 Found 69 hits with Last Name = 'sherrill' and Initial = 'j'
Found 69 hits with Last Name = 'sherrill' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
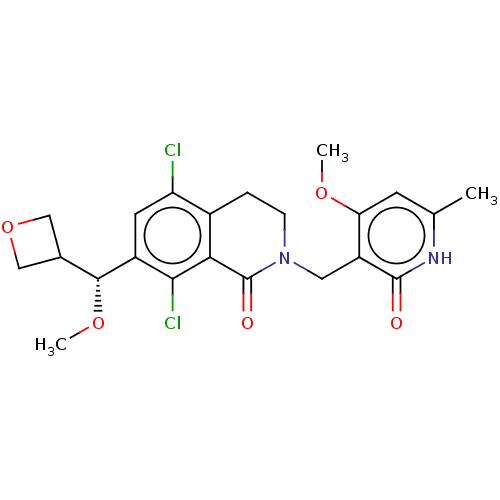
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibitory constant against human adenosne A3 receptor |
J Med Chem 50: 65-73 (2007)
Article DOI: 10.1021/jm061045z
BindingDB Entry DOI: 10.7270/Q2TD9Z40 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibitory constant aganist human adenosine A1 receptor |
J Med Chem 50: 65-73 (2007)
Article DOI: 10.1021/jm061045z
BindingDB Entry DOI: 10.7270/Q2TD9Z40 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50246967

(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH1 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50202002
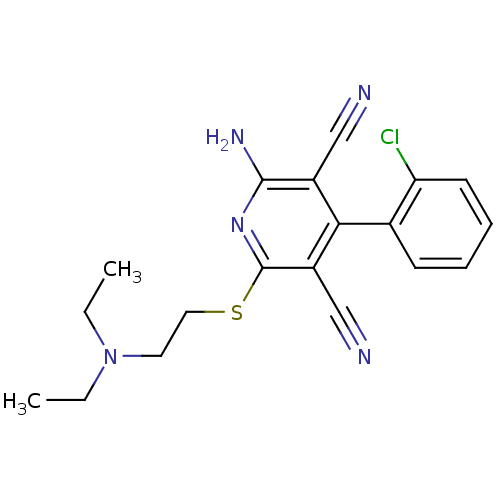
(2-amino-4-(2-chlorophenyl)-6-(2-(diethylamino)ethy...)Show SMILES CCN(CC)CCSc1nc(N)c(C#N)c(-c2ccccc2Cl)c1C#N |(30.4,-.59,;29.07,-1.36,;29.07,-2.9,;30.41,-3.67,;31.74,-2.89,;27.74,-3.68,;26.41,-2.92,;26.41,-1.38,;25.07,-.61,;23.74,-1.38,;22.4,-.61,;21.07,-1.38,;22.41,.93,;21.07,1.7,;19.74,2.48,;23.73,1.7,;23.73,3.24,;22.39,4,;22.38,5.54,;23.72,6.32,;25.05,5.55,;25.06,4.01,;26.39,3.24,;25.07,.94,;26.4,1.72,;27.73,2.49,)| Show InChI InChI=1S/C19H20ClN5S/c1-3-25(4-2)9-10-26-19-15(12-22)17(14(11-21)18(23)24-19)13-7-5-6-8-16(13)20/h5-8H,3-4,9-10H2,1-2H3,(H2,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibitory constant aganist human adenosine A1 receptor |
J Med Chem 50: 65-73 (2007)
Article DOI: 10.1021/jm061045z
BindingDB Entry DOI: 10.7270/Q2TD9Z40 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibitory constant against human adenosine A2a receptor |
J Med Chem 50: 65-73 (2007)
Article DOI: 10.1021/jm061045z
BindingDB Entry DOI: 10.7270/Q2TD9Z40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967

