

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280415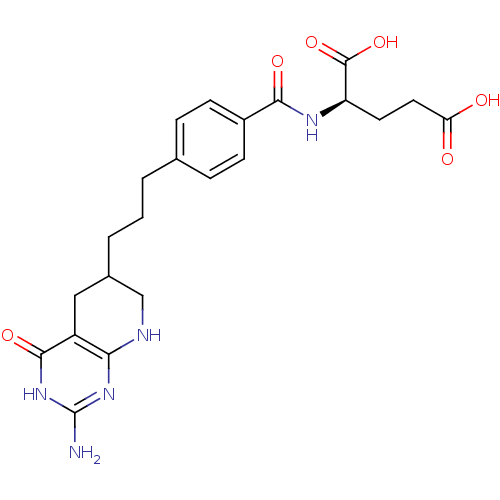 ((R)-2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.0000190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590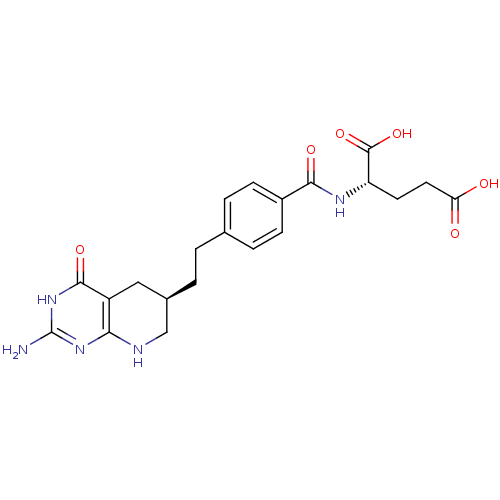 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 0.000120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280414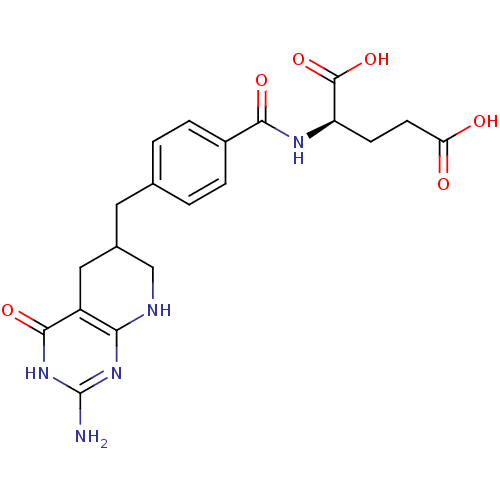 ((R)-2-[4-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50288820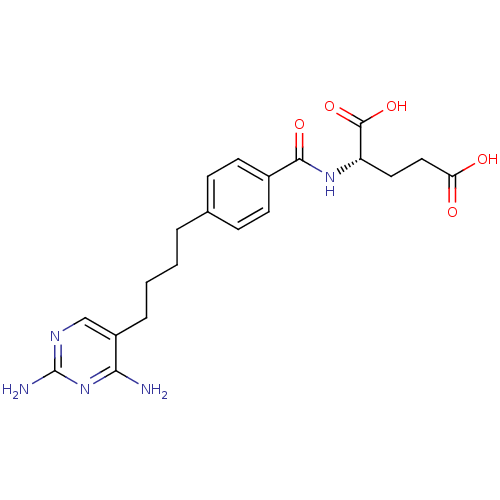 ((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 6: 473-476 (1996) Article DOI: 10.1016/0960-894X(96)00053-4 BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50288821 ((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 6: 473-476 (1996) Article DOI: 10.1016/0960-894X(96)00053-4 BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073754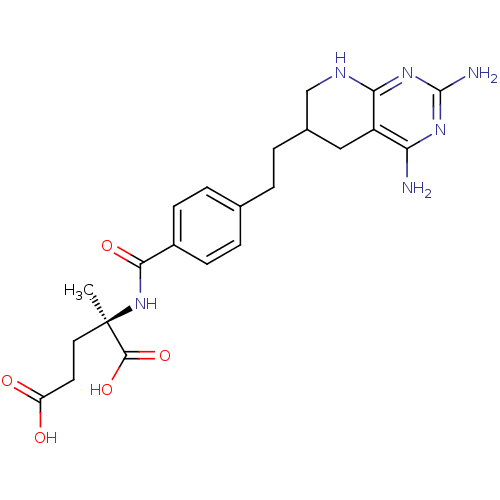 ((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073752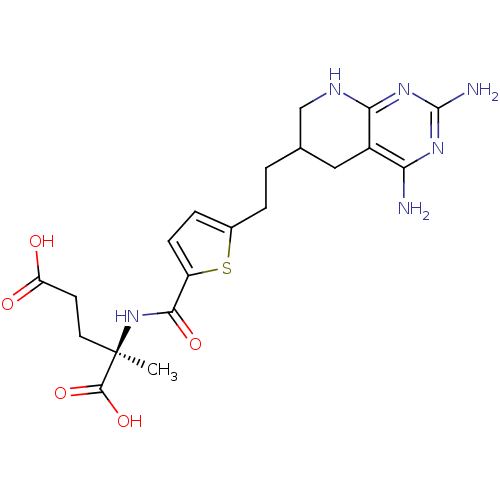 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429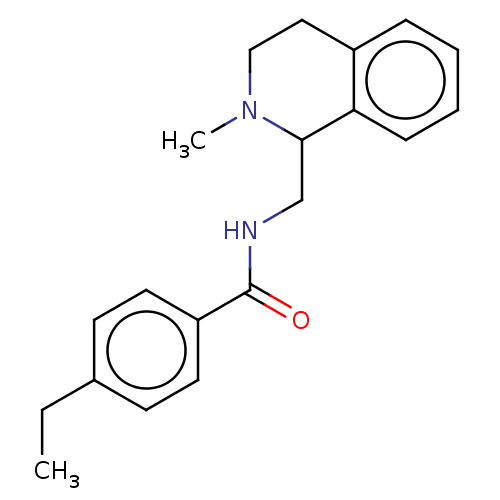 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435111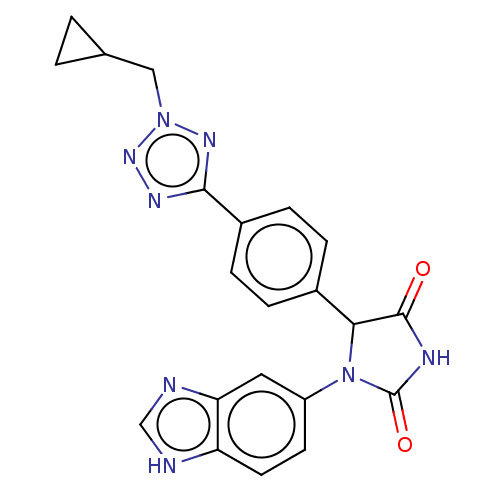 (US10584120, Compound 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435104 (US10584120, Compound 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435105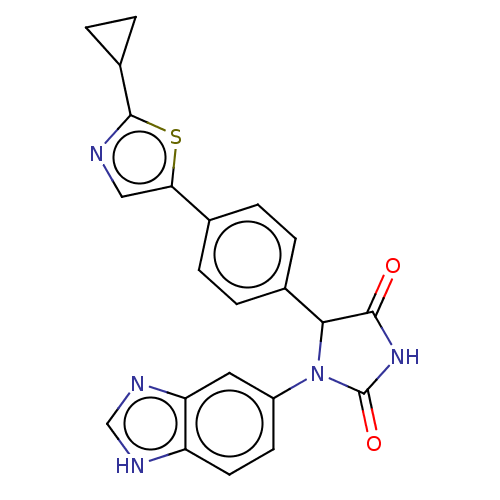 (US10584120, Compound 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985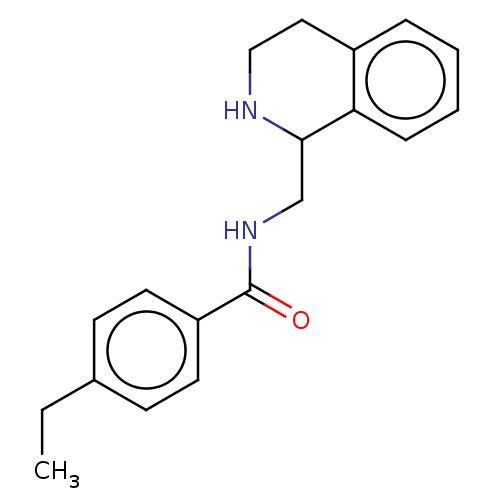 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435108 (US10584120, Compound 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064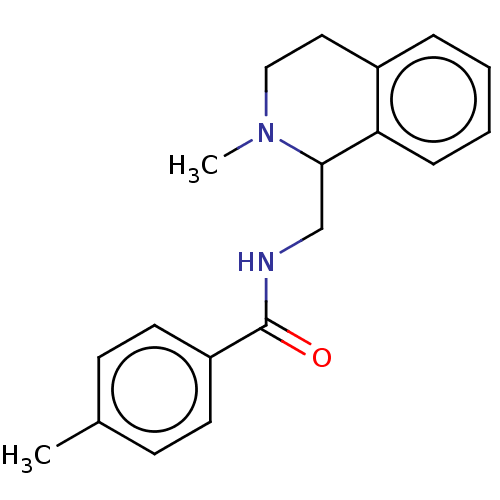 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435128 (US10584120, Compound 62) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435114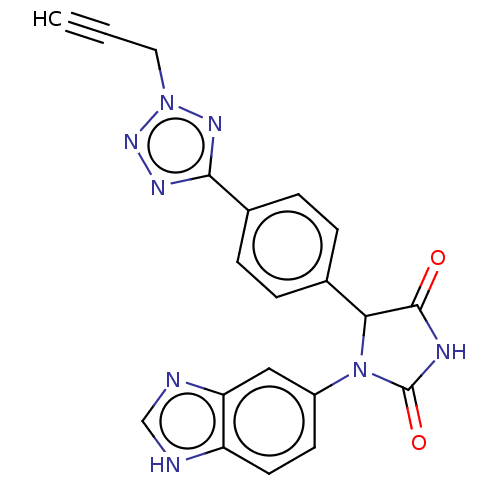 (US10584120, Compound 47) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ROCK1 by homogenous luciferase assay | J Med Chem 53: 759-77 (2010) Article DOI: 10.1021/jm9014263 BindingDB Entry DOI: 10.7270/Q2V125RD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426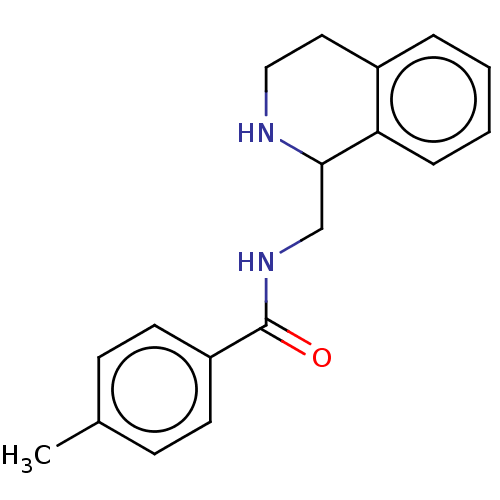 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239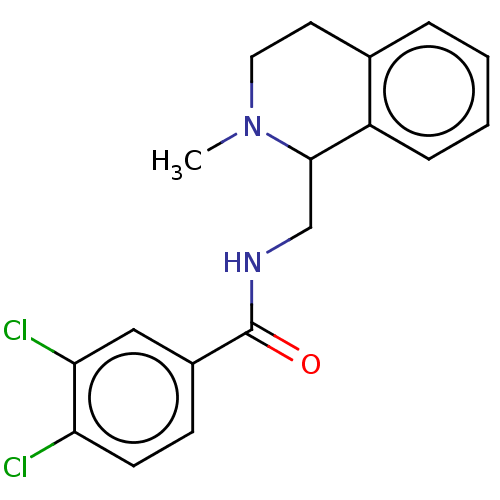 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435126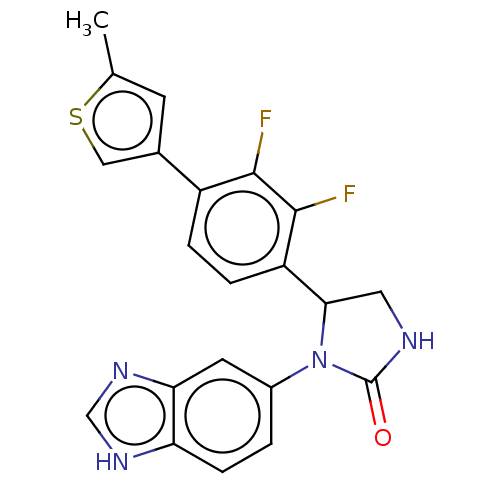 (US10584120, Compound 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435123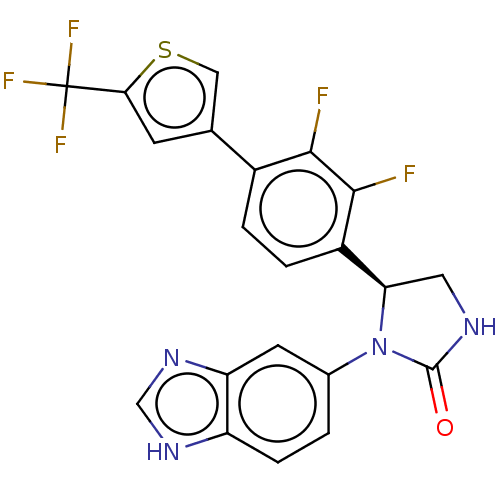 (US10584120, Compound 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435112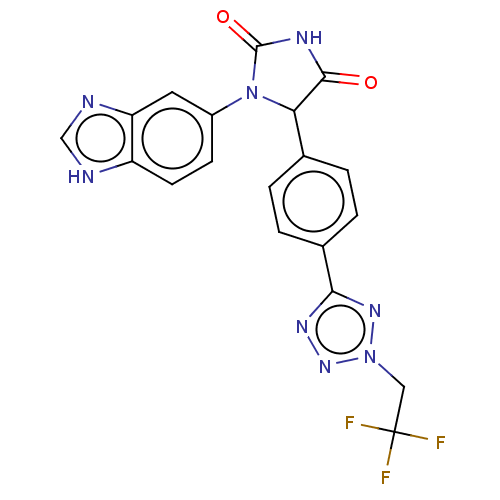 (US10584120, Compound 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435109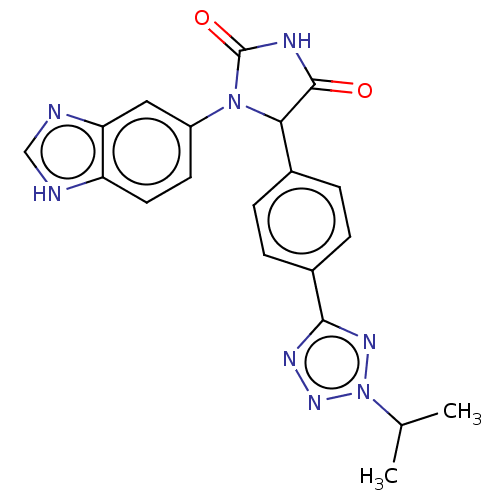 (US10584120, Compound 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435106 (US10584120, Compound 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073753 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435116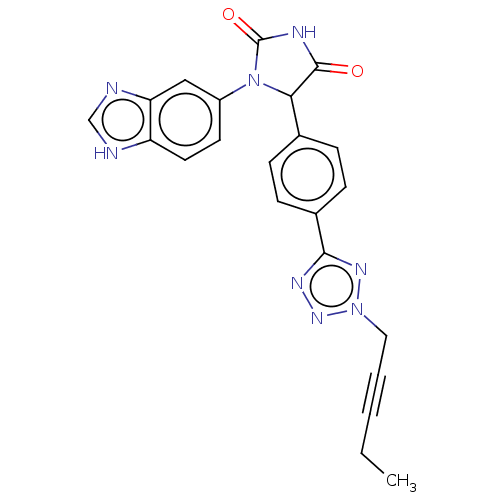 (US10584120, Compound 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435115 (US10584120, Compound 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435110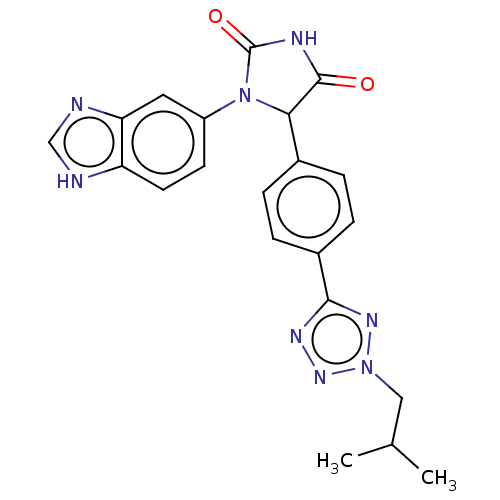 (US10584120, Compound 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435129 (US10584120, Compound 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435107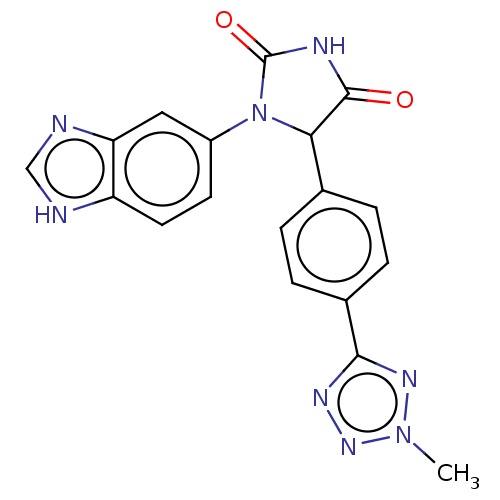 (US10584120, Compound 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435117 (US10584120, Compound 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435130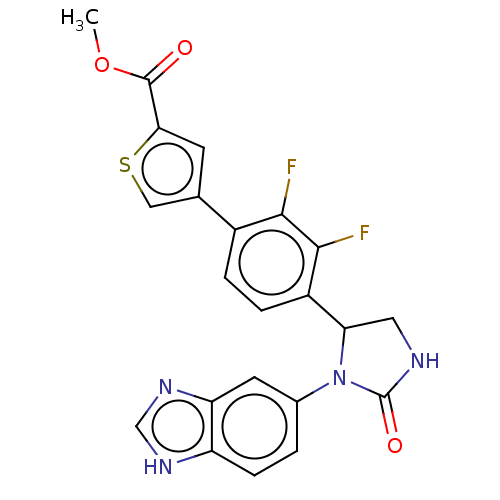 (US10584120, Compound 64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435127 (US10584120, Compound 61) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435078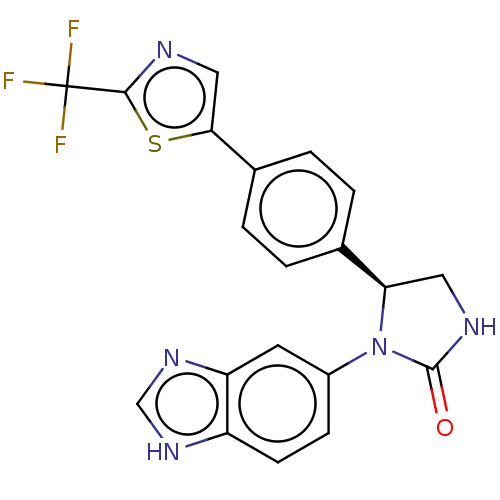 (US10584120, Compound 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435103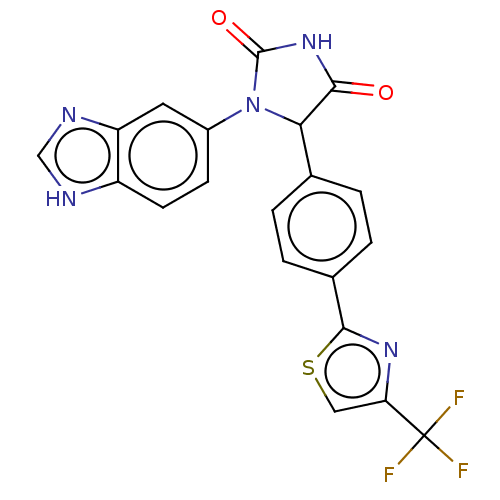 (US10584120, Compound 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435125 (US10584120, Compound 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435131 (US10584120, Compound 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435075 (US10584120, Compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435121 (US10584120, Compound 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435118 (US10584120, Compound 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human placental adenosine kinase | J Med Chem 36: 3424-30 (1993) BindingDB Entry DOI: 10.7270/Q2NG4R8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435102 (US10584120, Compound 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435090 (US10584120, Compound 