 Found 118 hits with Last Name = 'shutes' and Initial = 'a'
Found 118 hits with Last Name = 'shutes' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291573
(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291413

(1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC2CC3(C2)CN(C3)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-21(31)30-14-25(15-30)12-18(13-25)29-24-22(23(26)27-16-28-24)17-8-10-20(11-9-17)32-19-6-4-3-5-7-19/h2-11,16,18H,1,12-15H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291455

(N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC23CC(C2)(CC3)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |(-.82,-6.28,;-2.15,-5.51,;-3.48,-6.28,;-4.82,-5.51,;-4.82,-3.97,;-3.48,-3.2,;-3.48,-1.66,;-4.82,-.89,;-4.82,.65,;-6.28,1.13,;-5.88,-.36,;-7.19,-.12,;-6.28,-1.37,;-6.68,2.61,;-5.59,3.7,;-4.1,3.3,;-5.99,5.19,;-4.9,6.28,;-2.15,-3.97,;-.82,-3.2,;.52,-3.97,;1.85,-3.2,;1.85,-1.66,;3.19,-.89,;4.52,-1.66,;4.52,-3.2,;5.85,-3.97,;7.19,-3.2,;7.19,-1.66,;5.85,-.89,;.52,-.89,;-.82,-1.66,)| Show InChI InChI=1S/C25H25N5O2/c1-2-20(31)29-24-12-13-25(14-24,15-24)30-23-21(22(26)27-16-28-23)17-8-10-19(11-9-17)32-18-6-4-3-5-7-18/h2-11,16H,1,12-15H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291452
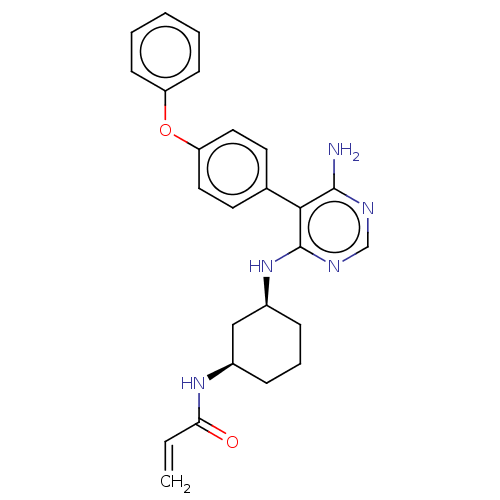
(N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...)Show SMILES Nc1ncnc(N[C@H]2CCC[C@H](C2)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-5,9-14,16,18-19H,1,6-8,15H2,(H,29,31)(H3,26,27,28,30)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291635
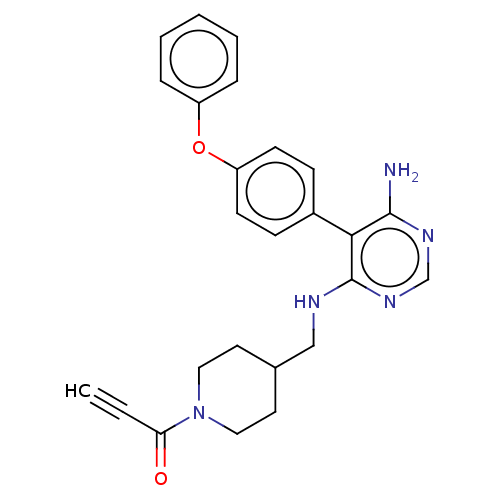
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C#C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h1,3-11,17-18H,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50519156
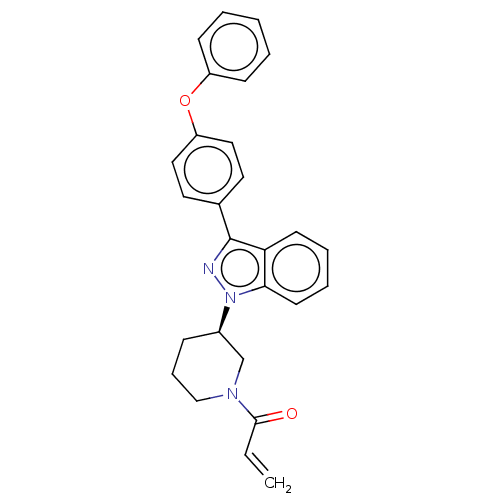
(CHEMBL4466205)Show SMILES C=CC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2)c2ccccc12 |r| Show InChI InChI=1S/C27H25N3O2/c1-2-26(31)29-18-8-9-21(19-29)30-25-13-7-6-12-24(25)27(28-30)20-14-16-23(17-15-20)32-22-10-4-3-5-11-22/h2-7,10-17,21H,1,8-9,18-19H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433630
(CHEMBL2380834)Show SMILES Fc1ccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c(c1)C(F)(F)F Show InChI InChI=1S/C26H16F4N4O2/c27-14-5-10-19(20(13-14)26(28,29)30)25(36)33-16-8-6-15(7-9-16)32-21-11-12-31-23-22(21)17-3-1-2-4-18(17)24(35)34-23/h1-13H,(H,33,36)(H2,31,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433630