(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246938
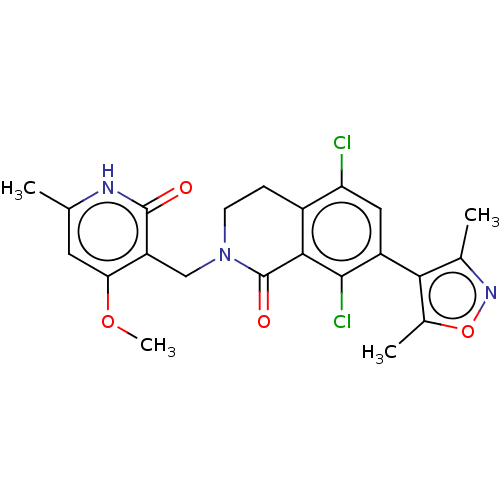
(CHEMBL4082565)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(36.87,-43.31,;36.88,-41.77,;38.22,-41.01,;39.54,-41.78,;40.88,-41.02,;42.21,-41.8,;40.89,-39.48,;39.56,-38.7,;39.56,-37.16,;38.23,-39.47,;36.9,-38.69,;35.57,-39.46,;35.57,-41,;34.24,-41.76,;32.91,-41,;31.59,-41.76,;31.59,-43.3,;30.26,-41,;30.26,-39.46,;28.91,-38.69,;27.51,-39.32,;27.19,-40.82,;26.48,-38.17,;27.25,-36.84,;28.75,-37.16,;29.9,-36.13,;31.59,-38.69,;31.59,-37.15,;32.91,-39.46,;34.24,-38.68,;34.24,-37.14,)| Show InChI InChI=1S/C22H21Cl2N3O4/c1-10-7-17(30-4)15(21(28)25-10)9-27-6-5-13-16(23)8-14(20(24)19(13)22(27)29)18-11(2)26-31-12(18)3/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246962
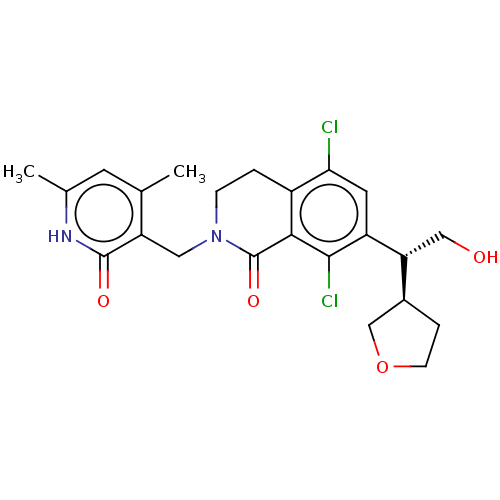
(CHEMBL4080043 | US10570121, Example 1)Show SMILES [H][C@@]1(CCOC1)[C@@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-7-13(2)26-22(29)17(12)9-27-5-3-15-19(24)8-16(21(25)20(15)23(27)30)18(10-28)14-4-6-31-11-14/h7-8,14,18,28H,3-6,9-11H2,1-2H3,(H,26,29)/t14-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246927
(CHEMBL4060447 | US10570121, Example 127)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-8-18(30-2)16(22(28)26-12)10-27-6-4-14-17(24)9-15(20(25)19(14)23(27)29)21(31-3)13-5-7-32-11-13/h8-9,13,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246968
(CHEMBL4093757 | US10570121, Example 13)Show SMILES [H][C@@]1(CCCO1)[C@@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(29)16(12)10-27-6-5-14-18(24)9-15(21(25)20(14)23(27)30)17(11-28)19-4-3-7-31-19/h8-9,17,19,28H,3-7,10-11H2,1-2H3,(H,26,29)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246952
(CHEMBL4073054 | US10570121, Example 12)Show SMILES [H][C@]1(CCCO1)[C@@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(29)16(12)10-27-6-5-14-18(24)9-15(21(25)20(14)23(27)30)17(11-28)19-4-3-7-31-19/h8-9,17,19,28H,3-7,10-11H2,1-2H3,(H,26,29)/t17-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246942
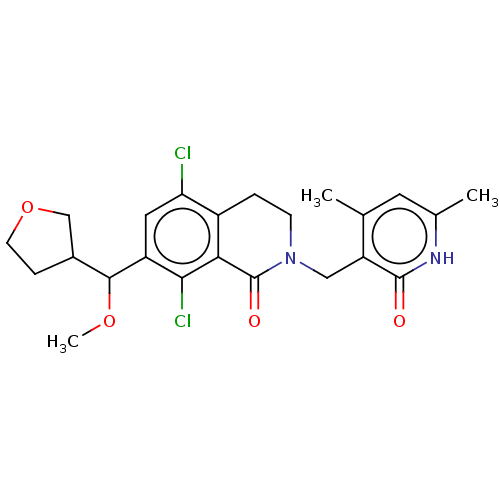
(CHEMBL4061264 | US10570121, Example 163)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(28)17(12)10-27-6-4-15-18(24)9-16(20(25)19(15)23(27)29)21(30-3)14-5-7-31-11-14/h8-9,14,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246966
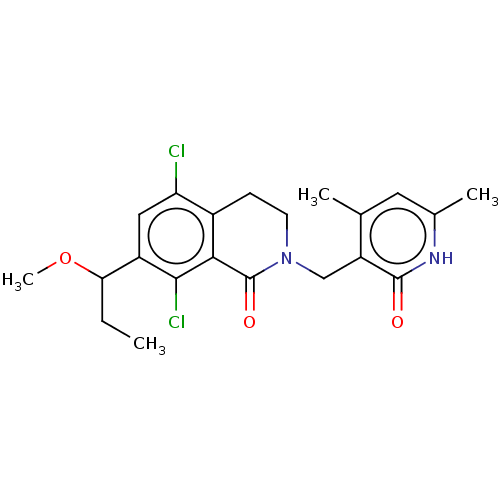
(CHEMBL4061098 | US10570121, Example 16)Show SMILES CCC(OC)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H24Cl2N2O3/c1-5-17(28-4)14-9-16(22)13-6-7-25(21(27)18(13)19(14)23)10-15-11(2)8-12(3)24-20(15)26/h8-9,17H,5-7,10H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246966

