 Found 263 hits with Last Name = 'selvakumar' and Initial = 'k'
Found 263 hits with Last Name = 'selvakumar' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50509860
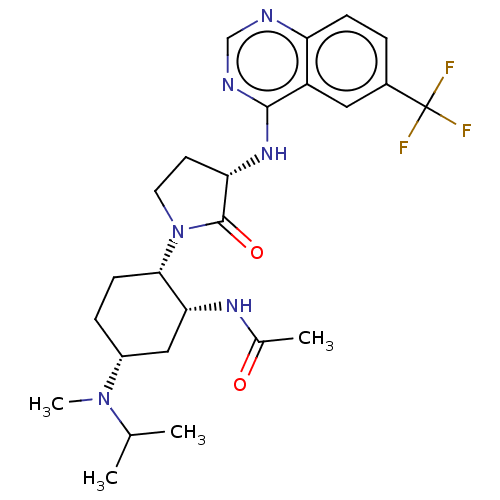
(CHEMBL4442783)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@@H](C1)NC(C)=O)N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H33F3N6O2/c1-14(2)33(4)17-6-8-22(21(12-17)31-15(3)35)34-10-9-20(24(34)36)32-23-18-11-16(25(26,27)28)5-7-19(18)29-13-30-23/h5,7,11,13-14,17,20-22H,6,8-10,12H2,1-4H3,(H,31,35)(H,29,30,32)/t17-,20+,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50557872
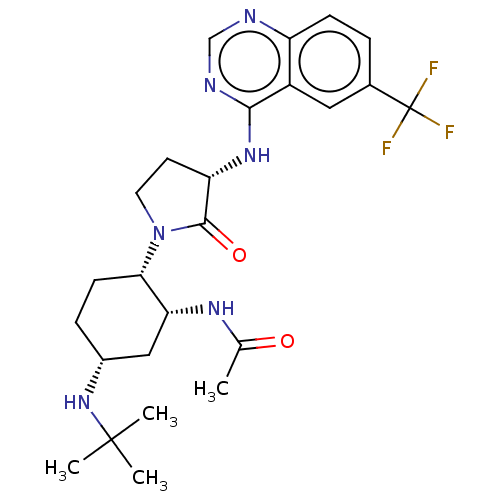
(CHEMBL4781426)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)NC(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50557872

(CHEMBL4781426)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)NC(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50557872

(CHEMBL4781426)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)NC(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 in human peripheral T cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100761

(US8507683, 12)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(cc(F)c1C)C(=O)NC1(COC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H23F3N4O4/c1-16-21(12-18(13-22(16)33)28(39)38-31(14-41-15-31)30-36-10-3-11-37-30)20-8-9-23-24(26(20)34)25(29(40)35-2)27(42-23)17-4-6-19(32)7-5-17/h3-13H,14-15H2,1-2H3,(H,35,40)(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-CCL2 from CCR2 in human PBMC cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100759

(US8507683, 10)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(COC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O4/c1-17-4-5-19(28(38)37-31(15-40-16-31)30-35-12-3-13-36-30)14-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(41-23)18-6-8-20(32)9-7-18/h3-14H,15-16H2,1-2H3,(H,34,39)(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100758
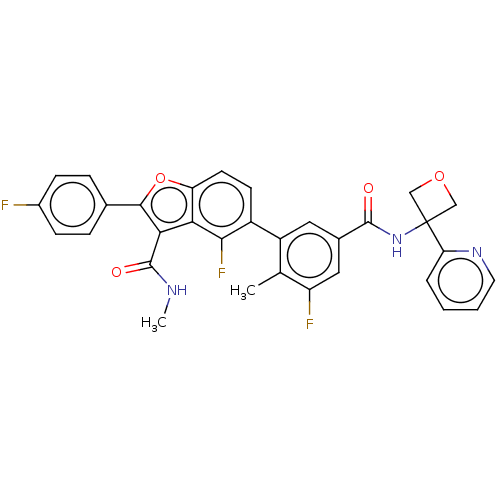
(US8507683, 9)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(cc(F)c1C)C(=O)NC1(COC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H24F3N3O4/c1-17-22(13-19(14-23(17)34)30(39)38-32(15-41-16-32)25-5-3-4-12-37-25)21-10-11-24-26(28(21)35)27(31(40)36-2)29(42-24)18-6-8-20(33)9-7-18/h3-14H,15-16H2,1-2H3,(H,36,40)(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100757
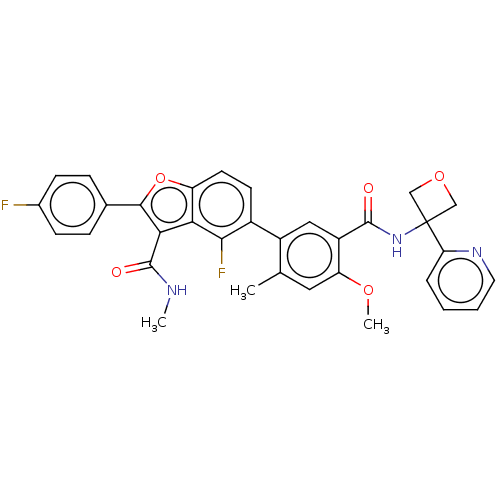
(US8507683, 8)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(C(=O)NC2(COC2)c2ccccn2)c(OC)cc1C)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27F2N3O5/c1-18-14-25(41-3)23(31(39)38-33(16-42-17-33)26-6-4-5-13-37-26)15-22(18)21-11-12-24-27(29(21)35)28(32(40)36-2)30(43-24)19-7-9-20(34)10-8-19/h4-15H,16-17H2,1-3H3,(H,36,40)(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100760