(CHEMBL2380834)Show SMILES Fc1ccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c(c1)C(F)(F)F Show InChI InChI=1S/C26H16F4N4O2/c27-14-5-10-19(20(13-14)26(28,29)30)25(36)33-16-8-6-15(7-9-16)32-21-11-12-31-23-22(21)17-3-1-2-4-18(17)24(35)34-23/h1-13H,(H,33,36)(H2,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433636
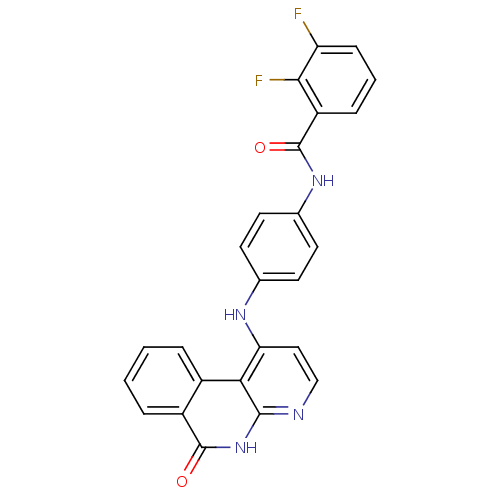
(CHEMBL2380829)Show SMILES Fc1cccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c1F Show InChI InChI=1S/C25H16F2N4O2/c26-19-7-3-6-18(22(19)27)25(33)30-15-10-8-14(9-11-15)29-20-12-13-28-23-21(20)16-4-1-2-5-17(16)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291634
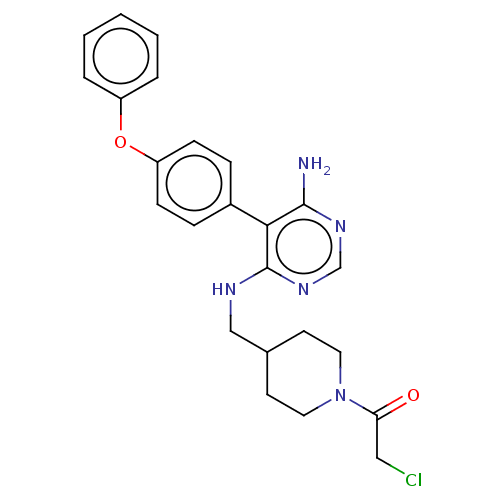
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)CCl)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H26ClN5O2/c25-14-21(31)30-12-10-17(11-13-30)15-27-24-22(23(26)28-16-29-24)18-6-8-20(9-7-18)32-19-4-2-1-3-5-19/h1-9,16-17H,10-15H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433634
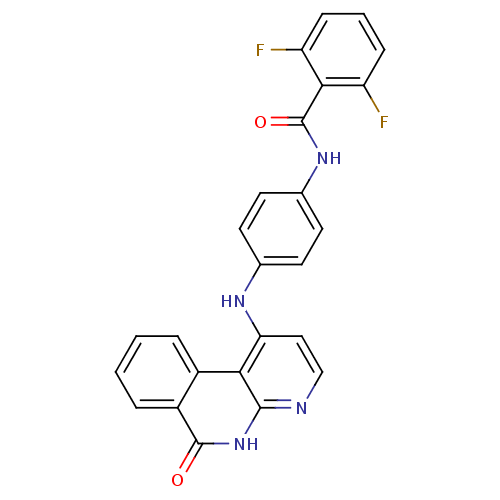
(CHEMBL2380831)Show SMILES Fc1cccc(F)c1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-18-6-3-7-19(27)22(18)25(33)30-15-10-8-14(9-11-15)29-20-12-13-28-23-21(20)16-4-1-2-5-17(16)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433632
(CHEMBL2380833)Show SMILES Fc1cc(F)cc(c1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-15-11-14(12-16(27)13-15)24(32)30-18-7-5-17(6-8-18)29-21-9-10-28-23-22(21)19-3-1-2-4-20(19)25(33)31-23/h1-13H,(H,30,32)(H2,28,29,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433633
(CHEMBL2380832)Show SMILES Fc1ccc(cc1F)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-19-10-5-14(13-20(19)27)24(32)30-16-8-6-15(7-9-16)29-21-11-12-28-23-22(21)17-3-1-2-4-18(17)25(33)31-23/h1-13H,(H,30,32)(H2,28,29,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291389
(5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...)Show SMILES Nc1ncnc(NCC2CCN(CC2)S(=O)(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H27N5O3S/c1-2-33(30,31)29-14-12-18(13-15-29)16-26-24-22(23(25)27-17-28-24)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433629
(CHEMBL2380845)Show SMILES O=C(Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1)c1ccccc1 Show InChI InChI=1S/C25H18N4O2/c30-24(16-6-2-1-3-7-16)28-18-12-10-17(11-13-18)27-21-14-15-26-23-22(21)19-8-4-5-9-20(19)25(31)29-23/h1-15H,(H,28,30)(H2,26,27,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM291512