(CHEMBL4061098 | US10570121, Example 16)Show SMILES CCC(OC)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H24Cl2N2O3/c1-5-17(28-4)14-9-16(22)13-6-7-25(21(27)18(13)19(14)23)10-15-11(2)8-12(3)24-20(15)26/h8-9,17H,5-7,10H2,1-4H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246926
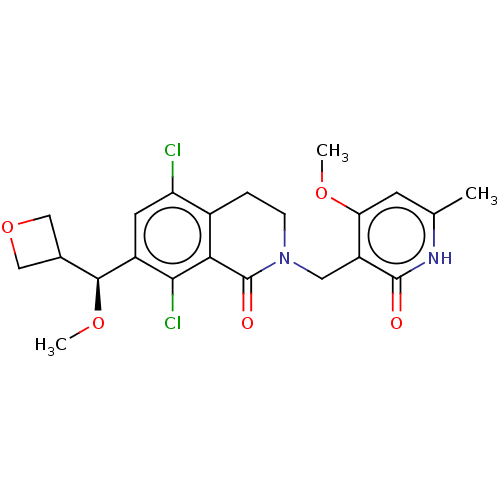
(CHEMBL4062204 | US10570121, Example 82)Show SMILES CO[C@@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246970
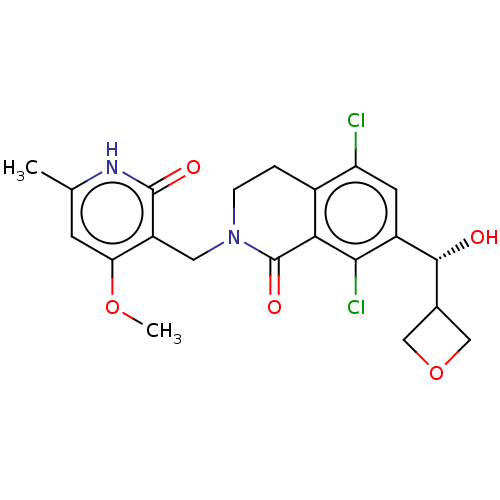
(CHEMBL4060378)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCc2c(Cl)cc([C@H](O)C3COC3)c(Cl)c2C1=O |r| Show InChI InChI=1S/C21H22Cl2N2O5/c1-10-5-16(29-2)14(20(27)24-10)7-25-4-3-12-15(22)6-13(18(23)17(12)21(25)28)19(26)11-8-30-9-11/h5-6,11,19,26H,3-4,7-9H2,1-2H3,(H,24,27)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246927

