

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110268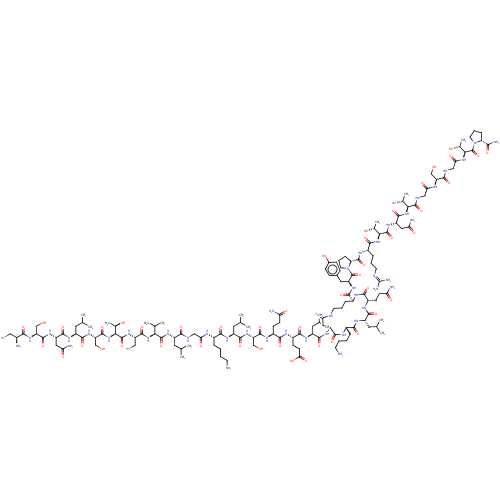 (CHEMBL2369895 | CSNLSTCVLGKLSQELc[DKLQK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50024170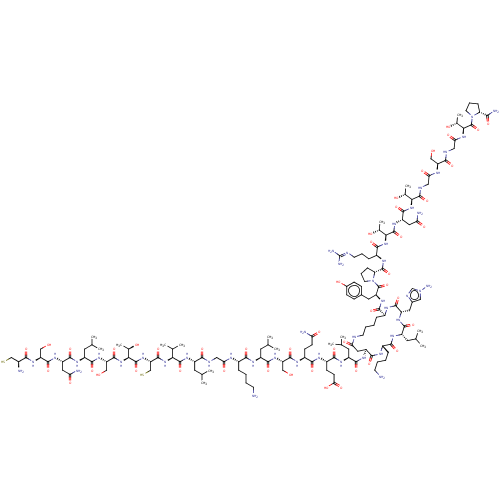 (CHEMBL2369912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110272 (CHEMBL2369907 | CSNLSTCVLGKLSQELc[DKLHK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110265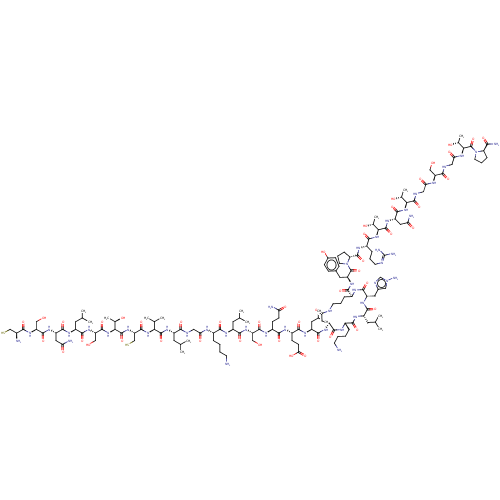 (CHEMBL2369886 | CSNLSTCVLGKLSQELc[DKLHO]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110275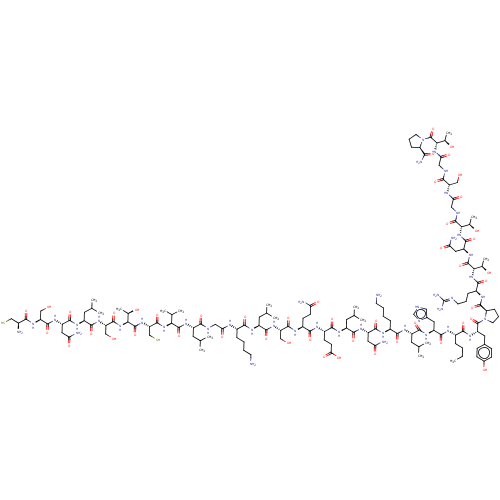 (CGNLSTCBLGTYTQDF[DKFHO]YPQTAIGVGAP-amide | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001107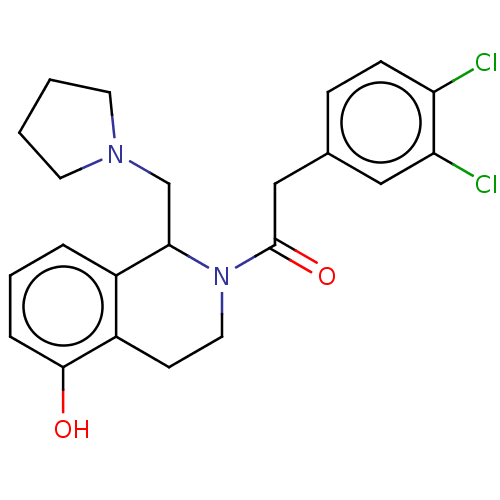 (2-(3,4-Dichloro-phenyl)-1-(5-hydroxy-1-pyrrolidin-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Receptor binding affinity towards opioid receptor kappa | J Med Chem 35: 2970-8 (1992) BindingDB Entry DOI: 