(US8507683, 11)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(C(=O)NC2(COC2)c2ncccn2)c(OC)cc1C)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26F2N4O5/c1-17-13-24(41-3)22(29(39)38-32(15-42-16-32)31-36-11-4-12-37-31)14-21(17)20-9-10-23-25(27(20)34)26(30(40)35-2)28(43-23)18-5-7-19(33)8-6-18/h4-14H,15-16H2,1-3H3,(H,35,40)(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100756

(US8507683, 7)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(COC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H25F2N3O4/c1-18-6-7-20(30(38)37-32(16-40-17-32)25-5-3-4-14-36-25)15-23(18)22-12-13-24-26(28(22)34)27(31(39)35-2)29(41-24)19-8-10-21(33)11-9-19/h3-15H,16-17H2,1-2H3,(H,35,39)(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50557872

(CHEMBL4781426)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(cc23)C(F)(F)F)C1=O)NC(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Mus musculus) | BDBM50578294

(Bms 813160 | Bms-813160)Show SMILES CC(=O)N[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3cc(nn23)C(C)(C)C)C1=O)NC(C)(C)C | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-CCL2 from mouse CCR2 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00373
BindingDB Entry DOI: 10.7270/Q2M04981 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM100755
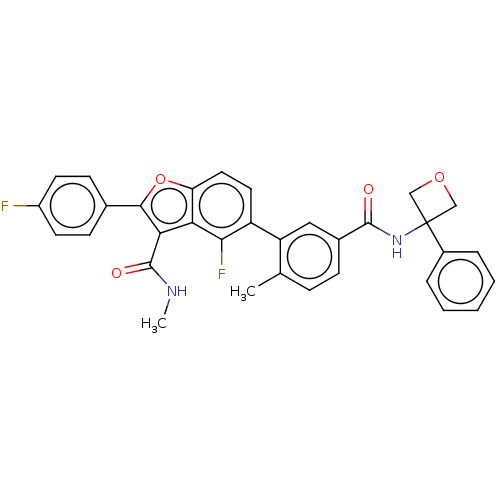
(US8507683, 6)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(COC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H26F2N2O4/c1-19-8-9-21(31(38)37-33(17-40-18-33)22-6-4-3-5-7-22)16-25(19)24-14-15-26-27(29(24)35)28(32(39)36-2)30(41-26)20-10-12-23(34)13-11-20/h3-16H,17-18H2,1-2H3,(H,36,39)(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
HCV NS5B enzyme assay. |
US Patent US8507683 (2013)
BindingDB Entry DOI: 10.7270/Q2C827XJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239253
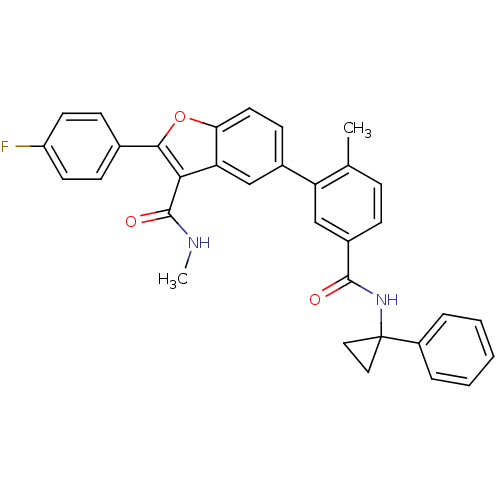
(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239253