(CHEMBL4060447 | US10570121, Example 127)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-8-18(30-2)16(22(28)26-12)10-27-6-4-14-17(24)9-15(20(25)19(14)23(27)29)21(31-3)13-5-7-32-11-13/h8-9,13,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246964
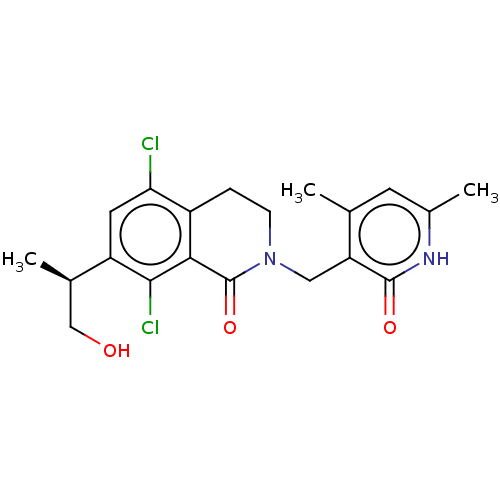
(CHEMBL4060640 | US10570121, Example 18)Show SMILES C[C@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C20H22Cl2N2O3/c1-10-6-12(3)23-19(26)15(10)8-24-5-4-13-16(21)7-14(11(2)9-25)18(22)17(13)20(24)27/h6-7,11,25H,4-5,8-9H2,1-3H3,(H,23,26)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246932
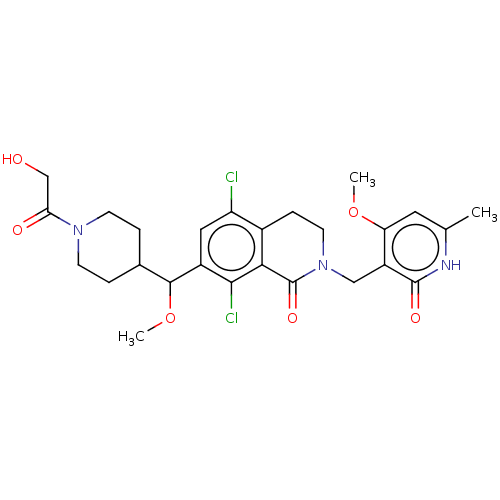
(CHEMBL4073864 | US10570121, Example 112)Show SMILES COC(C1CCN(CC1)C(=O)CO)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O6/c1-14-10-20(36-2)18(25(34)29-14)12-31-9-6-16-19(27)11-17(23(28)22(16)26(31)35)24(37-3)15-4-7-30(8-5-15)21(33)13-32/h10-11,15,24,32H,4-9,12-13H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246932

(CHEMBL4073864 | US10570121, Example 112)Show SMILES COC(C1CCN(CC1)C(=O)CO)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O6/c1-14-10-20(36-2)18(25(34)29-14)12-31-9-6-16-19(27)11-17(23(28)22(16)26(31)35)24(37-3)15-4-7-30(8-5-15)21(33)13-32/h10-11,15,24,32H,4-9,12-13H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246921
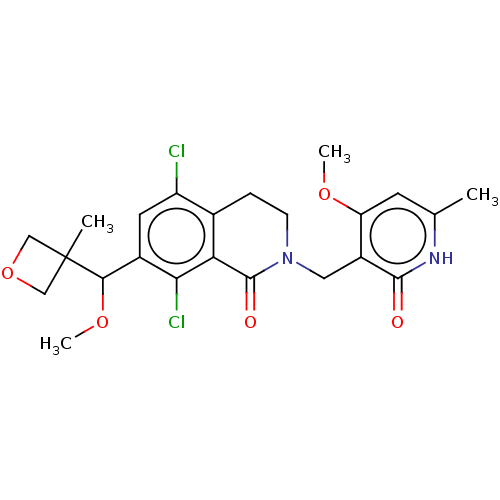
(CHEMBL4077043)Show SMILES COC(c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl)C1(C)COC1 Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-7-17(30-3)15(21(28)26-12)9-27-6-5-13-16(24)8-14(19(25)18(13)22(27)29)20(31-4)23(2)10-32-11-23/h7-8,20H,5-6,9-11H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246921

(CHEMBL4077043)Show SMILES COC(c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl)C1(C)COC1 Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-7-17(30-3)15(21(28)26-12)9-27-6-5-13-16(24)8-14(19(25)18(13)22(27)29)20(31-4)23(2)10-32-11-23/h7-8,20H,5-6,9-11H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246927

(CHEMBL4060447 | US10570121, Example 127)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-8-18(30-2)16(22(28)26-12)10-27-6-4-14-17(24)9-15(20(25)19(14)23(27)29)21(31-3)13-5-7-32-11-13/h8-9,13,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 391 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246927

