

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423789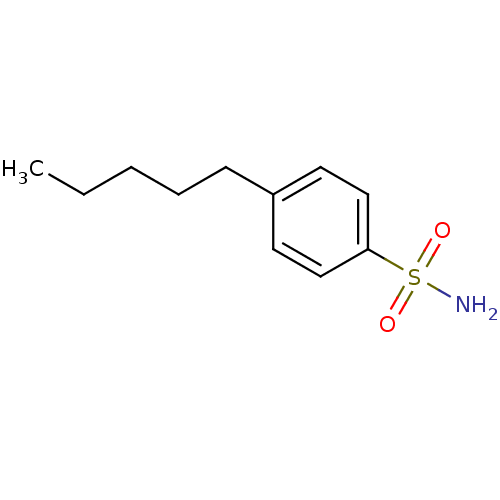 (4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423789 (4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423788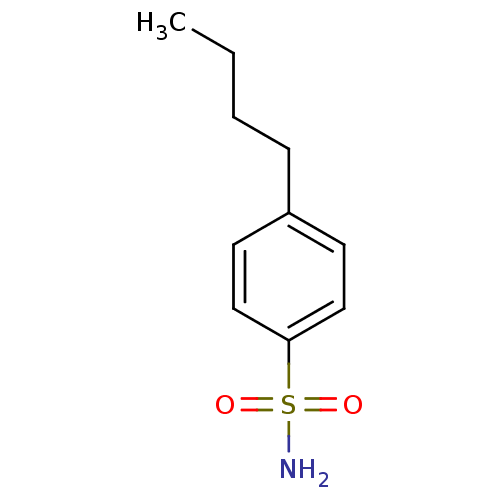 (4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423788 (4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423787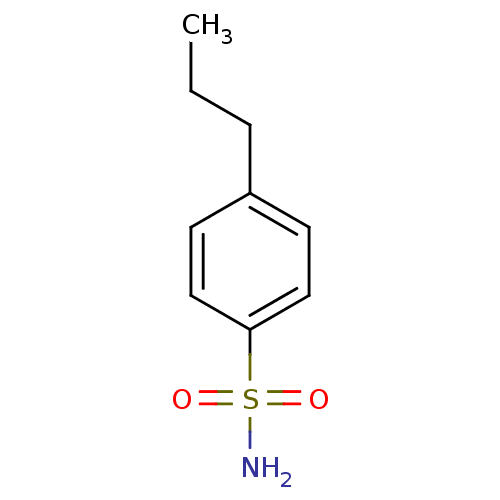 (4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50423787 (4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50415863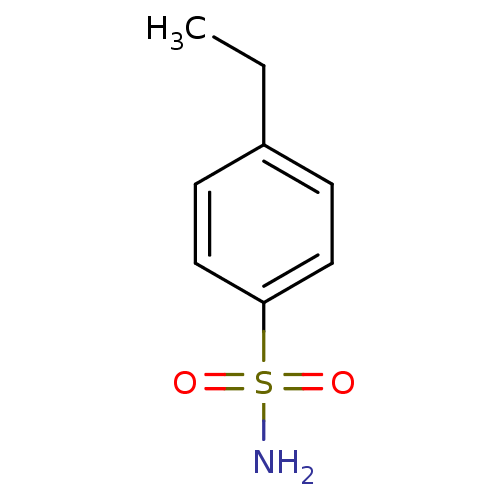 (CHEMBL358263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50415863 (CHEMBL358263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478141 (CHEMBL403526) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719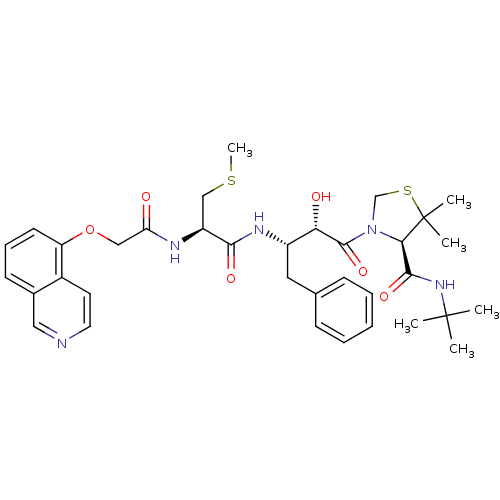 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194790 (US9206167, 11 | US9206167, 12 | USRE48059, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4227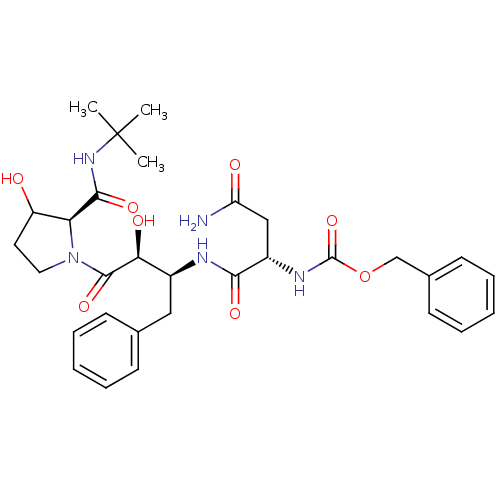 (AHPBA 35a | Z-Asn.