(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239248
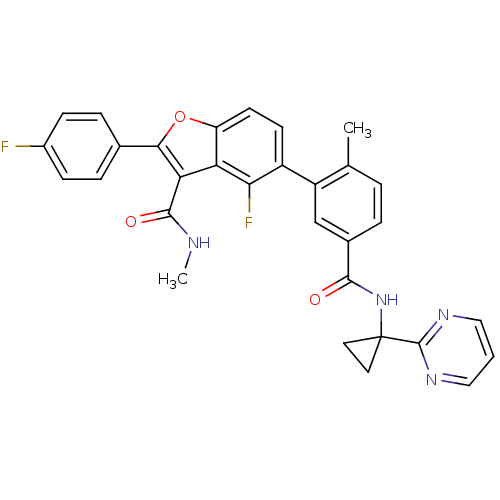
(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50509327
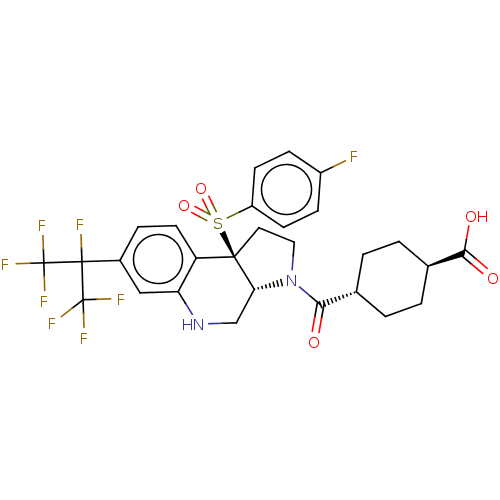
(CHEMBL4464711)Show SMILES [H][C@@]12CNc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(14.49,-6.66,;13.16,-7.44,;13.16,-5.88,;11.82,-5.1,;10.48,-5.88,;9.14,-5.12,;7.81,-5.89,;7.81,-7.43,;9.15,-8.2,;10.48,-7.44,;11.81,-8.21,;12.13,-9.73,;13.67,-9.9,;14.31,-8.49,;15.82,-8.17,;16.3,-6.71,;16.84,-9.32,;18.34,-9.01,;19.36,-10.15,;18.88,-11.62,;17.37,-11.93,;16.35,-10.79,;19.91,-12.77,;19.42,-14.24,;21.41,-12.46,;10.71,-9.29,;9.61,-10.38,;9.22,-8.89,;11.1,-10.78,;10,-11.86,;10.38,-13.35,;11.87,-13.76,;12.26,-15.25,;12.97,-12.67,;12.58,-11.19,;6.48,-5.12,;5.14,-4.35,;5.15,-5.89,;3.81,-5.12,;5.15,-7.43,;3.8,-6.65,;6.48,-3.58,;7.81,-2.81,;5.15,-2.81,;6.47,-2.04,)| Show InChI InChI=1S/C28H26F8N2O5S/c29-18-6-8-19(9-7-18)44(42,43)25-11-12-38(23(39)15-1-3-16(4-2-15)24(40)41)22(25)14-37-21-13-17(5-10-20(21)25)26(30,27(31,32)33)28(34,35)36/h5-10,13,15-16,22,37H,1-4,11-12,14H2,(H,40,41)/t15-,16-,22-,25-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM382173
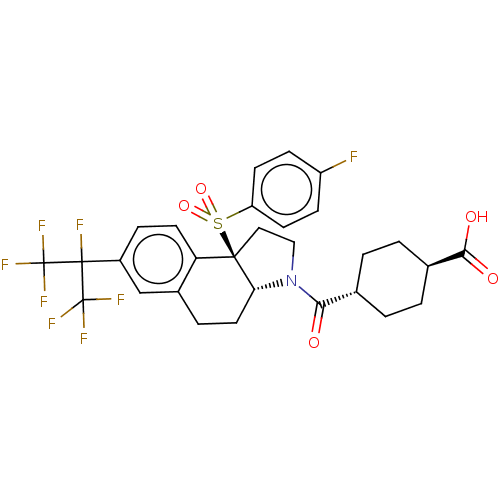
((1R,4r)-4-((3aR,9bR)-9b-((4-fluorophenyl)sulfonyl)...)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r,wU:15.16,3.2,wD:6.9,14.37,(-10.01,1.35,;-9.74,-.16,;-10.91,-1.16,;-8.29,-.68,;-7.11,.31,;-5.66,-.21,;-5.39,-1.73,;-6.57,-2.72,;-8.01,-2.2,;-3.94,-2.25,;-3.67,-3.76,;-2.76,-1.25,;-2.88,.28,;-1.45,.87,;-.46,-.31,;-1.27,-1.62,;-.54,-2.98,;1,-3.02,;1.81,-1.72,;3.35,-1.76,;4.16,-.46,;3.43,.9,;1.89,.95,;1.08,-.36,;5.7,-.5,;5.75,1.04,;7.24,-.55,;8.78,-.6,;7.19,-2.09,;7.29,.99,;5.65,-2.04,;4.29,-2.77,;6.96,-2.85,;5.6,-3.58,;.21,1.08,;1.74,1.24,;.84,2.48,;-.65,2.35,;-2.19,2.24,;-3.05,3.51,;-2.38,4.9,;-3.25,6.17,;-.85,5.01,;.02,3.74,)| Show InChI InChI=1S/C29H27F8NO5S/c30-20-7-9-21(10-8-20)44(42,43)26-13-14-38(24(39)16-1-3-17(4-2-16)25(40)41)23(26)12-5-18-15-19(6-11-22(18)26)27(31,28(32,33)34)29(35,36)37/h6-11,15-17,23H,1-5,12-14H2,(H,40,41)/t16-,17-,23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM382198
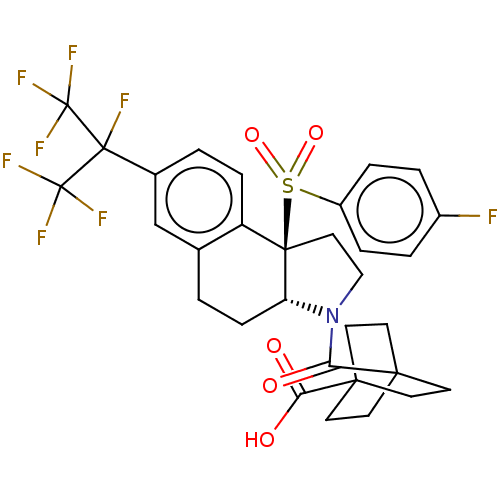
(US10273259, Example 12 | US10711020, Example 12)Show SMILES OC(=O)C12CCC(CC1)(CC2)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H29F8NO5S/c32-20-3-5-21(6-4-20)46(44,45)28-15-16-40(24(41)26-9-12-27(13-10-26,14-11-26)25(42)43)23(28)8-1-18-17-19(2-7-22(18)28)29(33,30(34,35)36)31(37,38)39/h2-7,17,23H,1,8-16H2,(H,42,43)/t23-,26?,27?,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50509330
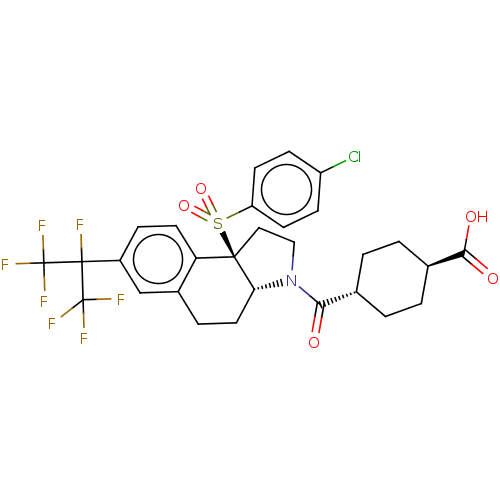
(CHEMBL4525527)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(Cl)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(39.12,-6.49,;37.79,-7.27,;37.79,-5.71,;36.45,-4.93,;35.11,-5.71,;33.77,-4.95,;32.44,-5.72,;32.44,-7.26,;33.78,-8.03,;35.11,-7.26,;36.44,-8.04,;36.76,-9.56,;38.3,-9.73,;38.94,-8.31,;40.45,-8,;40.93,-6.54,;41.47,-9.15,;42.97,-8.84,;43.99,-9.98,;43.51,-11.45,;42,-11.76,;40.98,-10.61,;44.54,-12.6,;44.05,-14.06,;46.04,-12.29,;35.34,-9.12,;34.24,-10.21,;33.85,-8.72,;35.73,-10.61,;34.63,-11.69,;35.01,-13.18,;36.5,-13.59,;36.89,-15.08,;37.6,-12.5,;37.21,-11.02,;31.11,-4.95,;29.77,-4.18,;29.78,-5.72,;28.44,-4.95,;29.78,-7.26,;28.43,-6.48,;31.11,-3.41,;32.44,-2.64,;29.78,-2.64,;31.1,-1.87,)| Show InChI InChI=1S/C29H27ClF7NO5S/c30-20-7-9-21(10-8-20)44(42,43)26-13-14-38(24(39)16-1-3-17(4-2-16)25(40)41)23(26)12-5-18-15-19(6-11-22(18)26)27(31,28(32,33)34)29(35,36)37/h6-11,15-17,23H,1-5,12-14H2,(H,40,41)/t16-,17-,23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM382198