(CHEMBL4060447 | US10570121, Example 127)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O5/c1-12-8-18(30-2)16(22(28)26-12)10-27-6-4-14-17(24)9-15(20(25)19(14)23(27)29)21(31-3)13-5-7-32-11-13/h8-9,13,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 391 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246931
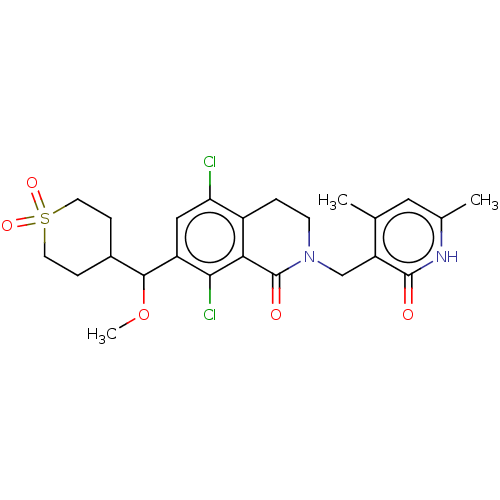
(CHEMBL4065249 | US10570121, Example 117)Show SMILES COC(C1CCS(=O)(=O)CC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C24H28Cl2N2O5S/c1-13-10-14(2)27-23(29)18(13)12-28-7-4-16-19(25)11-17(21(26)20(16)24(28)30)22(33-3)15-5-8-34(31,32)9-6-15/h10-11,15,22H,4-9,12H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246931

(CHEMBL4065249 | US10570121, Example 117)Show SMILES COC(C1CCS(=O)(=O)CC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C24H28Cl2N2O5S/c1-13-10-14(2)27-23(29)18(13)12-28-7-4-16-19(25)11-17(21(26)20(16)24(28)30)22(33-3)15-5-8-34(31,32)9-6-15/h10-11,15,22H,4-9,12H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246942

(CHEMBL4061264 | US10570121, Example 163)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(28)17(12)10-27-6-4-15-18(24)9-16(20(25)19(15)23(27)29)21(30-3)14-5-7-31-11-14/h8-9,14,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246963
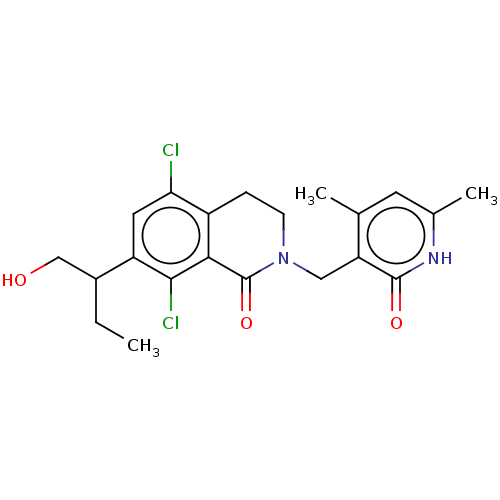
(CHEMBL4072040 | US10570121, Example 30)Show SMILES CCC(CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H24Cl2N2O3/c1-4-13(10-26)15-8-17(22)14-5-6-25(21(28)18(14)19(15)23)9-16-11(2)7-12(3)24-20(16)27/h7-8,13,26H,4-6,9-10H2,1-3H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 682 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246963

(CHEMBL4072040 | US10570121, Example 30)Show SMILES CCC(CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H24Cl2N2O3/c1-4-13(10-26)15-8-17(22)14-5-6-25(21(28)18(14)19(15)23)9-16-11(2)7-12(3)24-20(16)27/h7-8,13,26H,4-6,9-10H2,1-3H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 682 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246933
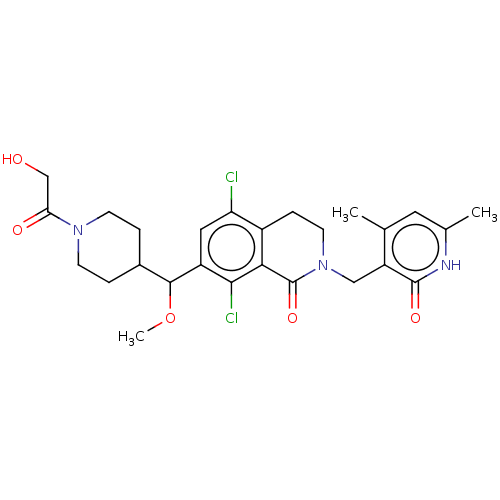
(CHEMBL4094432 | US10570121, Example 165)Show SMILES COC(C1CCN(CC1)C(=O)CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O5/c1-14-10-15(2)29-25(34)19(14)12-31-9-6-17-20(27)11-18(23(28)22(17)26(31)35)24(36-3)16-4-7-30(8-5-16)21(33)13-32/h10-11,16,24,32H,4-9,12-13H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 749 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246933