10.7270/Q2P26ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity of compound was determined against Opioid receptor delta 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027063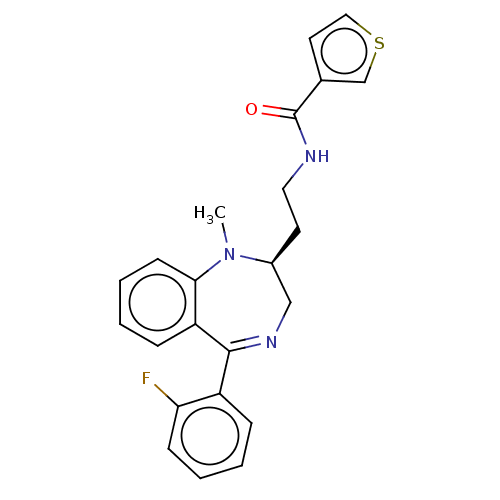 (CHEMBL2111838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027063 (CHEMBL2111838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027064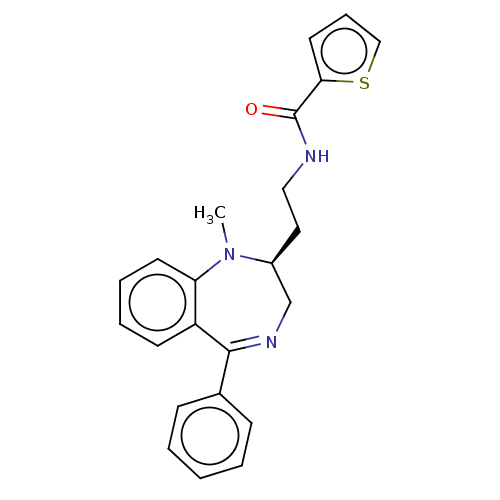 (CHEMBL2111836) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001108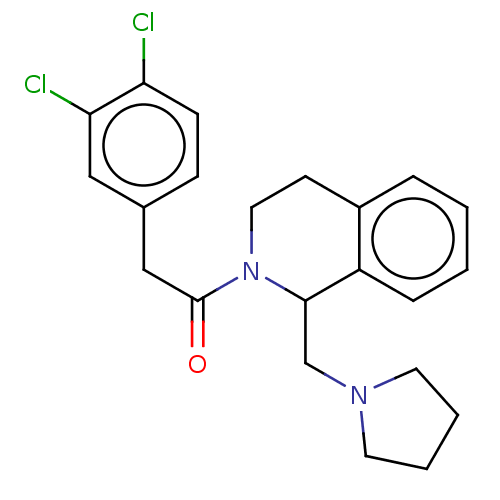 (2-(3,4-Dichloro-phenyl)-1-(1-pyrrolidin-1-ylmethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001108 (2-(3,4-Dichloro-phenyl)-1-(1-pyrrolidin-1-ylmethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Receptor binding affinity towards opioid receptor kappa | J Med Chem 35: 2970-8 (1992) BindingDB Entry DOI: 10.7270/Q2P26ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007159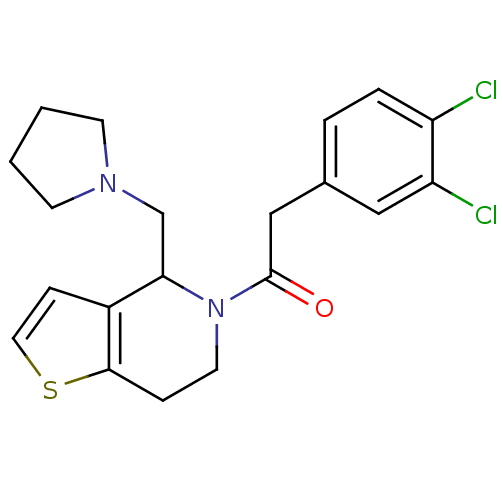 (2-(3,4-Dichloro-phenyl)-1-(4-pyrrolidin-1-ylmethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor delta 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50453089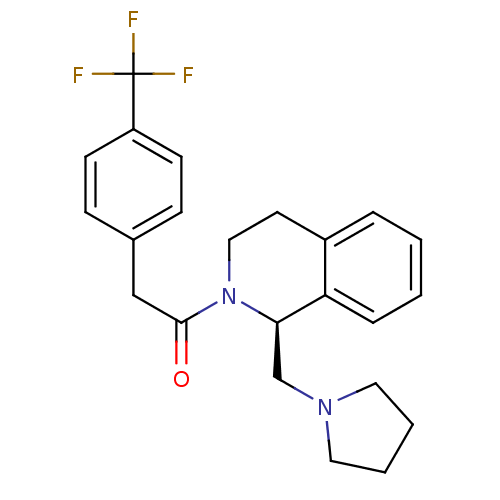 (CHEMBL2114225) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131967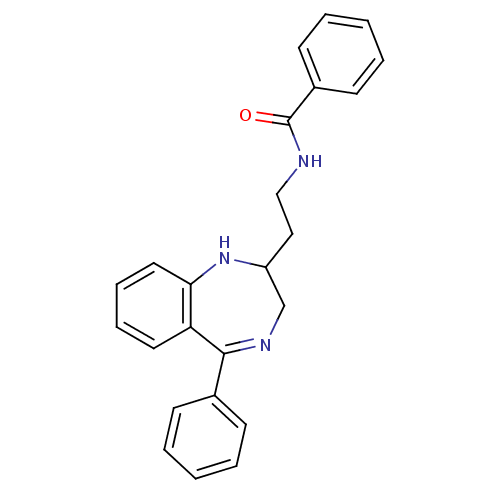 (CHEMBL123898 | N-[2-(5-Phenyl-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131967 (CHEMBL123898 | N-[2-(5-Phenyl-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131972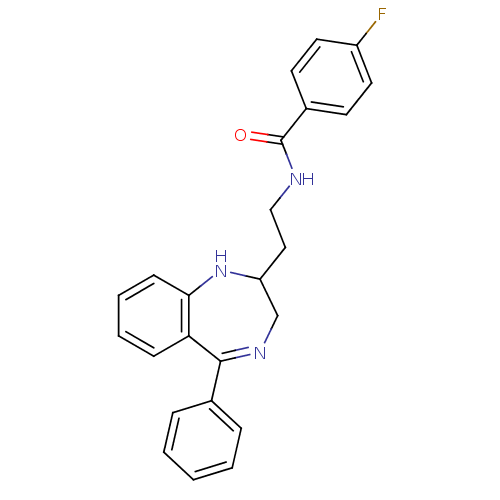 (4-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131972 (4-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049802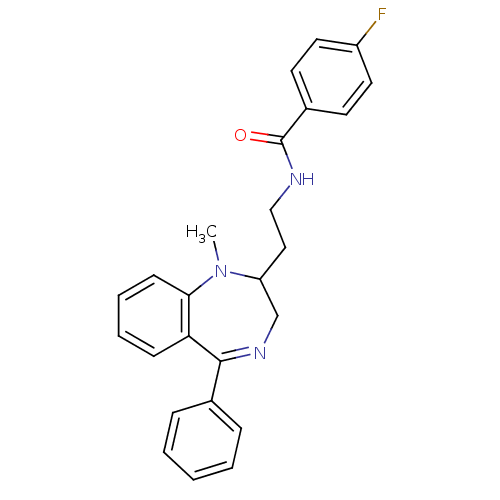 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049802 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007158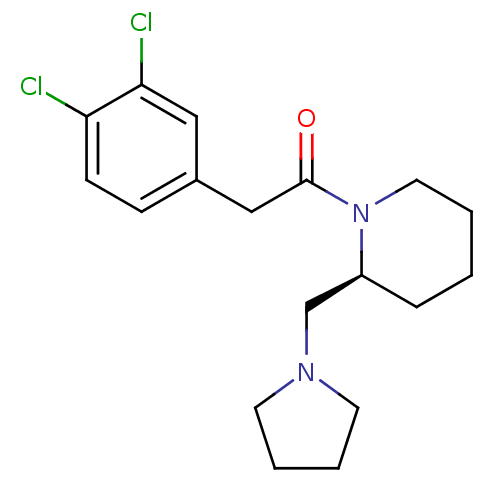 ((S)-2-(3,4-Dichloro-phenyl)-1-(2-pyrrolidin-1-ylme...