

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244370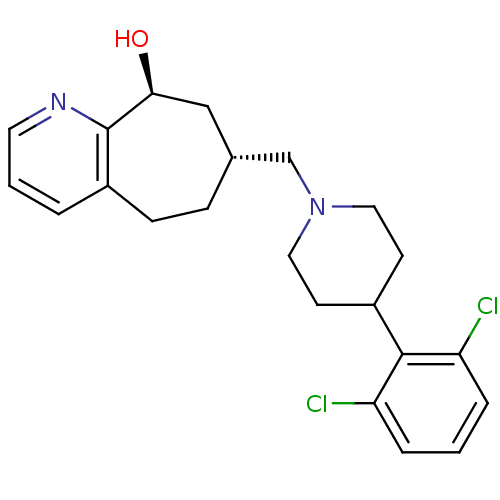 ((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244371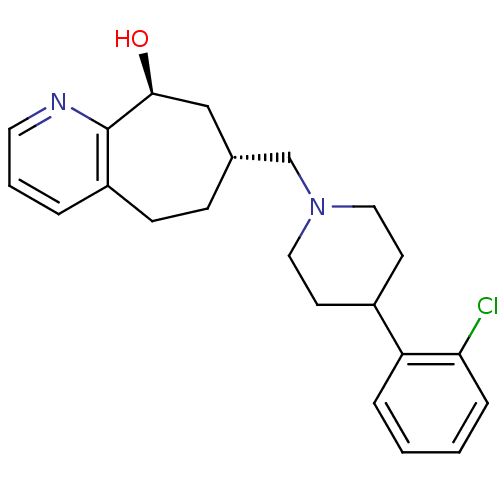 ((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243726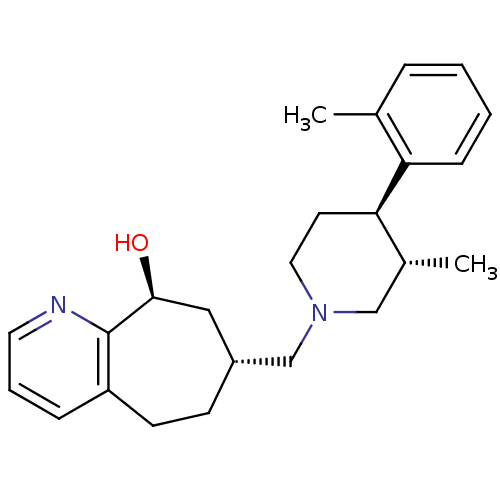 ((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243729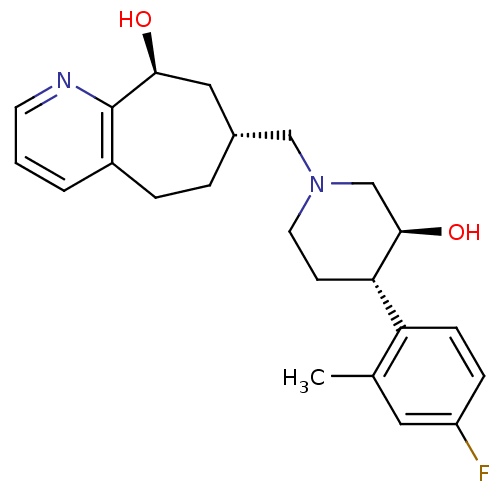 ((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243727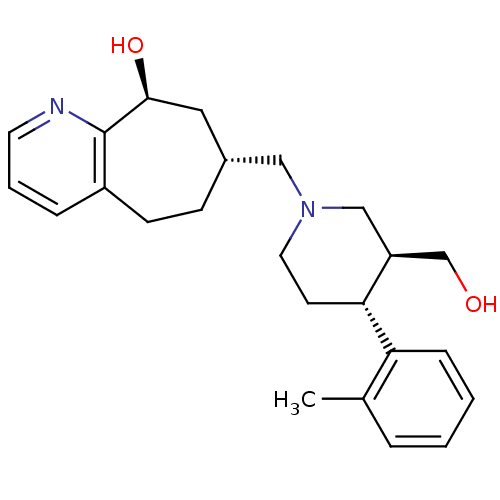 ((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243730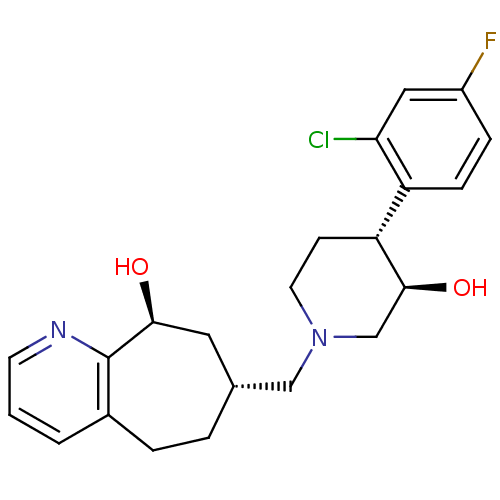 ((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244297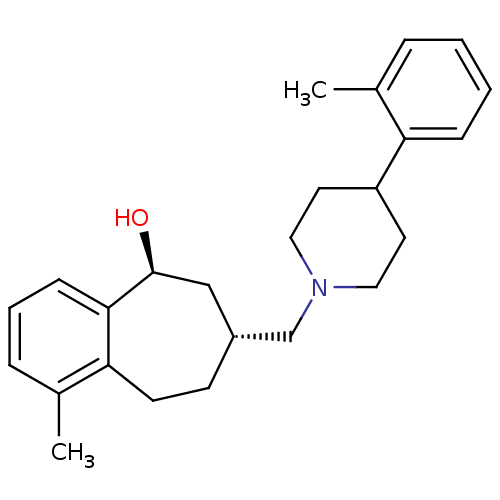 (CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243728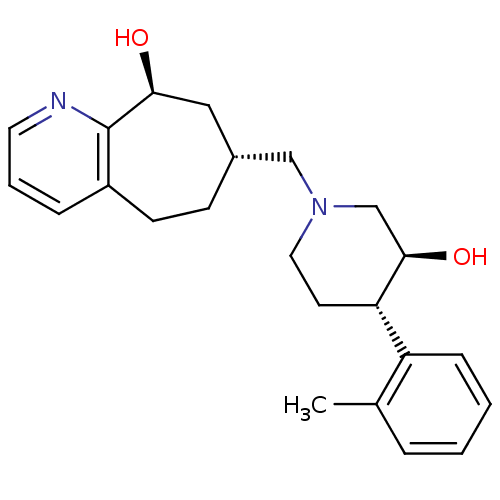 ((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243887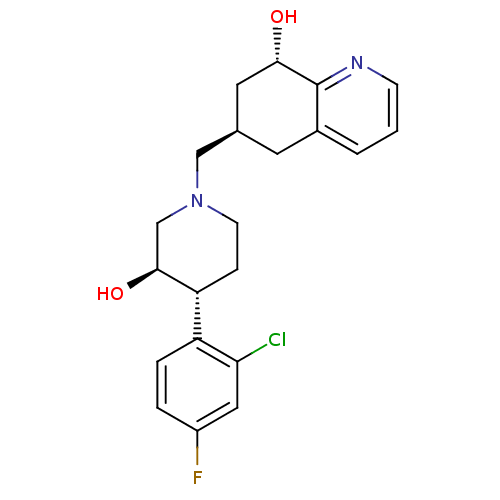 ((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244336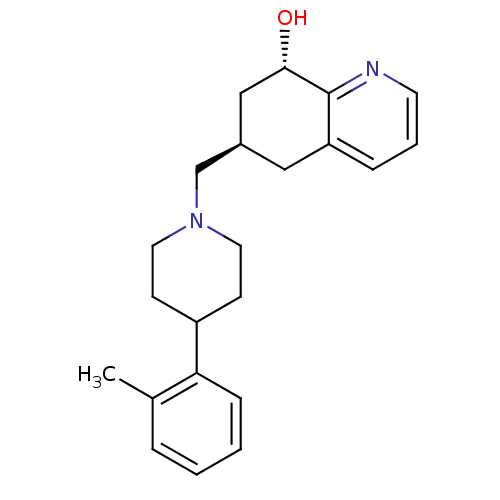 ((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334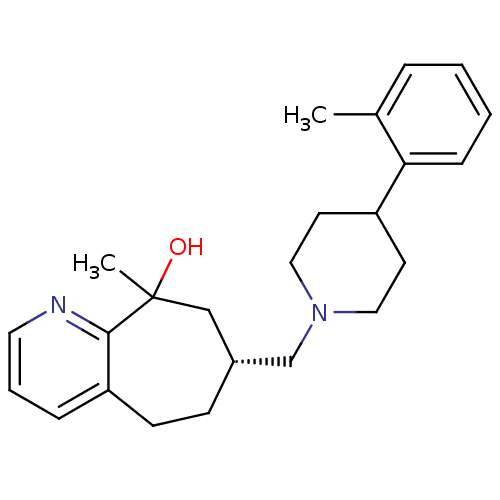 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295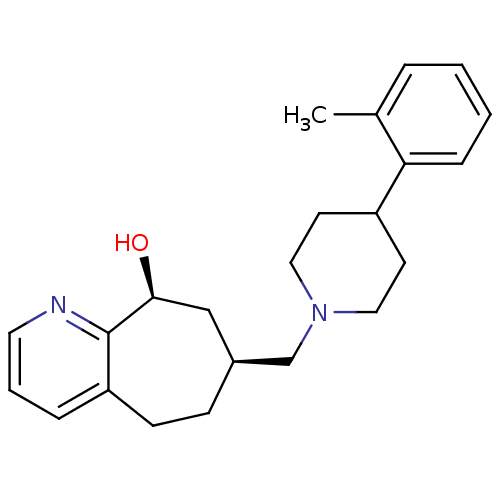 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244372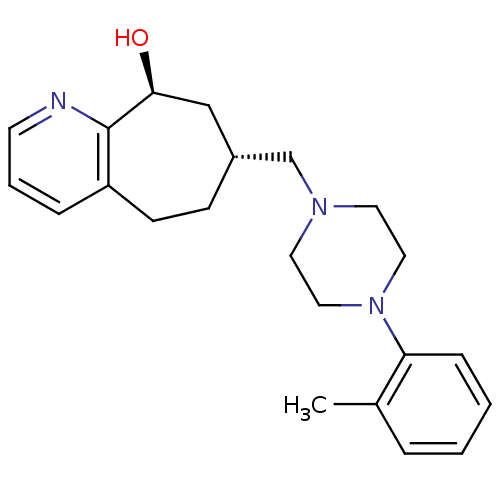 ((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243886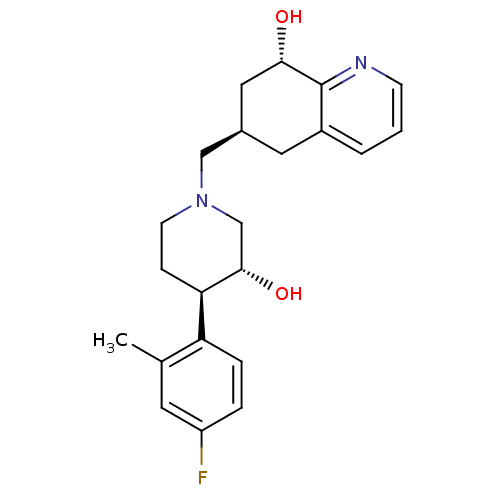 ((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244335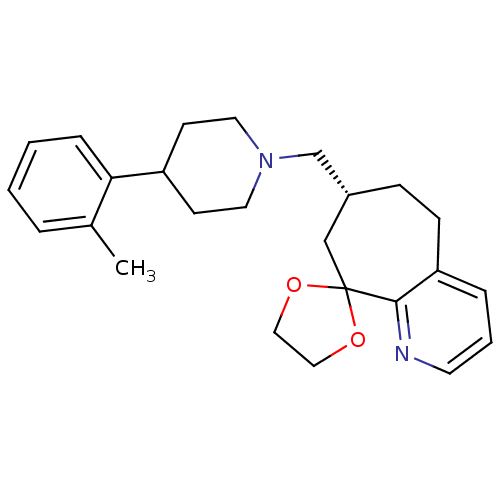 ((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244373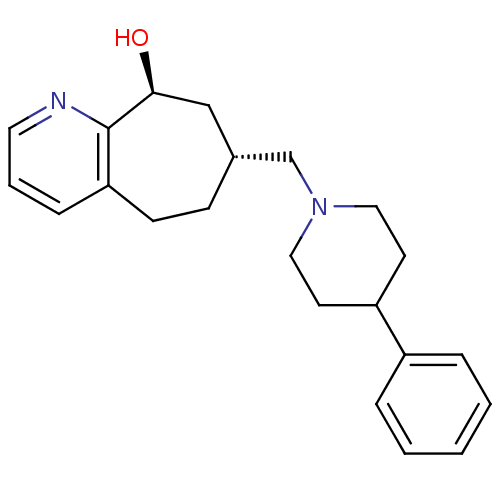 ((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244333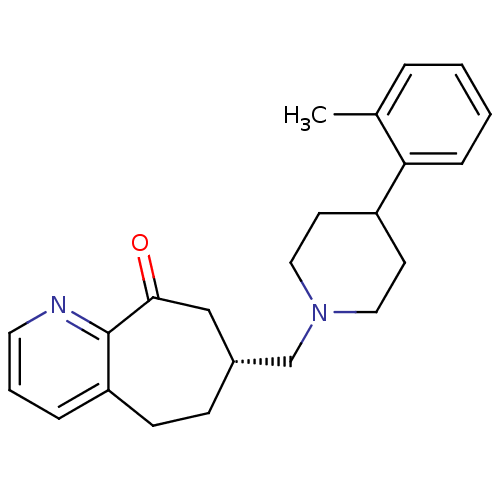 ((R)-7-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243797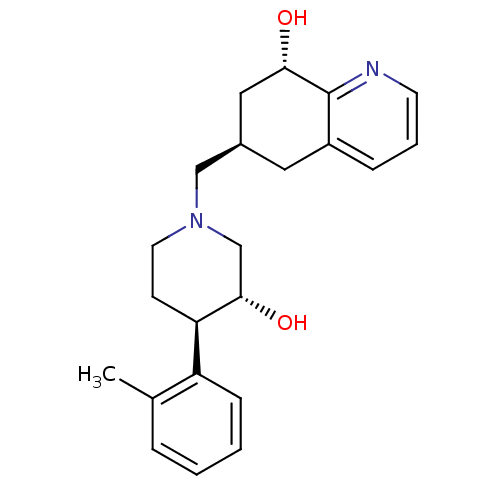 ((6R,8S)-6-(((3R,4R)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244374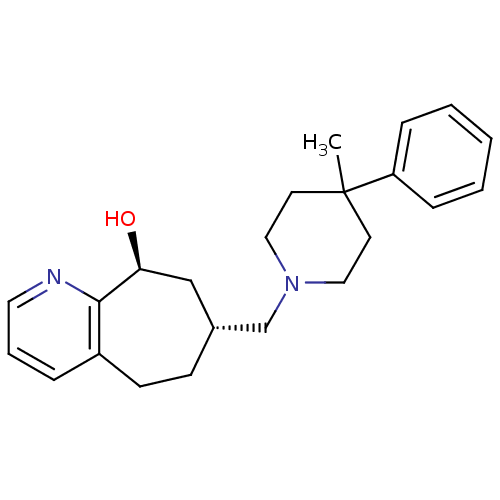 ((7R,9S)-7-((4-methyl-4-phenylpiperidin-1-yl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244296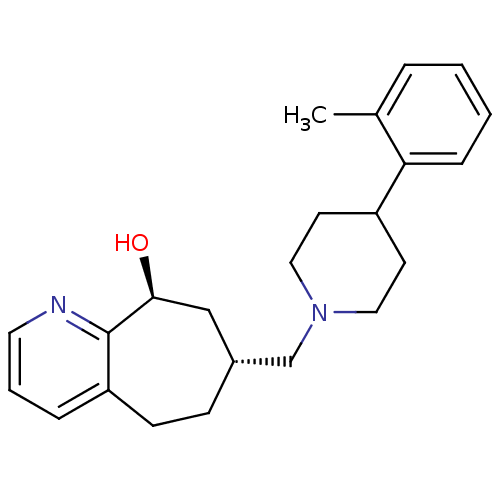 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442114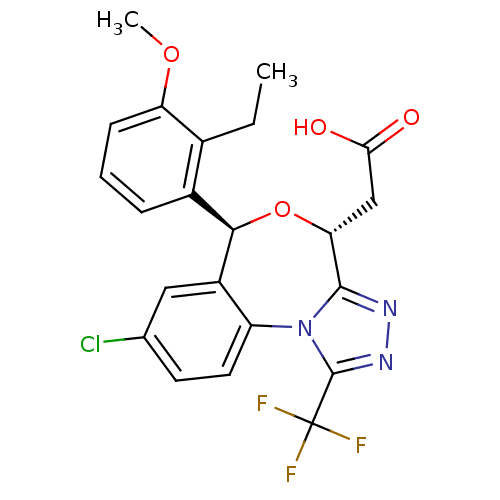 (CHEMBL2441090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499811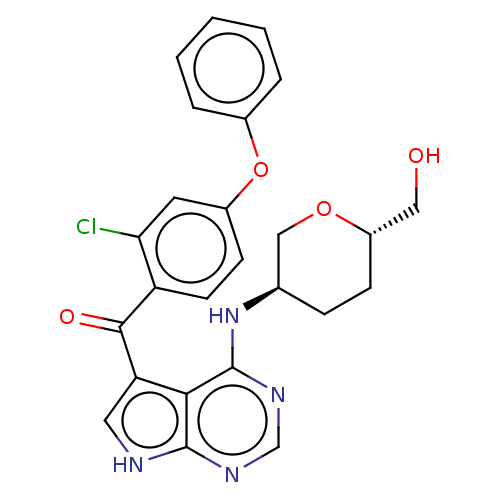 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614182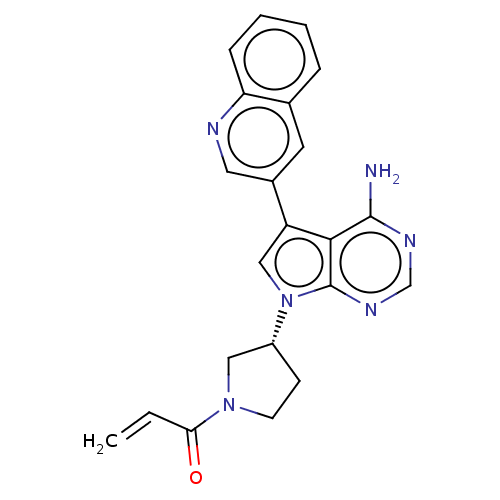 (CHEMBL5291403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501949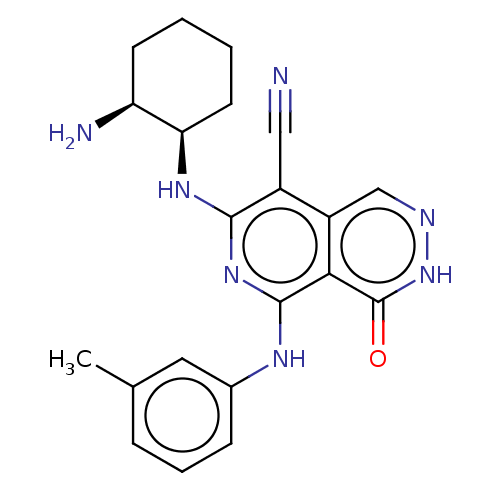 (CHEMBL4461851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442115 (CHEMBL2440128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499810 (N-((S)-1-((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499814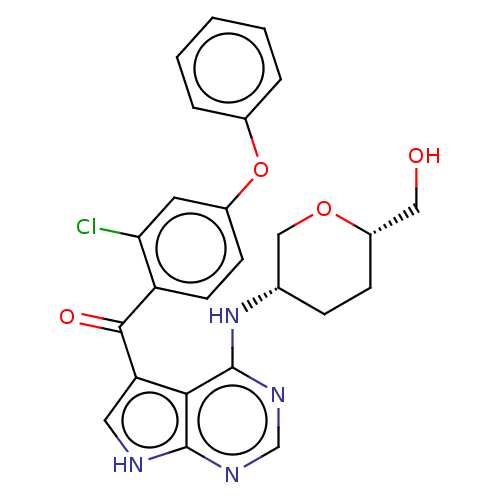 ((2-chloro-4-phenoxyphenyl)(4- (((3S,6S)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442113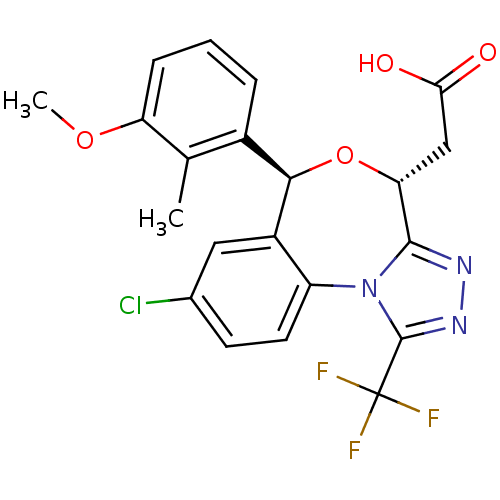 (CHEMBL2441083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499838 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-((R)-1- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499976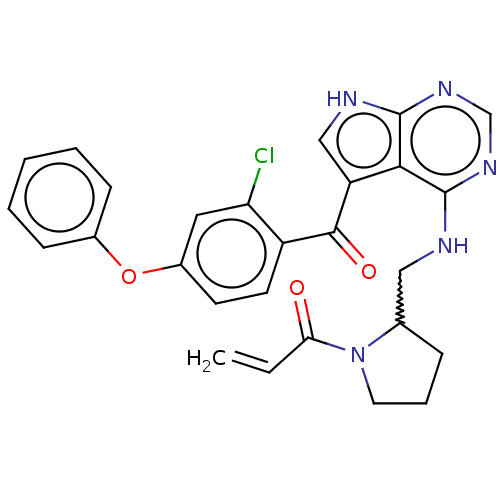 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499924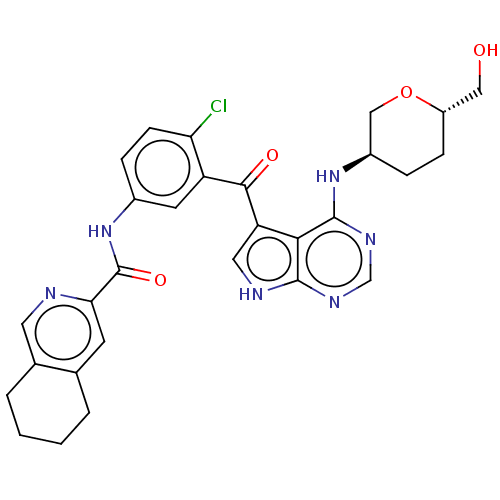 (N-(4-chloro-3-(4-(((3R,6S)-6- (hydroxymethyl)tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499974 