(CHEMBL4094432 | US10570121, Example 165)Show SMILES COC(C1CCN(CC1)C(=O)CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O5/c1-14-10-15(2)29-25(34)19(14)12-31-9-6-17-20(27)11-18(23(28)22(17)26(31)35)24(36-3)16-4-7-30(8-5-16)21(33)13-32/h10-11,16,24,32H,4-9,12-13H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 749 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246953
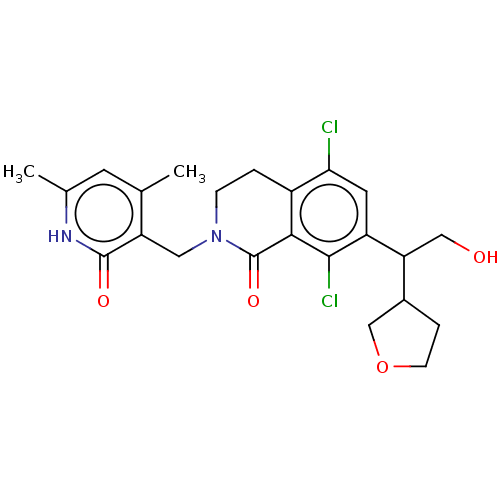
(CHEMBL4090352 | US10570121, Example 3)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(C(CO)C4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-7-13(2)26-22(29)17(12)9-27-5-3-15-19(24)8-16(21(25)20(15)23(27)30)18(10-28)14-4-6-31-11-14/h7-8,14,18,28H,3-6,9-11H2,1-2H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246953

(CHEMBL4090352 | US10570121, Example 3)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(C(CO)C4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-7-13(2)26-22(29)17(12)9-27-5-3-15-19(24)8-16(21(25)20(15)23(27)30)18(10-28)14-4-6-31-11-14/h7-8,14,18,28H,3-6,9-11H2,1-2H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246951
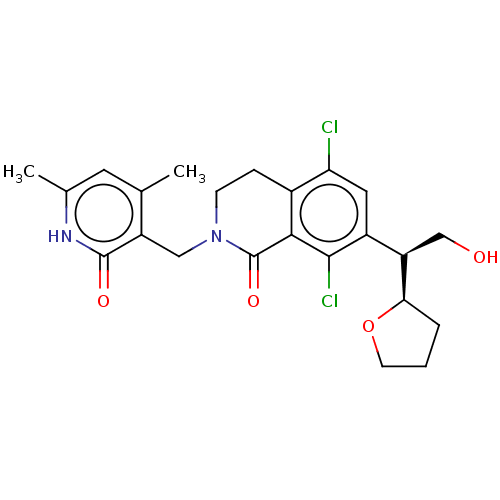
(CHEMBL4069930 | US10570121, Example 45)Show SMILES [H][C@@]1(CCCO1)[C@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(29)16(12)10-27-6-5-14-18(24)9-15(21(25)20(14)23(27)30)17(11-28)19-4-3-7-31-19/h8-9,17,19,28H,3-7,10-11H2,1-2H3,(H,26,29)/t17-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246969
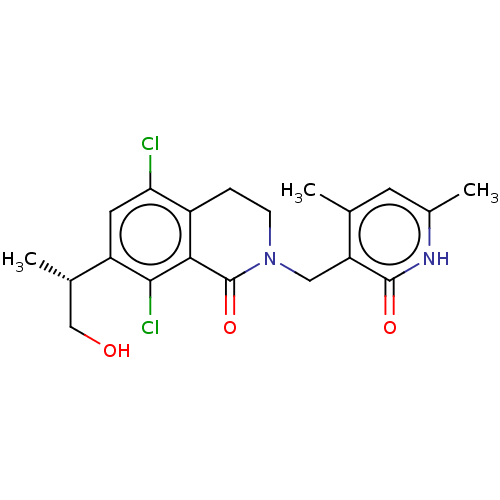
(CHEMBL4082516 | US10570121, Example 19)Show SMILES C[C@@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C20H22Cl2N2O3/c1-10-6-12(3)23-19(26)15(10)8-24-5-4-13-16(21)7-14(11(2)9-25)18(22)17(13)20(24)27/h6-7,11,25H,4-5,8-9H2,1-3H3,(H,23,26)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246942