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049807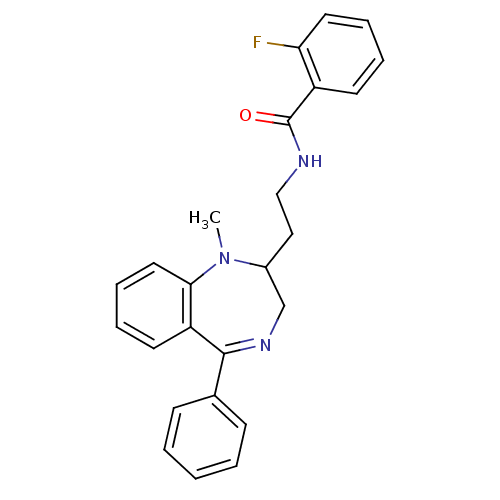 (2-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049807 (2-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131968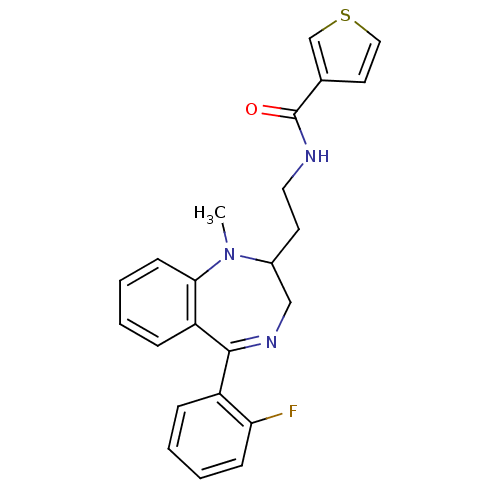 (CHEMBL127828 | Thiophene-3-carboxylic acid {2-[5-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001104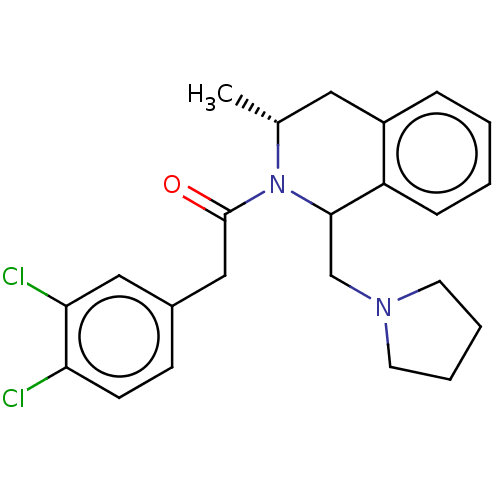 (2-(3,4-Dichloro-phenyl)-1-(3-methyl-1-pyrrolidin-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Receptor binding affinity towards opioid receptor kappa | J Med Chem 35: 2970-8 (1992) BindingDB Entry DOI: 10.7270/Q2P26ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027064 (CHEMBL2111836) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027064 (CHEMBL2111836) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131969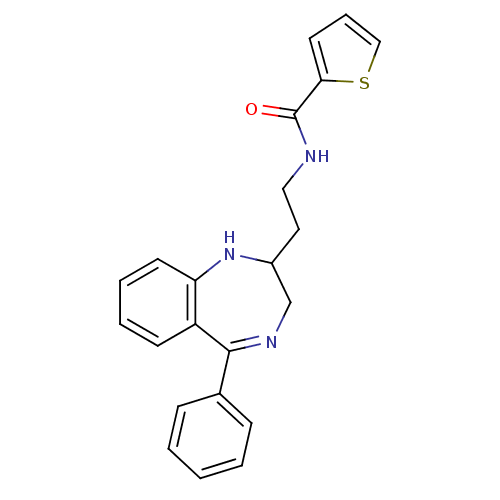 (CHEMBL126688 | Thiophene-2-carboxylic acid [2-(5-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131969 (CHEMBL126688 | Thiophene-2-carboxylic acid [2-(5-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007166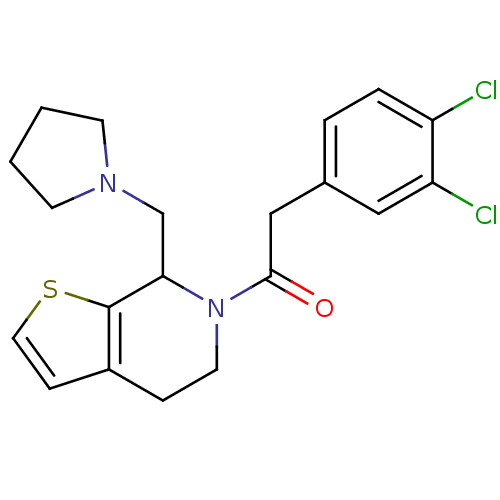 