(US10273259, Example 12 | US10711020, Example 12)Show SMILES OC(=O)C12CCC(CC1)(CC2)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H29F8NO5S/c32-20-3-5-21(6-4-20)46(44,45)28-15-16-40(24(41)26-9-12-27(13-10-26,14-11-26)25(42)43)23(28)8-1-18-17-19(2-7-22(18)28)29(33,30(34,35)36)31(37,38)39/h2-7,17,23H,1,8-16H2,(H,42,43)/t23-,26?,27?,28-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50509331
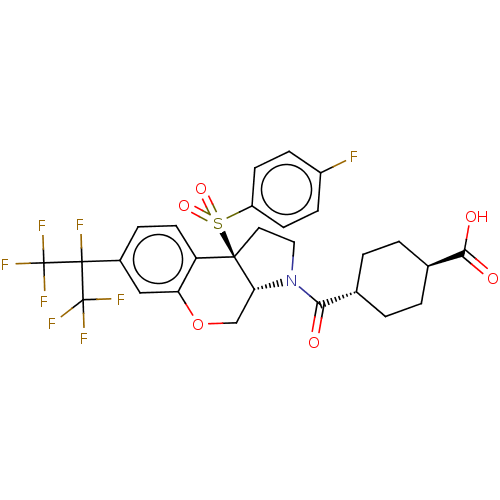
(CHEMBL4527625)Show SMILES [H][C@@]12COc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(38.73,-26.8,;37.4,-27.58,;37.4,-26.03,;36.05,-25.25,;34.71,-26.03,;33.38,-25.26,;32.05,-26.03,;32.05,-27.58,;33.38,-28.35,;34.71,-27.58,;36.05,-28.35,;36.36,-29.88,;37.91,-30.05,;38.55,-28.63,;40.06,-28.32,;40.54,-26.86,;41.08,-29.47,;42.57,-29.15,;43.6,-30.3,;43.12,-31.77,;41.61,-32.08,;40.58,-30.93,;44.14,-32.92,;43.66,-34.38,;45.65,-32.6,;34.95,-29.44,;33.85,-30.53,;33.45,-29.03,;35.33,-30.93,;34.23,-32.01,;34.62,-33.49,;36.11,-33.91,;36.49,-35.4,;37.21,-32.82,;36.81,-31.33,;30.72,-25.26,;29.38,-24.49,;29.38,-26.03,;28.05,-25.26,;29.38,-27.57,;28.04,-26.79,;30.72,-23.72,;32.05,-22.95,;29.38,-22.95,;30.71,-22.18,)| Show InChI InChI=1S/C28H25F8NO6S/c29-18-6-8-19(9-7-18)44(41,42)25-11-12-37(23(38)15-1-3-16(4-2-15)24(39)40)22(25)14-43-21-13-17(5-10-20(21)25)26(30,27(31,32)33)28(34,35)36/h5-10,13,15-16,22H,1-4,11-12,14H2,(H,39,40)/t15-,16-,22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50509327