(N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...)Show SMILES Nc1ncnc(Nc2ccc(Oc3ccccc3)cc2)c1-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-19-8-6-7-17(15-19)23-24(26)27-16-28-25(23)30-18-11-13-21(14-12-18)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433641

(CHEMBL2380824)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H17F3N4O2/c27-26(28,29)20-8-4-3-7-19(20)25(35)32-16-11-9-15(10-12-16)31-21-13-14-30-23-22(21)17-5-1-2-6-18(17)24(34)33-23/h1-14H,(H,32,35)(H2,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433635

(CHEMBL2380830)Show SMILES Fc1ccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c(F)c1 Show InChI InChI=1S/C25H16F2N4O2/c26-14-5-10-19(20(27)13-14)25(33)30-16-8-6-15(7-9-16)29-21-11-12-28-23-22(21)17-3-1-2-4-18(17)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433652
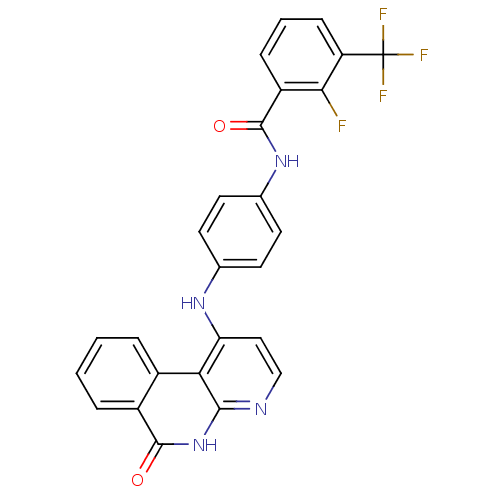
(CHEMBL2380835)Show SMILES Fc1c(cccc1C(F)(F)F)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H16F4N4O2/c27-22-18(6-3-7-19(22)26(28,29)30)25(36)33-15-10-8-14(9-11-15)32-20-12-13-31-23-21(20)16-4-1-2-5-17(16)24(35)34-23/h1-13H,(H,33,36)(H2,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433638
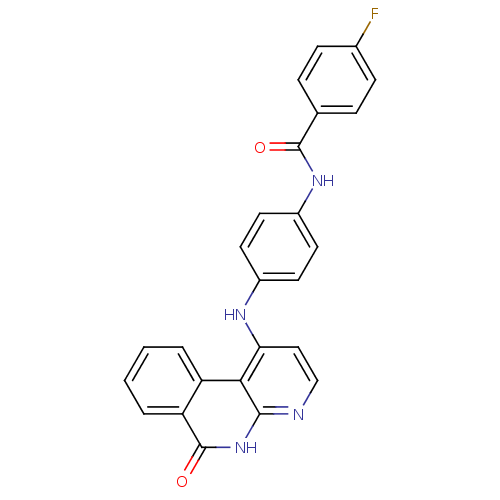
(CHEMBL2380827)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H17FN4O2/c26-16-7-5-15(6-8-16)24(31)29-18-11-9-17(10-12-18)28-21-13-14-27-23-22(21)19-3-1-2-4-20(19)25(32)30-23/h1-14H,(H,29,31)(H2,27,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433640
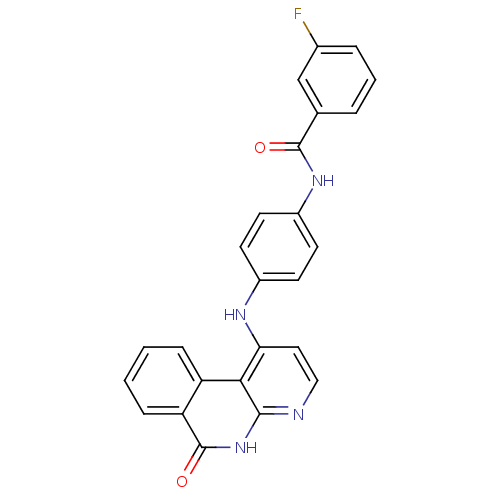
