

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50253952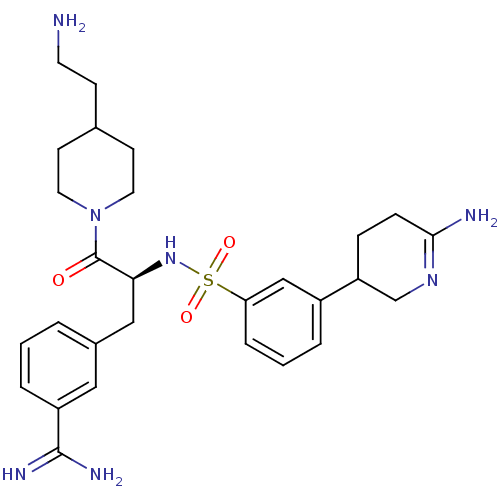 (3-{(S)-3-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-[3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108110 (US8598206, Table 6, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108117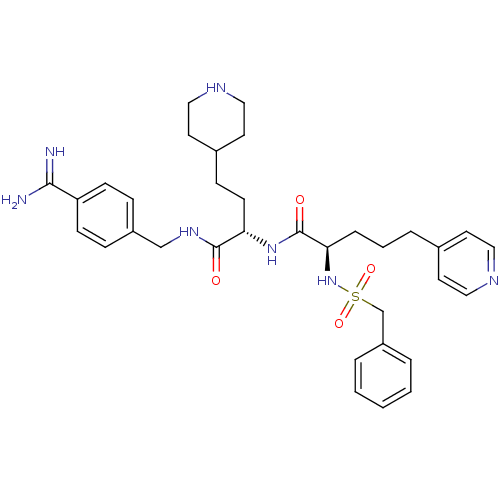 (US8598206, Table 6, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108109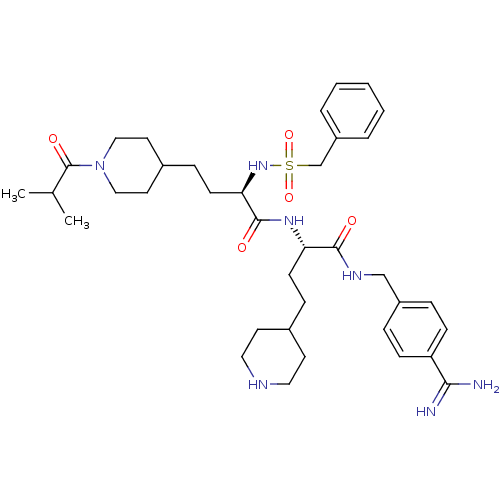 (US8598206, 118 | US8598206, 122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108106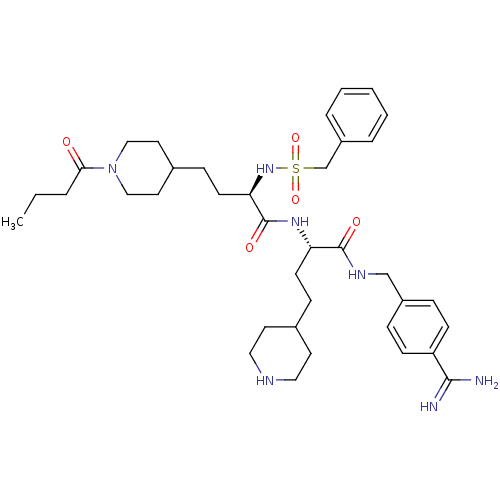 (US8598206, Table 6, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108109 (US8598206, 118 | US8598206, 122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108107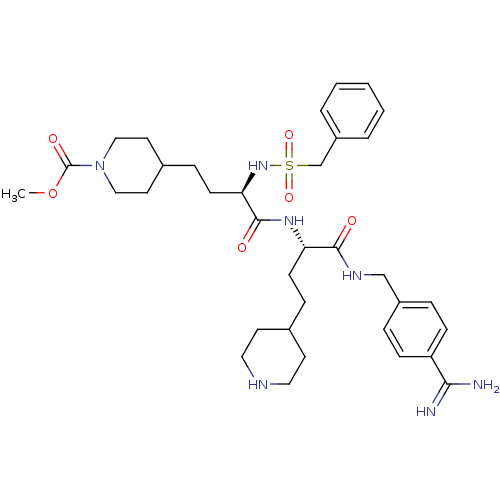 (US8598206, Table 6, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108099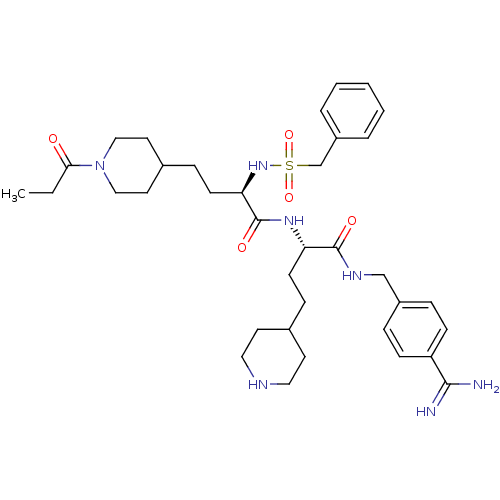 (US8598206, Table 6, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108104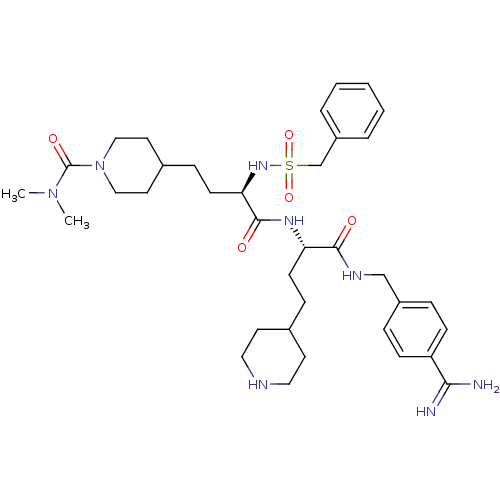 (US8598206, Table 6, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108099 (US8598206, Table 6, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM104908 (CHEMBL468270 | US8569313, Inhibitor 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108115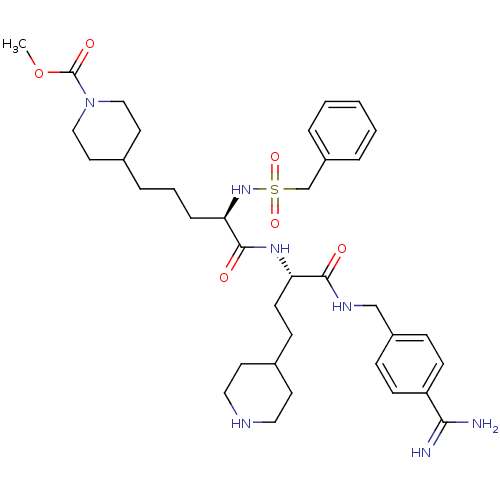 (US8598206, Table 6, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108108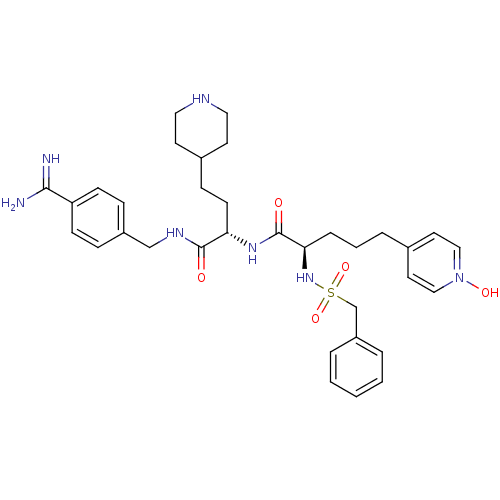 (US8598206, 117 | US8598206, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108105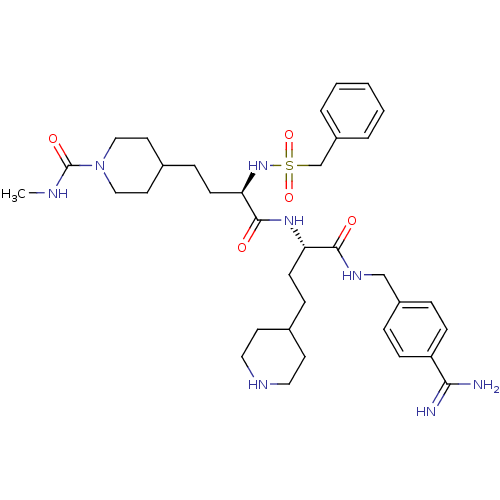 (US8598206, Table 6, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108108 (US8598206, 117 | US8598206, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108102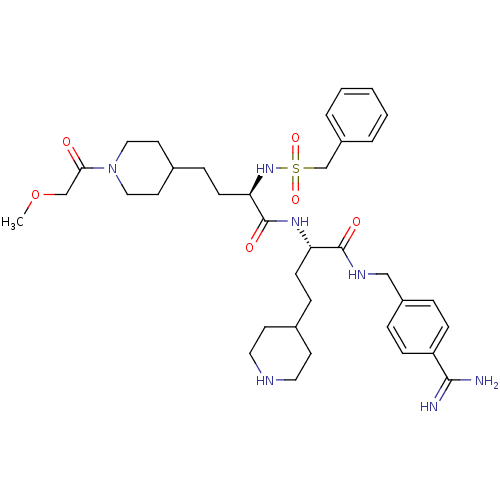 (US8598206, Table 6, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108102 (US8598206, Table 6, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108109 (US8598206, 118 | US8598206, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108110 (US8598206, Table 6, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM47178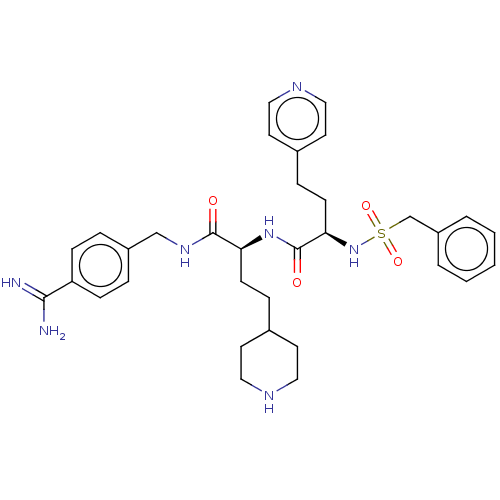 (BDBM108103 | US8598206, Table 6, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108115 (US8598206, Table 6, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108109 (US8598206, 118 | US8598206, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM47177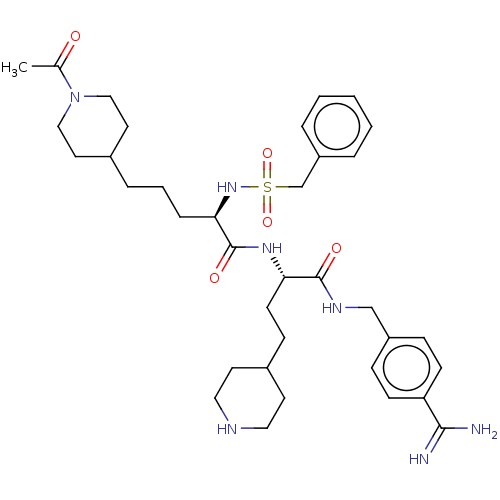 (BDBM108100 | US8598206, Table 6, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50254410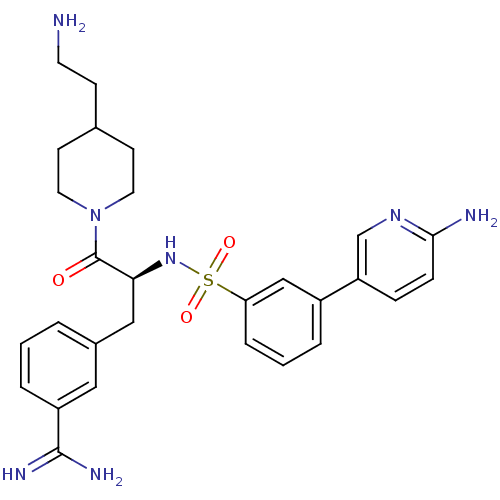 (3-{(S)-3-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-[3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108096 (US8598206, Table 6, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23916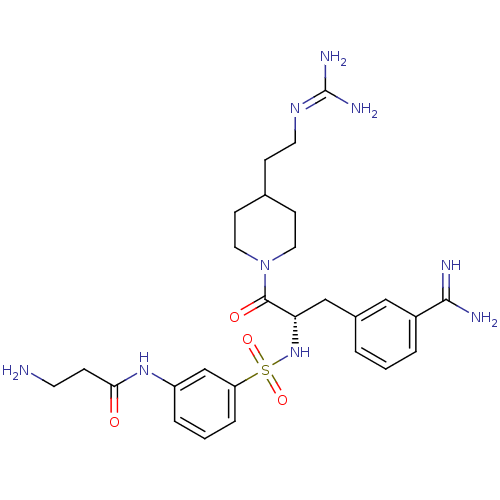 (3-amidinophenylalanine deriv., 12 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 1960-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.047 BindingDB Entry DOI: 10.7270/Q2J96681 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM47177 (BDBM108100 | US8598206, Table 6, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108105 (US8598206, Table 6, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108106 (US8598206, Table 6, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108097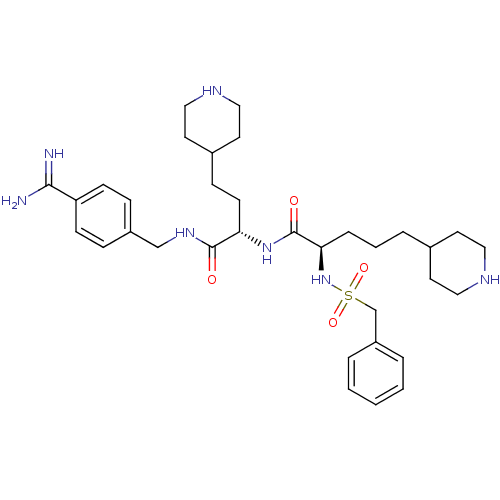 (US8598206, Table 6, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108107 (US8598206, Table 6, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108111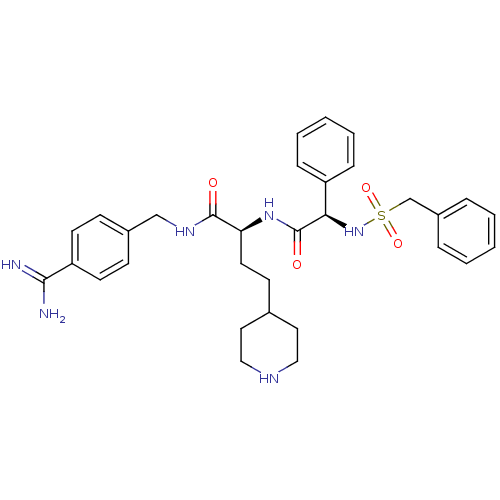 (US8598206, Table 6, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50563125 (CHEMBL4742132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50254368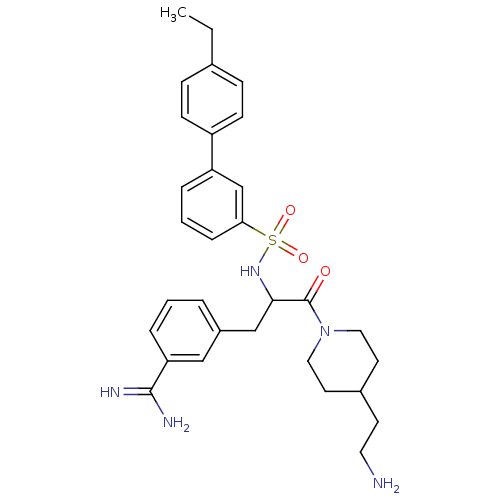 ((+/-)-3-[3-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108117 (US8598206, Table 6, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108116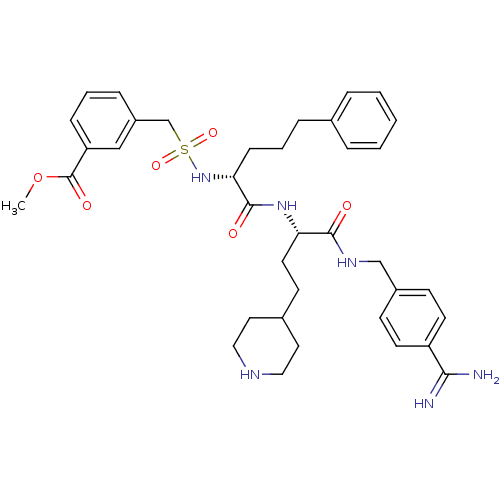 (US8598206, Table 6, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50563134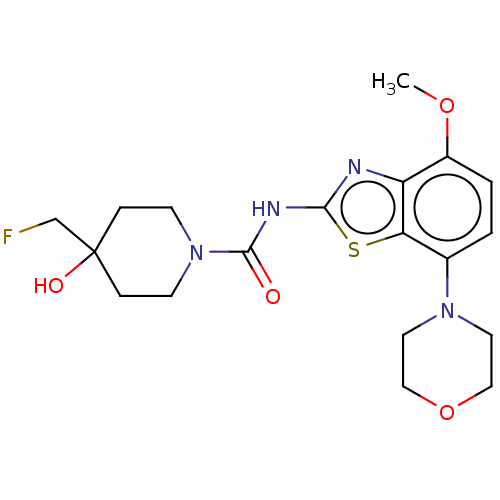 (CHEMBL4746388) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50254219 (Azetidine-3-carboxylic acid {3-[(S)-2-[4-(2-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108095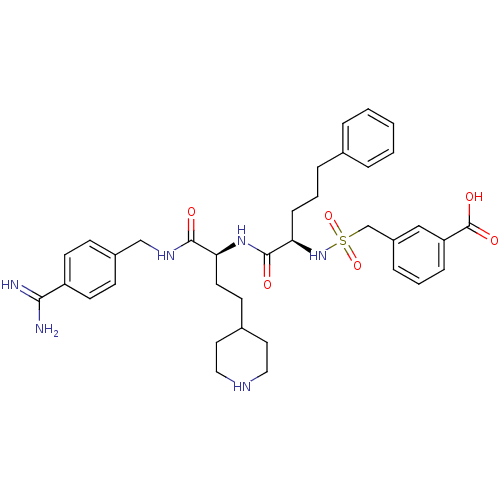 (US8598206, Table 6, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108101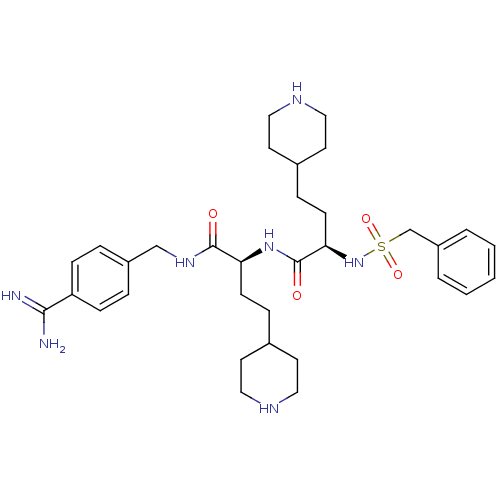 (US8598206, Table 6, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50563133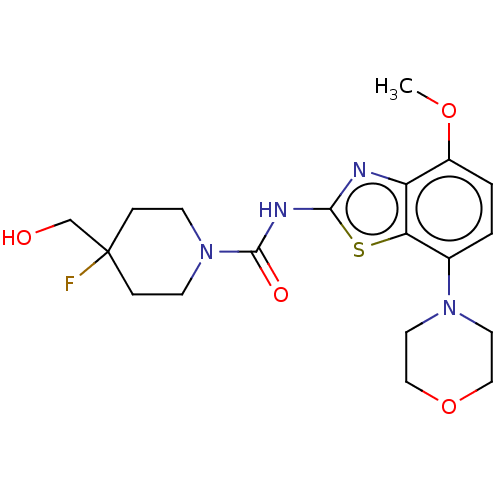 (CHEMBL4784681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50563130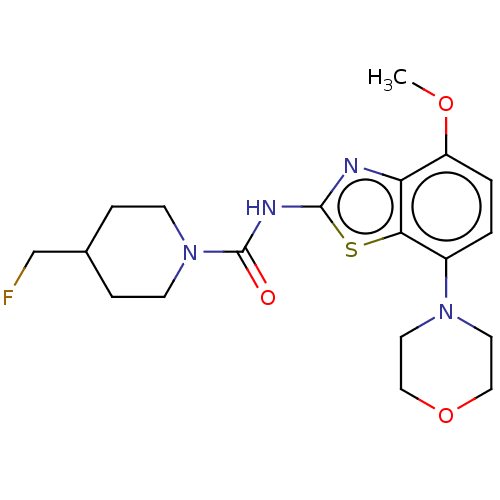 (CHEMBL4753657) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108095 (US8598206, Table 6, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23917 (3-amidinophenylalanine deriv., 59 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) | Bioorg Med Chem Lett 19: 67-73 (2008) Article DOI: 10.1016/j.bmcl.2008.11.019 BindingDB Entry DOI: 10.7270/Q2HH6JXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50563126 (CHEMBL4783617) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011294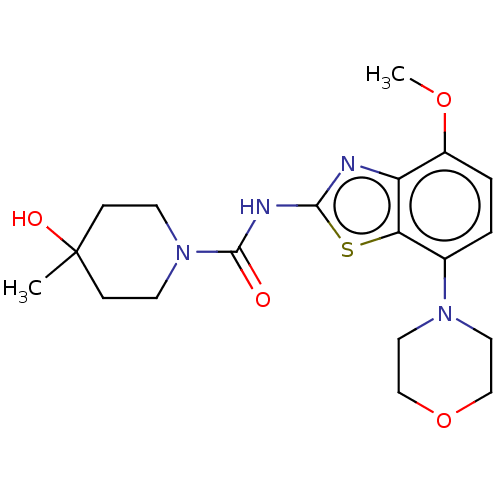 (A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-ZM241385 from recombinant human A2A receptor expressed in CHO-K1 cells incubated for 70 mins by liquid scintillation counting me... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113214 BindingDB Entry DOI: 10.7270/Q2B2801W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108108 (US8598206, 117 | US8598206, 123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108104 (US8598206, Table 6, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 527 total ) | Next | Last >> |