(CHEMBL4464711)Show SMILES [H][C@@]12CNc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(14.49,-6.66,;13.16,-7.44,;13.16,-5.88,;11.82,-5.1,;10.48,-5.88,;9.14,-5.12,;7.81,-5.89,;7.81,-7.43,;9.15,-8.2,;10.48,-7.44,;11.81,-8.21,;12.13,-9.73,;13.67,-9.9,;14.31,-8.49,;15.82,-8.17,;16.3,-6.71,;16.84,-9.32,;18.34,-9.01,;19.36,-10.15,;18.88,-11.62,;17.37,-11.93,;16.35,-10.79,;19.91,-12.77,;19.42,-14.24,;21.41,-12.46,;10.71,-9.29,;9.61,-10.38,;9.22,-8.89,;11.1,-10.78,;10,-11.86,;10.38,-13.35,;11.87,-13.76,;12.26,-15.25,;12.97,-12.67,;12.58,-11.19,;6.48,-5.12,;5.14,-4.35,;5.15,-5.89,;3.81,-5.12,;5.15,-7.43,;3.8,-6.65,;6.48,-3.58,;7.81,-2.81,;5.15,-2.81,;6.47,-2.04,)| Show InChI InChI=1S/C28H26F8N2O5S/c29-18-6-8-19(9-7-18)44(42,43)25-11-12-38(23(39)15-1-3-16(4-2-15)24(40)41)22(25)14-37-21-13-17(5-10-20(21)25)26(30,27(31,32)33)28(34,35)36/h5-10,13,15-16,22,37H,1-4,11-12,14H2,(H,40,41)/t15-,16-,22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50509332
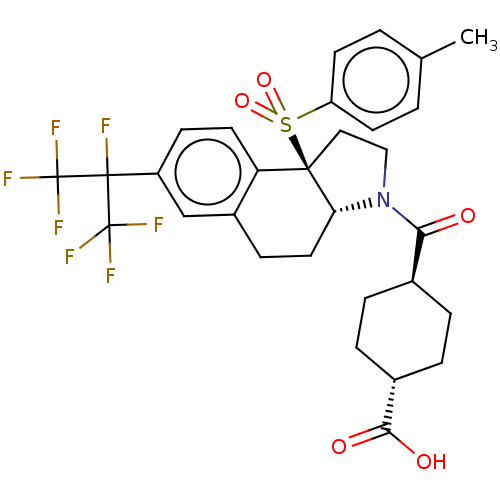
(CHEMBL4462790)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(C)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(64.23,-6.6,;62.9,-7.39,;62.9,-5.83,;61.56,-5.05,;60.21,-5.83,;58.88,-5.06,;57.55,-5.83,;57.55,-7.38,;58.88,-8.15,;60.21,-7.38,;61.55,-8.15,;61.86,-9.68,;63.41,-9.85,;64.05,-8.43,;65.56,-8.12,;66.04,-6.66,;66.58,-9.27,;68.07,-8.96,;69.1,-10.1,;68.62,-11.57,;67.11,-11.88,;66.09,-10.73,;69.64,-12.72,;69.16,-14.18,;71.15,-12.41,;60.45,-9.24,;59.35,-10.33,;58.96,-8.83,;60.83,-10.73,;59.74,-11.81,;60.12,-13.3,;61.61,-13.71,;62,-15.2,;62.71,-12.62,;62.32,-11.13,;56.22,-5.06,;54.88,-4.29,;54.88,-5.83,;53.55,-5.06,;54.88,-7.37,;53.54,-6.6,;56.22,-3.52,;57.55,-2.75,;54.88,-2.75,;56.21,-1.98,)| Show InChI InChI=1S/C30H30F7NO5S/c1-17-2-10-22(11-3-17)44(42,43)27-14-15-38(25(39)18-4-6-19(7-5-18)26(40)41)24(27)13-8-20-16-21(9-12-23(20)27)28(31,29(32,33)34)30(35,36)37/h2-3,9-12,16,18-19,24H,4-8,13-15H2,1H3,(H,40,41)/t18-,19-,24-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM382181
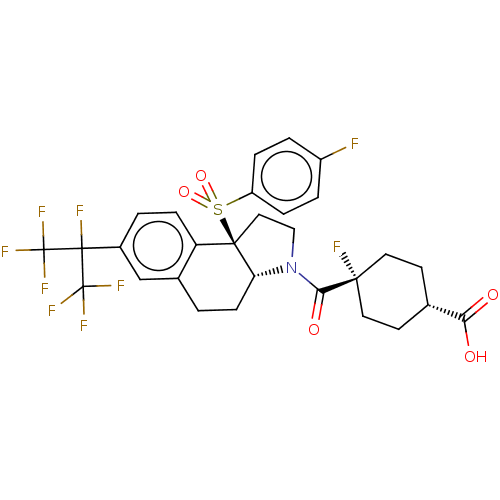
((1S,4s)-4-fluoro-4-((3aR,9bR)-9b-((4-fluorophenyl)...)Show SMILES OC(=O)[C@H]1CC[C@@](F)(CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r,wU:16.17,3.2,6.6,wD:6.10,15.38,(-6.98,10.68,;-7.74,9.34,;-9.28,9.33,;-6.97,8.01,;-5.43,8.01,;-4.65,6.68,;-5.42,5.35,;-5.68,3.83,;-6.96,5.34,;-7.73,6.67,;-3.97,4.83,;-3.7,3.31,;-2.79,5.82,;-2.91,7.36,;-1.48,7.94,;-.49,6.76,;-1.3,5.45,;-.57,4.1,;.97,4.05,;1.78,5.36,;3.32,5.31,;4.13,6.62,;3.4,7.97,;1.86,8.02,;1.05,6.71,;5.67,6.57,;5.72,8.11,;7.21,6.52,;8.75,6.47,;7.16,4.98,;7.26,8.06,;5.62,5.03,;4.27,4.3,;6.93,4.22,;5.58,3.49,;.18,8.15,;1.65,8.62,;.66,9.61,;-.68,9.42,;-2.22,9.31,;-3.08,10.58,;-2.41,11.97,;-3.28,13.24,;-.88,12.08,;-.01,10.81,)| Show InChI InChI=1S/C29H26F9NO5S/c30-19-3-5-20(6-4-19)45(43,44)26-13-14-39(24(42)25(31)11-9-16(10-12-25)23(40)41)22(26)8-1-17-15-18(2-7-21(17)26)27(32,28(33,34)35)29(36,37)38/h2-7,15-16,22H,1,8-14H2,(H,40,41)/t16-,22-,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at LXRbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM382356