(2S,3S)-AHPBA-[3(R)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478138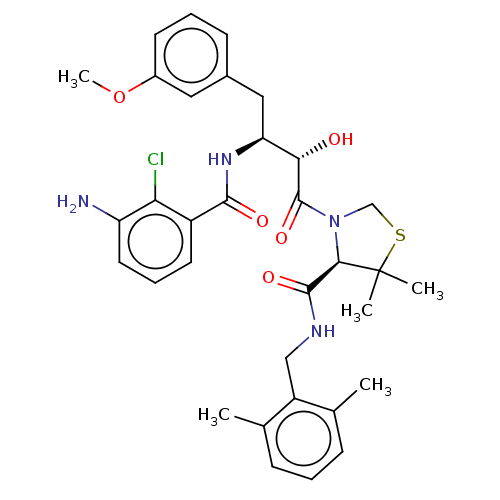 (CHEMBL256934 | SM-309515) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021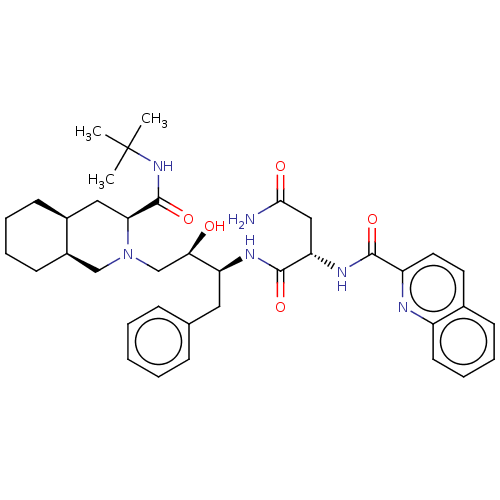 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478135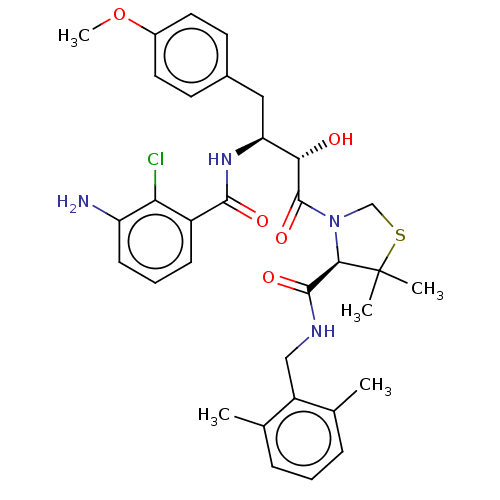 (CHEMBL409007) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478136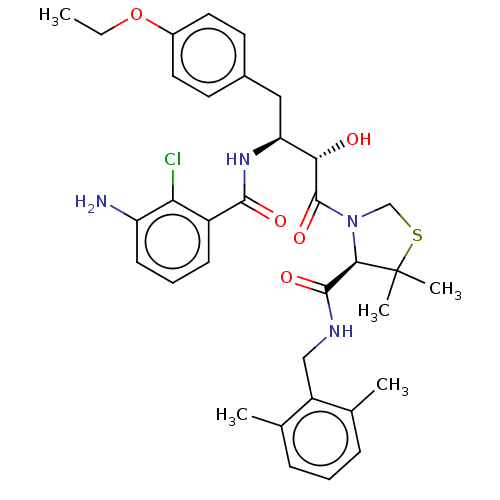 (CHEMBL257257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478137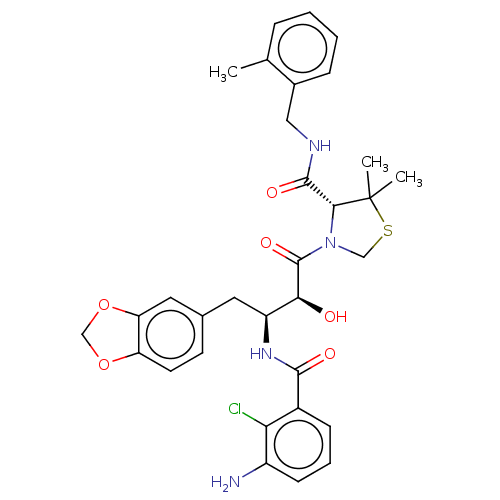 (CHEMBL271391) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194794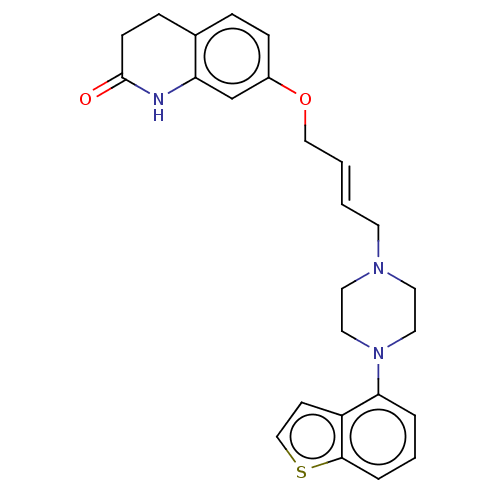 (US9206167, 15 | USRE48059, Compound of Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM448338 (USRE48059, Compound of Example 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194794 (US9206167, 15 | USRE48059, Compound of Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194772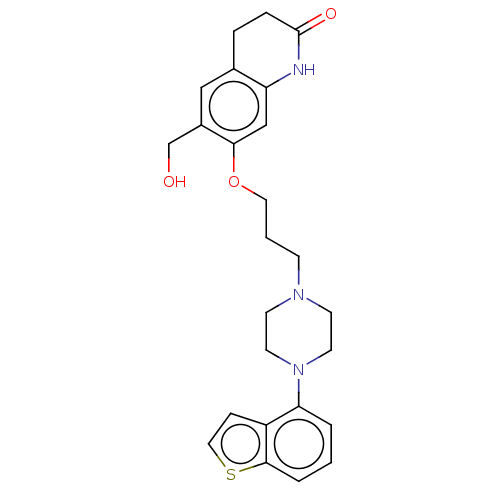 (US9206167, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262566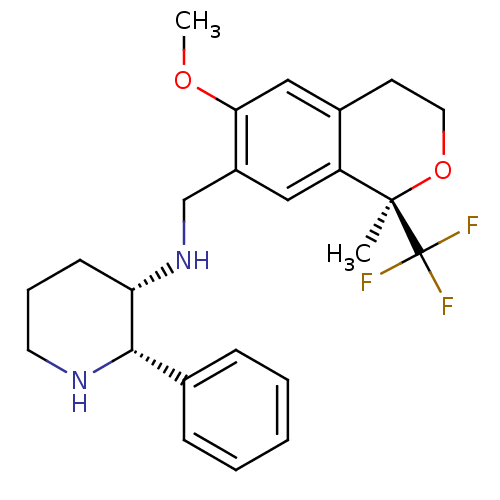 ((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50160668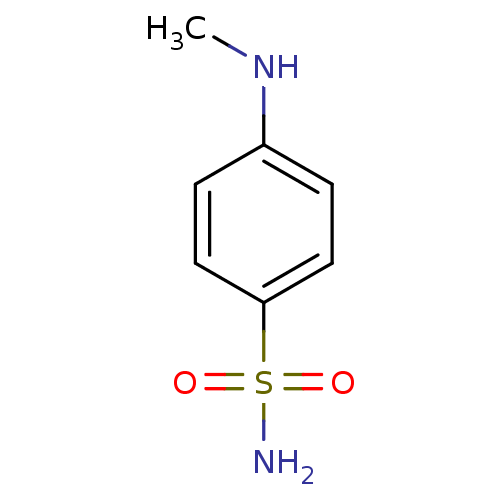 (4-Methylamino-benzenesulfonamide | CHEMBL174847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50160668 (4-Methylamino-benzenesulfonamide | CHEMBL174847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 | Bioorg Med Chem Lett 21: 141-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.050 BindingDB Entry DOI: 10.7270/Q2K938T6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478143 (CHEMBL269904) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478144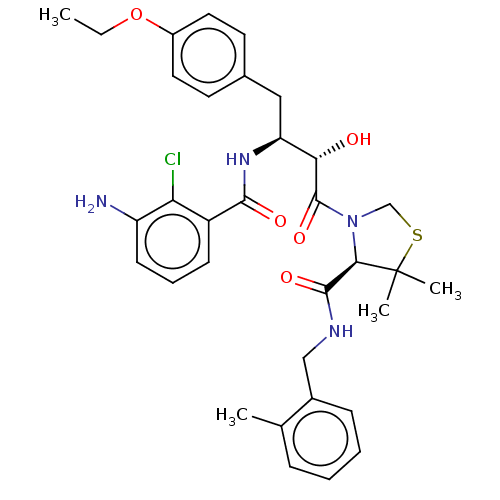 (CHEMBL272025) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478142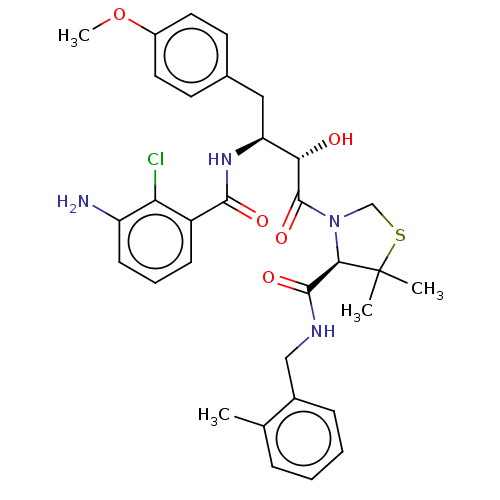 (CHEMBL272797) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478140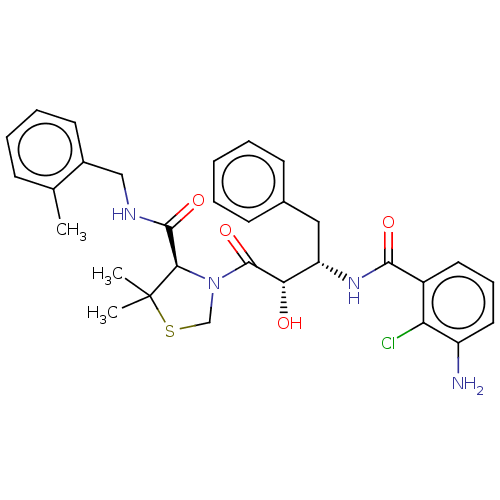 (CHEMBL404154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194789 (US9206167, 10 | USRE48059, Compound of Example 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194789 (US9206167, 10 | USRE48059, Compound of Example 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194764 (US9206167, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM448330 (USRE48059, Compound