(CHEMBL4061264 | US10570121, Example 163)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(28)17(12)10-27-6-4-15-18(24)9-16(20(25)19(15)23(27)29)21(30-3)14-5-7-31-11-14/h8-9,14,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246942

(CHEMBL4061264 | US10570121, Example 163)Show SMILES COC(C1CCOC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(28)17(12)10-27-6-4-15-18(24)9-16(20(25)19(15)23(27)29)21(30-3)14-5-7-31-11-14/h8-9,14,21H,4-7,10-11H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246940
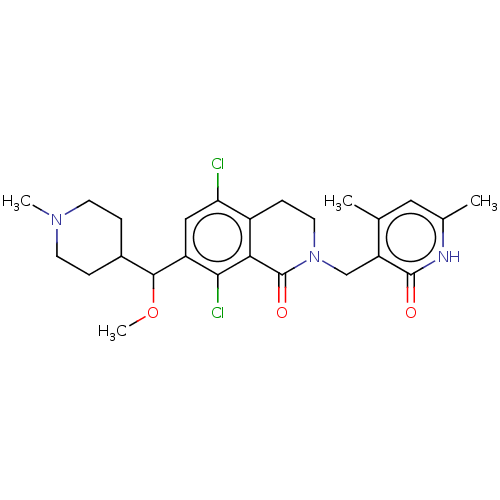
(CHEMBL4071056 | US10570121, Example 137)Show SMILES COC(C1CCN(C)CC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C25H31Cl2N3O3/c1-14-11-15(2)28-24(31)19(14)13-30-10-7-17-20(26)12-18(22(27)21(17)25(30)32)23(33-4)16-5-8-29(3)9-6-16/h11-12,16,23H,5-10,13H2,1-4H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246940

(CHEMBL4071056 | US10570121, Example 137)Show SMILES COC(C1CCN(C)CC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C25H31Cl2N3O3/c1-14-11-15(2)28-24(31)19(14)13-30-10-7-17-20(26)12-18(22(27)21(17)25(30)32)23(33-4)16-5-8-29(3)9-6-16/h11-12,16,23H,5-10,13H2,1-4H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246943
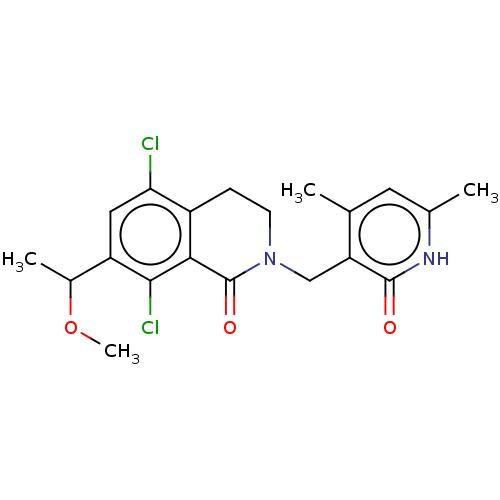
(CHEMBL4081551 | US10570121, Example 44)Show SMILES COC(C)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C20H22Cl2N2O3/c1-10-7-11(2)23-19(25)15(10)9-24-6-5-13-16(21)8-14(12(3)27-4)18(22)17(13)20(24)26/h7-8,12H,5-6,9H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246943

(CHEMBL4081551 | US10570121, Example 44)Show SMILES COC(C)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C20H22Cl2N2O3/c1-10-7-11(2)23-19(25)15(10)9-24-6-5-13-16(21)8-14(12(3)27-4)18(22)17(13)20(24)26/h7-8,12H,5-6,9H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246937