(US10273259, Example 69 | US10711020, Example 69)Show SMILES OC1(CCS(=O)(=O)CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H25F8NO6S2/c28-18-3-5-19(6-4-18)44(41,42)24-9-12-36(22(37)23(38)10-13-43(39,40)14-11-23)21(24)8-1-16-15-17(2-7-20(16)24)25(29,26(30,31)32)27(33,34)35/h2-7,15,21,38H,1,8-14H2/t21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C9 (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM382356

(US10273259, Example 69 | US10711020, Example 69)Show SMILES OC1(CCS(=O)(=O)CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H25F8NO6S2/c28-18-3-5-19(6-4-18)44(41,42)24-9-12-36(22(37)23(38)10-13-43(39,40)14-11-23)21(24)8-1-16-15-17(2-7-20(16)24)25(29,26(30,31)32)27(33,34)35/h2-7,15,21,38H,1,8-14H2/t21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50509328
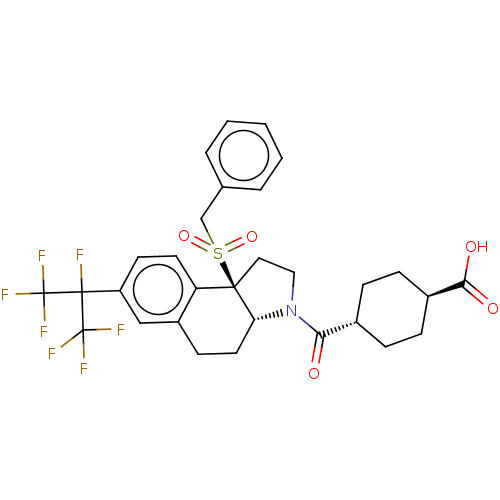
(CHEMBL4452650)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)Cc1ccccc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(38.9,-47.28,;37.57,-48.06,;37.57,-46.51,;36.22,-45.72,;34.88,-46.5,;33.55,-45.74,;32.22,-46.51,;32.22,-48.05,;33.55,-48.82,;34.88,-48.06,;36.22,-48.83,;36.53,-50.35,;38.08,-50.52,;38.72,-49.11,;40.23,-48.8,;40.71,-47.34,;41.25,-49.95,;42.74,-49.63,;43.77,-50.78,;43.29,-52.25,;41.78,-52.56,;40.75,-51.41,;44.31,-53.4,;43.83,-54.86,;45.82,-53.08,;35.12,-49.92,;34.02,-51.01,;33.62,-49.51,;35.5,-51.41,;34.4,-52.49,;32.93,-52.07,;31.83,-53.15,;32.21,-54.64,;33.71,-55.05,;34.8,-53.97,;30.89,-45.74,;29.55,-44.97,;29.55,-46.51,;28.22,-45.74,;29.55,-48.05,;28.21,-47.27,;30.89,-44.2,;32.22,-43.43,;29.55,-43.43,;30.88,-42.66,)| Show InChI InChI=1S/C30H30F7NO5S/c31-28(29(32,33)34,30(35,36)37)22-11-12-23-21(16-22)10-13-24-27(23,44(42,43)17-18-4-2-1-3-5-18)14-15-38(24)25(39)19-6-8-20(9-7-19)26(40)41/h1-5,11-12,16,19-20,24H,6-10,13-15,17H2,(H,40,41)/t19-,20-,24-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50509331