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499869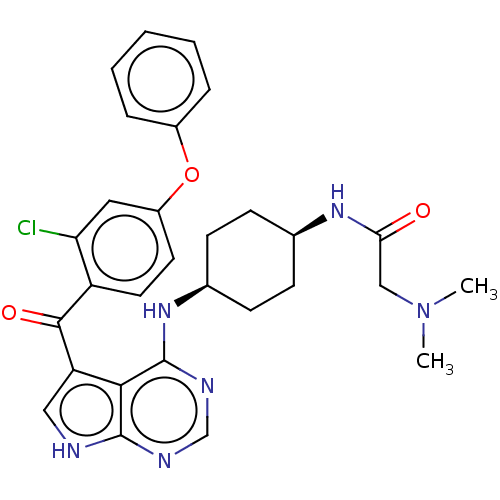 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161389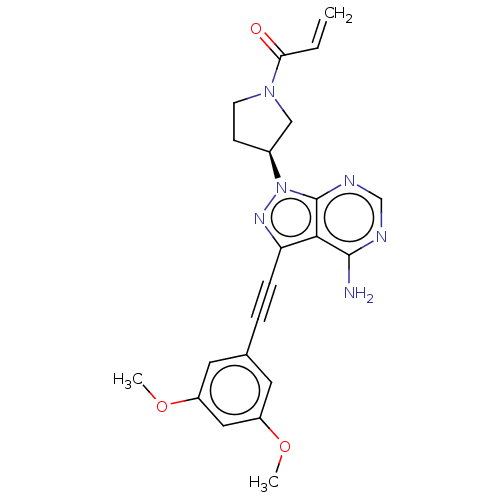 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499890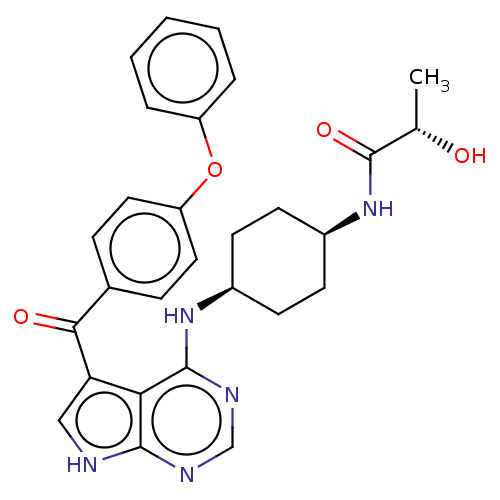 ((S)-2-hydroxy-N-(cis-4-((5-(4- phenoxybenzoyl)-7H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499991 (US11020398, Compound I-360e | racemic-cis-(2-chlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442112 (CHEMBL2441084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499892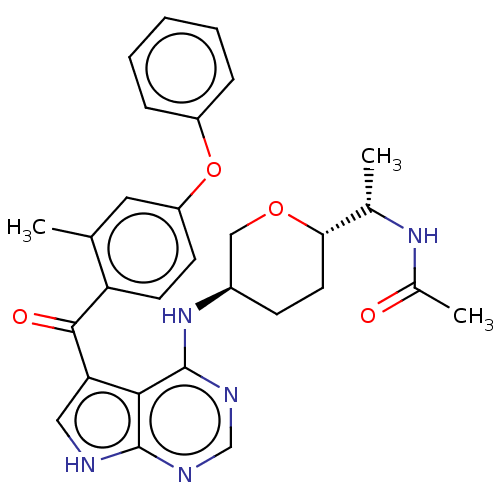 (N-((S)-1-((2S,5R)-5-((5-(2-methyl-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499854 ((4-(((3R,6S)-6-((S)-1- aminoethyl)tetrahydro-2H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614181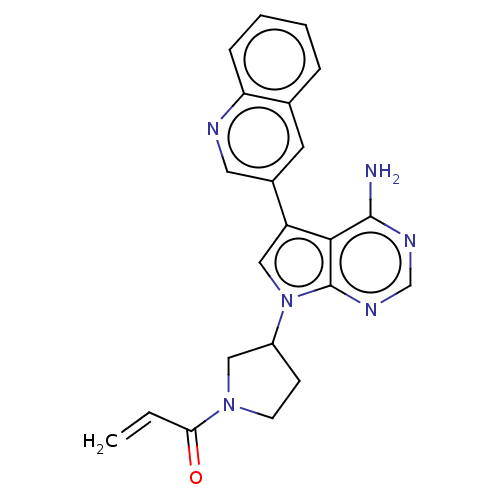 (CHEMBL5265881) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499881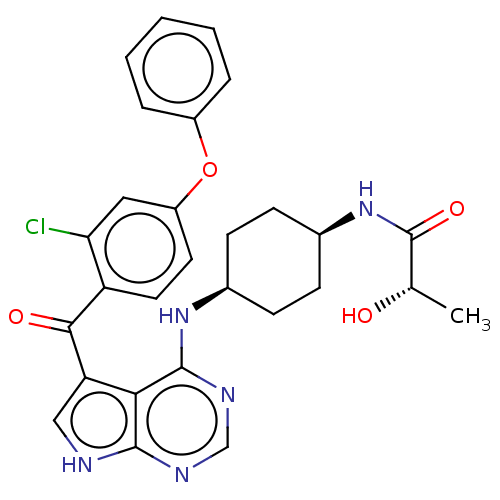 ((S)-N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499839 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499891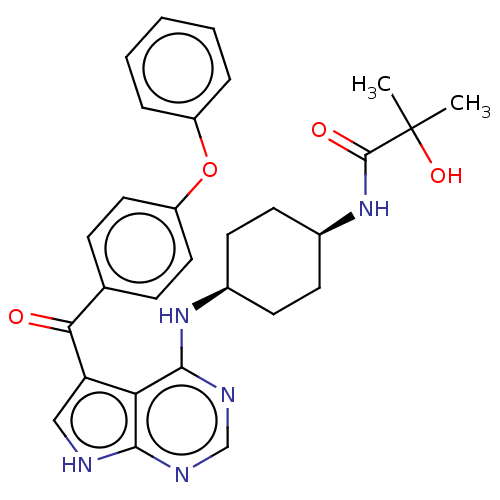 (2-hydroxy-2-methyl-N-(cis-4-((5-(4- phenoxybenzoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 554 total ) | Next | Last >> |