(CHEMBL4079181 | US10570121, Example 157)Show SMILES COC(C1CCN(CC1)C1COC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C27H33Cl2N3O4/c1-15-10-16(2)30-26(33)21(15)12-32-9-6-19-22(28)11-20(24(29)23(19)27(32)34)25(35-3)17-4-7-31(8-5-17)18-13-36-14-18/h10-11,17-18,25H,4-9,12-14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246937

(CHEMBL4079181 | US10570121, Example 157)Show SMILES COC(C1CCN(CC1)C1COC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C27H33Cl2N3O4/c1-15-10-16(2)30-26(33)21(15)12-32-9-6-19-22(28)11-20(24(29)23(19)27(32)34)25(35-3)17-4-7-31(8-5-17)18-13-36-14-18/h10-11,17-18,25H,4-9,12-14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246941

(CHEMBL4062421 | US10570121, Example 51)Show SMILES COC(C1CCOCC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C24H28Cl2N2O4/c1-13-10-14(2)27-23(29)18(13)12-28-7-4-16-19(25)11-17(21(26)20(16)24(28)30)22(31-3)15-5-8-32-9-6-15/h10-11,15,22H,4-9,12H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246941

(CHEMBL4062421 | US10570121, Example 51)Show SMILES COC(C1CCOCC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C24H28Cl2N2O4/c1-13-10-14(2)27-23(29)18(13)12-28-7-4-16-19(25)11-17(21(26)20(16)24(28)30)22(31-3)15-5-8-32-9-6-15/h10-11,15,22H,4-9,12H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246945
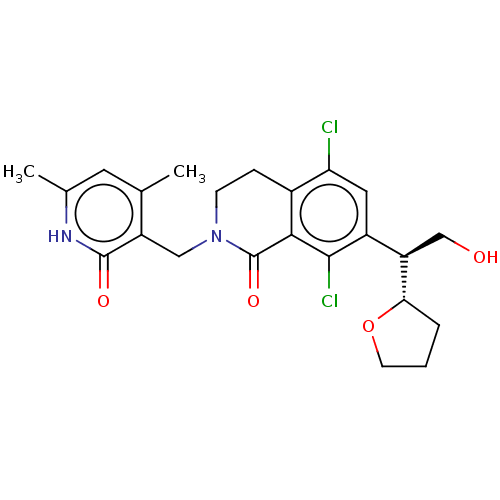
(CHEMBL4097129 | US10570121, Example 46)Show SMILES [H][C@]1(CCCO1)[C@H](CO)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C23H26Cl2N2O4/c1-12-8-13(2)26-22(29)16(12)10-27-6-5-14-18(24)9-15(21(25)20(14)23(27)30)17(11-28)19-4-3-7-31-19/h8-9,17,19,28H,3-7,10-11H2,1-2H3,(H,26,29)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246939
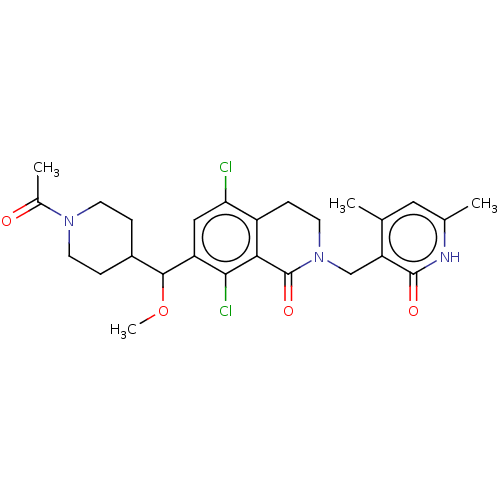
(CHEMBL4072089 | US10570121, Example 110)Show SMILES COC(C1CCN(CC1)C(C)=O)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O4/c1-14-11-15(2)29-25(33)20(14)13-31-10-7-18-21(27)12-19(23(28)22(18)26(31)34)24(35-4)17-5-8-30(9-6-17)16(3)32/h11-12,17,24H,5-10,13H2,1-4H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246939

(CHEMBL4072089 | US10570121, Example 110)Show SMILES COC(C1CCN(CC1)C(C)=O)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C26H31Cl2N3O4/c1-14-11-15(2)29-25(33)20(14)13-31-10-7-18-21(27)12-19(23(28)22(18)26(31)34)24(35-4)17-5-8-30(9-6-17)16(3)32/h11-12,17,24H,5-10,13H2,1-4H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data