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007165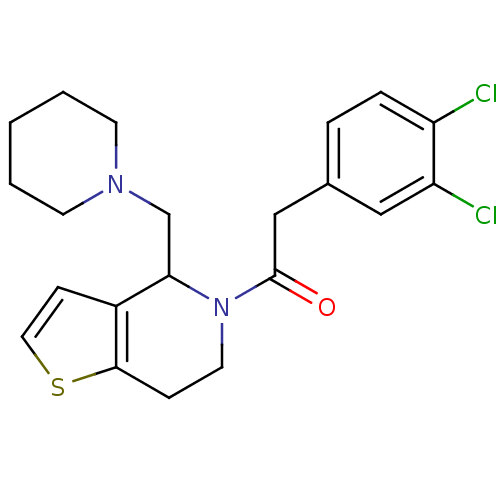 (2-(3,4-Dichloro-phenyl)-1-(4-piperidin-1-ylmethyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131973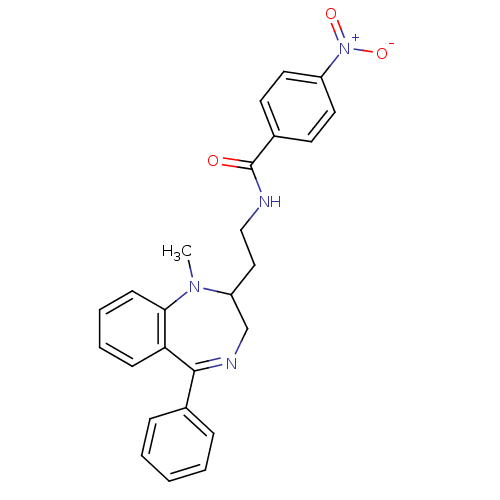 (CHEMBL338391 | N-[2-(1-Methyl-5-phenyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131973 (CHEMBL338391 | N-[2-(1-Methyl-5-phenyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001107 (2-(3,4-Dichloro-phenyl)-1-(5-hydroxy-1-pyrrolidin-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Receptor binding affinity towards opioid receptor mu | J Med Chem 35: 2970-8 (1992) BindingDB Entry DOI: 10.7270/Q2P26ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049806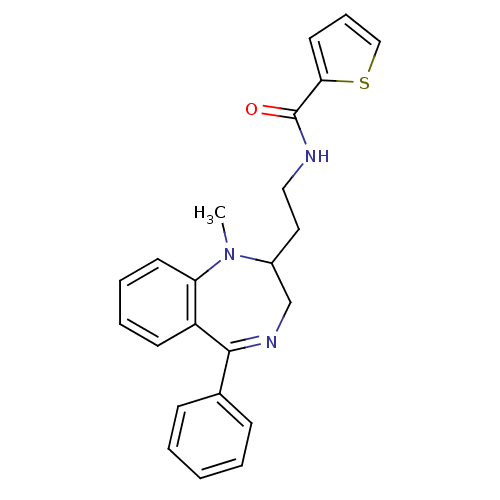 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049806 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049806 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50453088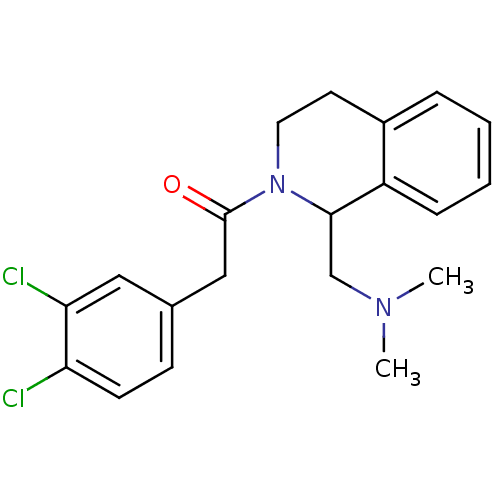 (CHEMBL2114226) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131970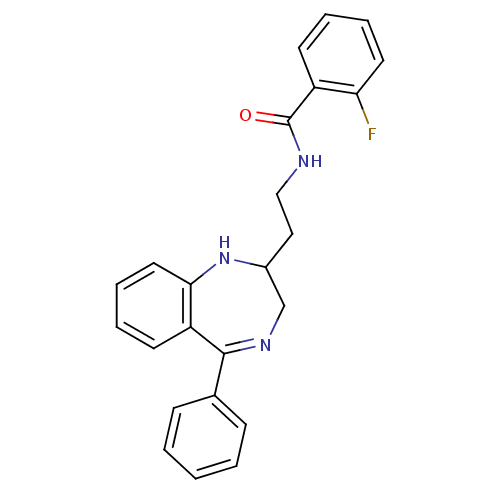 (2-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131970 (2-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049808 (CHEMBL127968 | N-[2-(7-Chloro-1-methyl-5-phenyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110273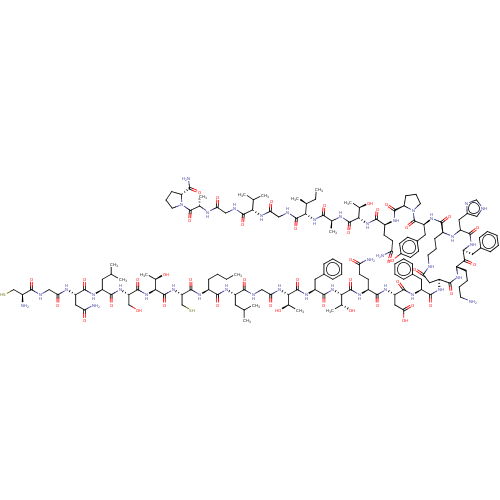 (CGNLSTCMLGTYTQDFc[DKFHK]FPQTAIGVGAP-amide | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049802 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049802 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007167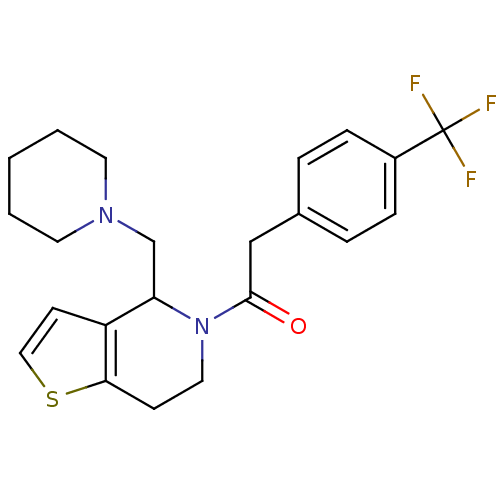 (1-(4-Piperidin-1-ylmethyl-6,7-dihydro-4H-thieno[3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001105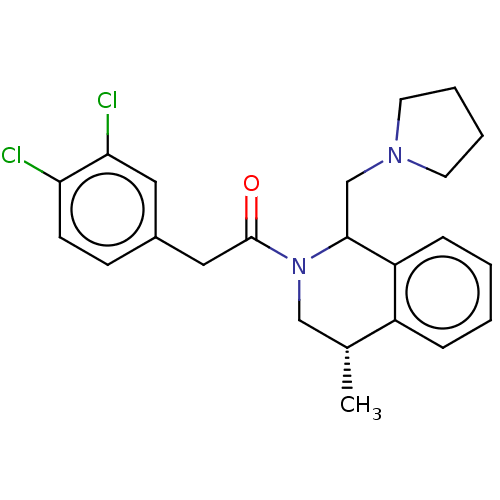 (2-(3,4-Dichloro-phenyl)-1-(4-methyl-1-pyrrolidin-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Receptor binding affinity towards opioid