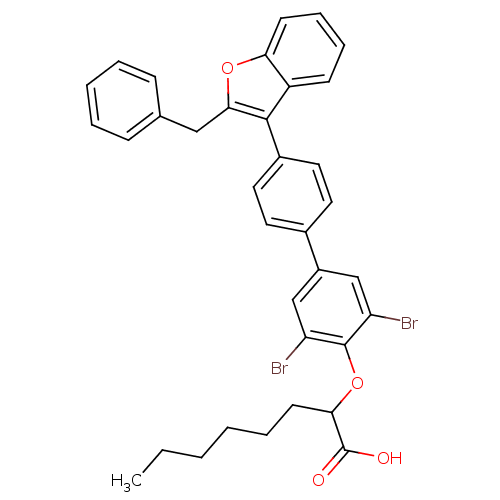 (2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086896 (4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3-bromo-bi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086972 (2-{4-[4-(2-benzyl-1-benzothiophen-3-yl)phenyl]-2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16486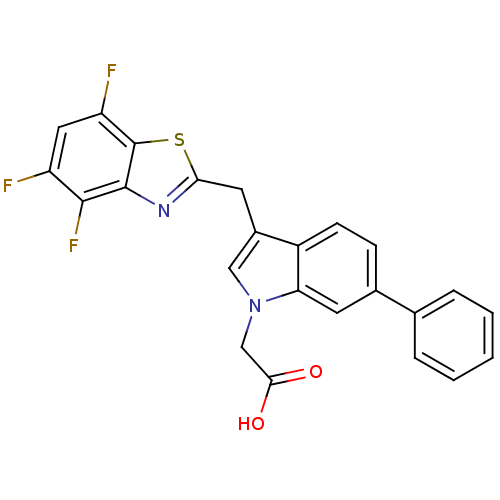 (2-{6-phenyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086947 (2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibromo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086950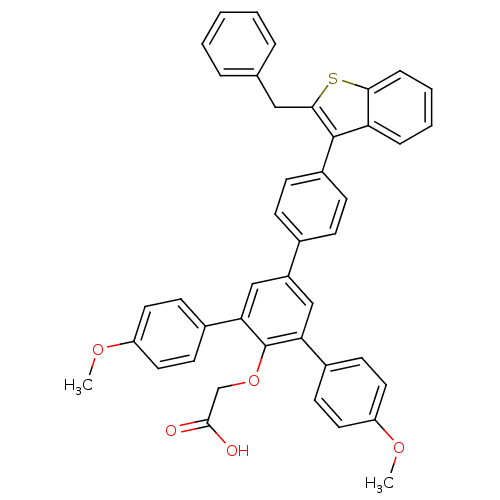 (2-[4-[4-(2-benzylbenzo[b]thiophen-3-yl)phenyl]-2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086911 (5-[4''-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086970 (4-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-biphenyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086923 (4-[4''-(2-Benzyl-benzofuran-3-yl)-3-cyclopentyl-bi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086975 (Benzothiophene derivative | CHEMBL25628 | [4-(2-Be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086981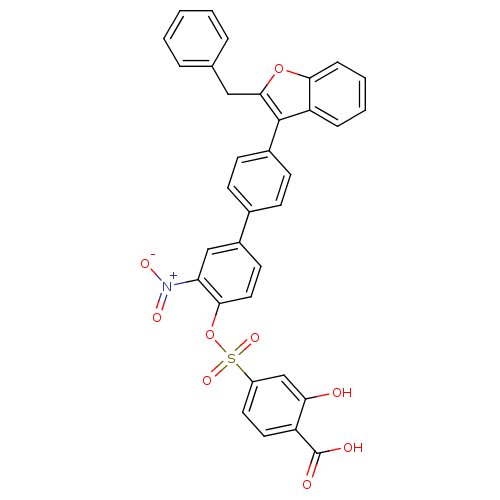 (4-[4''-(2-Benzyl-benzofuran-3-yl)-3-nitro-biphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086954 (CHEMBL278092 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086955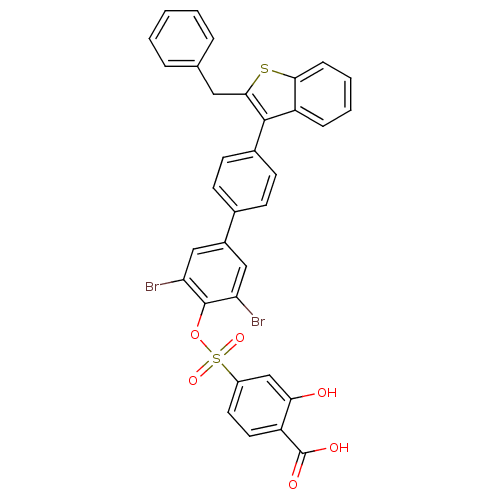 (4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibrom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16479 (2-{5-phenoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086951 (4-[4'-(2-Benzyl-4,5-dimethyl-thiophen-3-yl)-biphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16463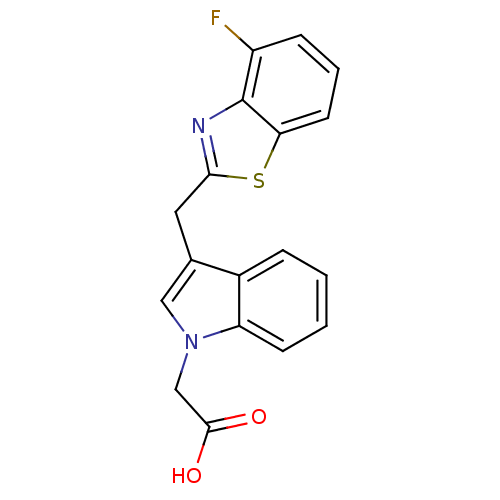 (2-{3-[(4-fluoro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086902 (4-[4''-(2-Benzyl-benzofuran-3-yl)-3,5-dimethyl-bip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086892 (2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086963 (4-[4'-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxys...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086973 (4-[4'-(2-Benzoyl-benzofuran-3-yl)-3-cyclopentyl-bi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086988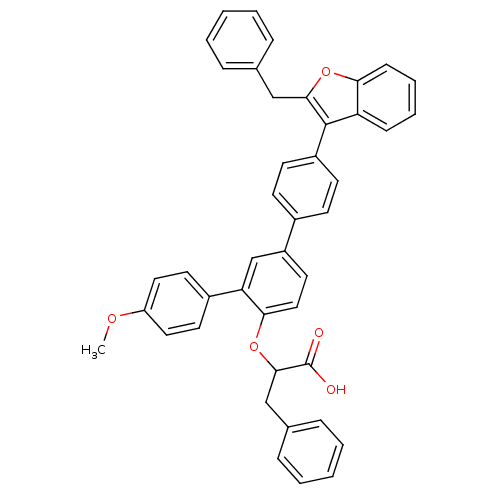 (2-[4-(2-Benzyl-benzofuran-3-yl)-4''-methoxy-[1,1';...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086952 (2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibromo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086969 (CHEMBL24607 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086903 (2-[4-(2-Benzyl-benzo[b]thiophen-3-yl)-4''-chloro-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) | J Med Chem 43: 1293-310 (2001) BindingDB Entry DOI: 10.7270/Q2W958FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 264 total ) | Next | Last >> |