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433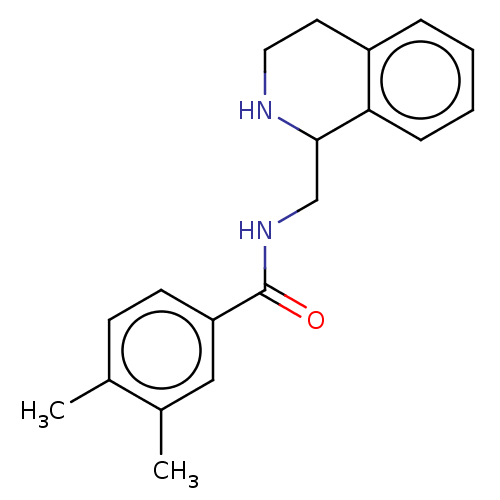 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435097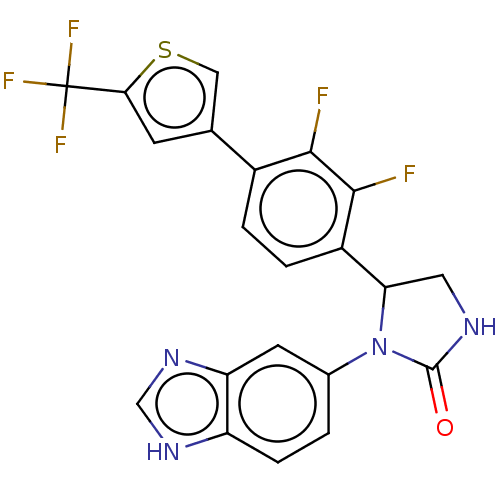 (US10584120, Compound 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50368712 (CHEMBL608051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human placental adenosine kinase | J Med Chem 36: 3424-30 (1993) BindingDB Entry DOI: 10.7270/Q2NG4R8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM435076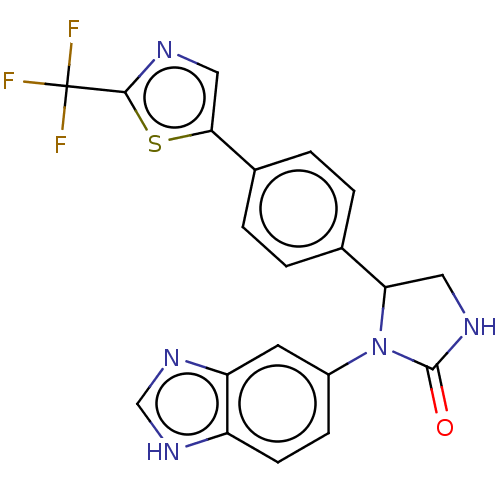 (US10584120, Compound 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes; Academia Sinica US Patent | Assay Description An inhibition activity assay of QC inhibitors was conducted. See Huang et al., J. Biol. Chem. 2011, 286, 12439-12449. A reaction mixture containing 3... | US Patent US10584120 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5K20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3235 total ) | Next | Last >> |