receptor kappa | J Med Chem 35: 2970-8 (1992) BindingDB Entry DOI: 10.7270/Q2P26ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007157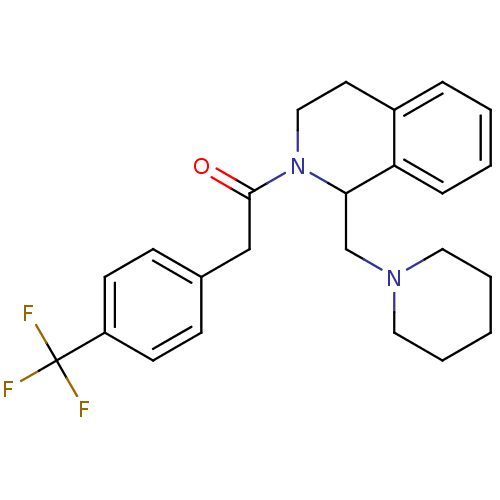 ((-)-1-(1-Piperidin-1-ylmethyl-3,4-dihydro-1H-isoqu...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007164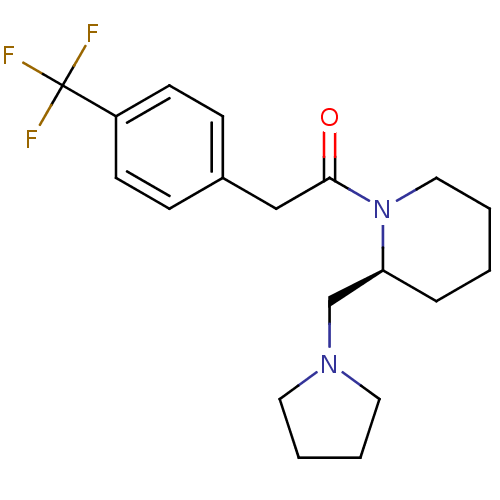 ((S)-1-(2-Pyrrolidin-1-ylmethyl-piperidin-1-yl)-2-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zambeletti Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-BRL 52537 from opioid receptor kappa site in guinea pig | J Med Chem 34: 2624-33 (1991) BindingDB Entry DOI: 10.7270/Q2CV4GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007164 ((S)-1-(2-Pyrrolidin-1-ylmethyl-piperidin-1-yl)-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Evaluated for the binding affinity at kappa receptor | J Med Chem 37: 3482-91 (1994) BindingDB Entry DOI: 10.7270/Q2W37VB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 246 total ) | Next | Last >> |