(CHEMBL4527625)Show SMILES [H][C@@]12COc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(38.73,-26.8,;37.4,-27.58,;37.4,-26.03,;36.05,-25.25,;34.71,-26.03,;33.38,-25.26,;32.05,-26.03,;32.05,-27.58,;33.38,-28.35,;34.71,-27.58,;36.05,-28.35,;36.36,-29.88,;37.91,-30.05,;38.55,-28.63,;40.06,-28.32,;40.54,-26.86,;41.08,-29.47,;42.57,-29.15,;43.6,-30.3,;43.12,-31.77,;41.61,-32.08,;40.58,-30.93,;44.14,-32.92,;43.66,-34.38,;45.65,-32.6,;34.95,-29.44,;33.85,-30.53,;33.45,-29.03,;35.33,-30.93,;34.23,-32.01,;34.62,-33.49,;36.11,-33.91,;36.49,-35.4,;37.21,-32.82,;36.81,-31.33,;30.72,-25.26,;29.38,-24.49,;29.38,-26.03,;28.05,-25.26,;29.38,-27.57,;28.04,-26.79,;30.72,-23.72,;32.05,-22.95,;29.38,-22.95,;30.71,-22.18,)| Show InChI InChI=1S/C28H25F8NO6S/c29-18-6-8-19(9-7-18)44(41,42)25-11-12-37(23(38)15-1-3-16(4-2-15)24(39)40)22(25)14-43-21-13-17(5-10-20(21)25)26(30,27(31,32)33)28(34,35)36/h5-10,13,15-16,22H,1-4,11-12,14H2,(H,39,40)/t15-,16-,22-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50509329

(CHEMBL4578712)Show SMILES [H][C@@]12COc3cc(ccc3[C@@]1(CCN2C(=O)C1(O)CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H23F8NO7S2/c27-16-2-4-17(5-3-16)44(40,41)23-7-10-35(21(36)22(37)8-11-43(38,39)12-9-22)20(23)14-42-19-13-15(1-6-18(19)23)24(28,25(29,30)31)26(32,33)34/h1-6,13,20,37H,7-12,14H2/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50509334

(CHEMBL4436417)Show SMILES [H][C@@]12CNc3cc(ccc3[C@@]1(CCN2C(=O)C1(O)CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H24F8N2O6S2/c27-16-2-4-17(5-3-16)44(41,42)23-7-10-36(21(37)22(38)8-11-43(39,40)12-9-22)20(23)14-35-19-13-15(1-6-18(19)23)24(28,25(29,30)31)26(32,33)34/h1-6,13,20,35,38H,7-12,14H2/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORalpha (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM382173