of Example 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263407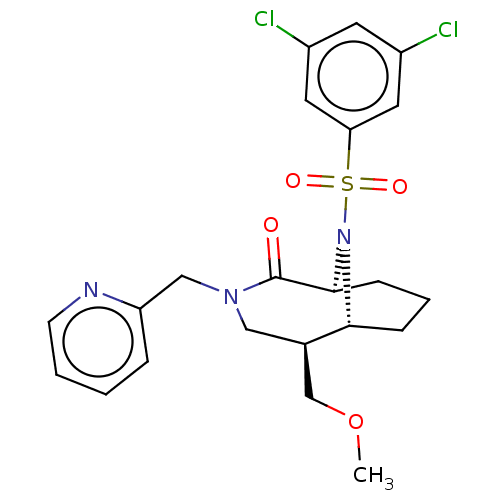 (CHEMBL4090599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194782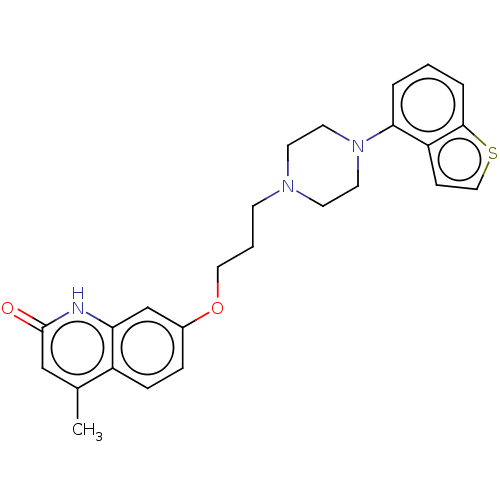 (US9206167, 3 | USRE48059, Compound of Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194783 (US9206167, 4 | USRE48059, Compound of Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co., Ltd. US Patent | Assay Description Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)... | US Patent USRE48059 (2020) BindingDB Entry DOI: 10.7270/Q2833W3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194786 (US9206167, 7 | USRE48059, Compound of Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM194782 (US9206167, 3 | USRE48059, Compound of Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description The assay was performed according to the method by Kohler et al. (Kohler C, Hall H, Ogren S O and Gawell L, Specific in vitro and in vivo binding of ... | US Patent US9206167 (2015) BindingDB Entry DOI: 10.7270/Q2XD10GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3865 total ) | Next | Last >> |