((1R,4r)-4-((3aR,9bR)-9b-((4-fluorophenyl)sulfonyl)...)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r,wU:15.16,3.2,wD:6.9,14.37,(-10.01,1.35,;-9.74,-.16,;-10.91,-1.16,;-8.29,-.68,;-7.11,.31,;-5.66,-.21,;-5.39,-1.73,;-6.57,-2.72,;-8.01,-2.2,;-3.94,-2.25,;-3.67,-3.76,;-2.76,-1.25,;-2.88,.28,;-1.45,.87,;-.46,-.31,;-1.27,-1.62,;-.54,-2.98,;1,-3.02,;1.81,-1.72,;3.35,-1.76,;4.16,-.46,;3.43,.9,;1.89,.95,;1.08,-.36,;5.7,-.5,;5.75,1.04,;7.24,-.55,;8.78,-.6,;7.19,-2.09,;7.29,.99,;5.65,-2.04,;4.29,-2.77,;6.96,-2.85,;5.6,-3.58,;.21,1.08,;1.74,1.24,;.84,2.48,;-.65,2.35,;-2.19,2.24,;-3.05,3.51,;-2.38,4.9,;-3.25,6.17,;-.85,5.01,;.02,3.74,)| Show InChI InChI=1S/C29H27F8NO5S/c30-20-7-9-21(10-8-20)44(42,43)26-13-14-38(24(39)16-1-3-17(4-2-16)25(40)41)23(26)12-5-18-15-19(6-11-22(18)26)27(31,28(32,33)34)29(35,36)37/h6-11,15-17,23H,1-5,12-14H2,(H,40,41)/t16-,17-,23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50509337
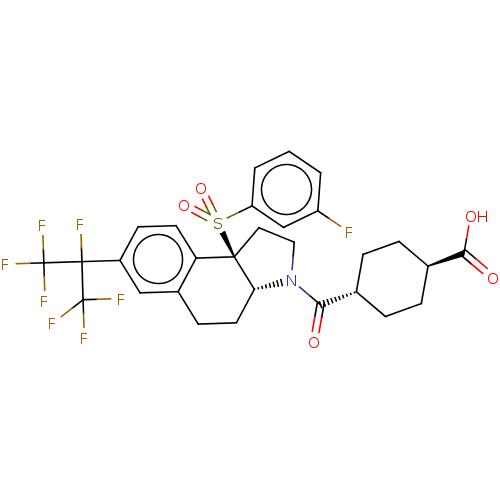
(CHEMBL4555822)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1cccc(F)c1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(15.64,-25.66,;14.31,-26.44,;14.32,-24.88,;12.97,-24.1,;11.63,-24.88,;10.29,-24.12,;8.96,-24.89,;8.96,-26.43,;10.3,-27.2,;11.63,-26.44,;12.96,-27.21,;13.28,-28.73,;14.82,-28.9,;15.46,-27.49,;16.97,-27.17,;17.45,-25.71,;17.99,-28.33,;19.49,-28.01,;20.51,-29.15,;20.03,-30.62,;18.52,-30.94,;17.5,-29.79,;21.06,-31.77,;20.57,-33.24,;22.56,-31.46,;11.86,-28.29,;10.76,-29.38,;10.37,-27.89,;12.25,-29.78,;11.15,-30.86,;11.54,-32.35,;13.02,-32.76,;14.12,-31.67,;15.61,-32.08,;13.73,-30.19,;7.63,-24.12,;6.29,-23.35,;6.3,-24.89,;4.96,-24.12,;6.3,-26.43,;4.95,-25.65,;7.63,-22.58,;8.96,-21.81,;6.3,-21.81,;7.62,-21.04,)| Show InChI InChI=1S/C29H27F8NO5S/c30-20-2-1-3-21(15-20)44(42,43)26-12-13-38(24(39)16-4-6-17(7-5-16)25(40)41)23(26)11-8-18-14-19(9-10-22(18)26)27(31,28(32,33)34)29(35,36)37/h1-3,9-10,14-17,23H,4-8,11-13H2,(H,40,41)/t16-,17-,23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50509336
(CHEMBL4458283)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CCN2C(=O)[C@H]1CC[C@@H](CC1)C(O)=O)S(=O)(=O)c1cccc(C)c1)C(F)(C(F)(F)F)C(F)(F)F |r,wU:16.18,wD:19.25,1.0,10.28,(65.64,-25.66,;64.32,-26.44,;64.32,-24.89,;62.97,-24.1,;61.63,-24.88,;60.3,-24.12,;58.97,-24.89,;58.97,-26.43,;60.3,-27.2,;61.63,-26.44,;62.97,-27.21,;63.28,-28.73,;64.83,-28.9,;65.47,-27.49,;66.97,-27.18,;67.46,-25.71,;68,-28.33,;69.49,-28.01,;70.52,-29.15,;70.04,-30.63,;68.53,-30.94,;67.5,-29.79,;71.06,-31.77,;70.58,-33.24,;72.57,-31.46,;61.86,-28.3,;60.77,-29.38,;60.37,-27.89,;62.25,-29.79,;61.15,-30.86,;61.54,-32.35,;63.02,-32.76,;64.12,-31.67,;65.61,-32.08,;63.73,-30.19,;57.63,-24.12,;56.29,-23.35,;56.3,-24.89,;54.97,-24.12,;56.3,-26.43,;54.96,-25.65,;57.63,-22.58,;58.97,-21.81,;56.3,-21.81,;57.62,-21.04,)| Show InChI InChI=1S/C30H30F7NO5S/c1-17-3-2-4-22(15-17)44(42,43)27-13-14-38(25(39)18-5-7-19(8-6-18)26(40)41)24(27)12-9-20-16-21(10-11-23(20)27)28(31,29(32,33)34)30(35,36)37/h2-4,10-11,15-16,18-19,24H,5-9,12-14H2,1H3,(H,40,41)/t18-,19-,24-,27-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM382280

(US10273259, Example 49 | US10711020, Example 49)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 |r,wU:15.16,3.2,wD:6.9,14.37,(-10.97,-1.49,;-9.8,-.49,;-10.07,1.02,;-8.35,-1.01,;-7.17,-.02,;-5.72,-.54,;-5.45,-2.06,;-6.63,-3.05,;-8.07,-2.53,;-4,-2.58,;-3.73,-4.09,;-2.82,-1.58,;-2.94,-.05,;-1.51,.53,;-.52,-.64,;-1.33,-1.95,;-.6,-3.31,;.94,-3.36,;1.75,-2.05,;3.29,-2.09,;4.1,-.79,;3.37,.57,;1.83,.62,;1.02,-.69,;5.64,-.83,;5.69,.71,;7.18,-.88,;8.72,-.93,;7.13,-2.42,;7.23,.66,;5.59,-2.37,;4.23,-3.1,;6.9,-3.18,;5.54,-3.91,;.15,.74,;1.66,1.07,;.31,2.28,;-.71,2.02,;-2.25,1.91,;-3.11,3.18,;-2.44,4.57,;-.91,4.68,;-.04,3.41,)| Show InChI InChI=1S/C29H28F7NO5S/c30-27(28(31,32)33,29(34,35)36)20-11-12-22-19(16-20)10-13-23-26(22,43(41,42)21-4-2-1-3-5-21)14-15-37(23)24(38)17-6-8-18(9-7-17)25(39)40/h1-5,11-12,16-18,23H,6-10,13-15H2,(H,39,40)/t17-,18-,23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Activity at RORbeta (unknown origin) |
J Med Chem 62: 9931-9946 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01369
BindingDB Entry DOI: 10.7270/Q2G44TKD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data