(CHEMBL2380825)Show SMILES Fc1cccc(c1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H17FN4O2/c26-16-5-3-4-15(14-16)24(31)29-18-10-8-17(9-11-18)28-21-12-13-27-23-22(21)19-6-1-2-7-20(19)25(32)30-23/h1-14H,(H,29,31)(H2,27,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433631
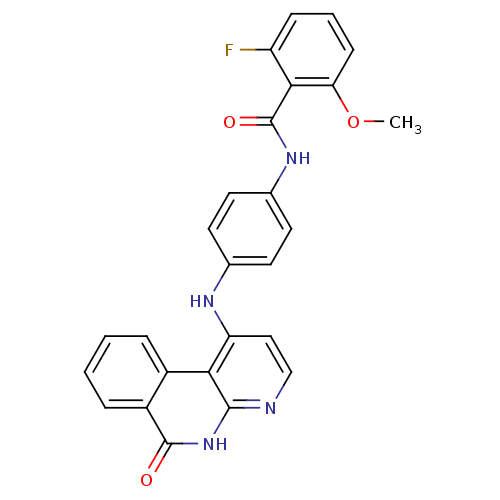
(CHEMBL2380838)Show SMILES COc1cccc(F)c1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H19FN4O3/c1-34-21-8-4-7-19(27)23(21)26(33)30-16-11-9-15(10-12-16)29-20-13-14-28-24-22(20)17-5-2-3-6-18(17)25(32)31-24/h2-14H,1H3,(H,30,33)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433643
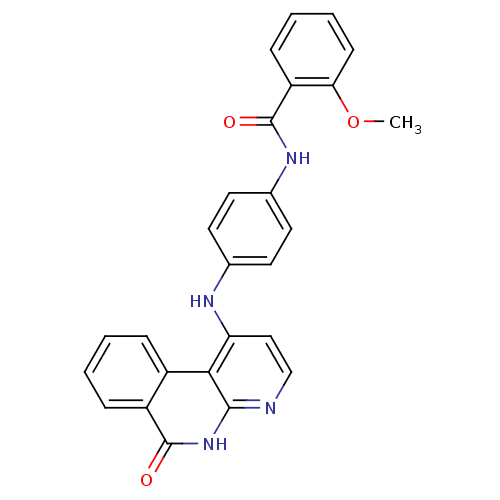
(CHEMBL2380839)Show SMILES COc1ccccc1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H20N4O3/c1-33-22-9-5-4-8-20(22)26(32)29-17-12-10-16(11-13-17)28-21-14-15-27-24-23(21)18-6-2-3-7-19(18)25(31)30-24/h2-15H,1H3,(H,29,32)(H2,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433636

(CHEMBL2380829)Show SMILES Fc1cccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c1F Show InChI InChI=1S/C25H16F2N4O2/c26-19-7-3-6-18(22(19)27)25(33)30-15-10-8-14(9-11-15)29-20-12-13-28-23-21(20)16-4-1-2-5-17(16)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433635

(CHEMBL2380830)Show SMILES Fc1ccc(C(=O)Nc2ccc(Nc3ccnc4[nH]c(=O)c5ccccc5c34)cc2)c(F)c1 Show InChI InChI=1S/C25H16F2N4O2/c26-14-5-10-19(20(27)13-14)25(33)30-16-8-6-15(7-9-16)29-21-11-12-28-23-22(21)17-3-1-2-4-18(17)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433642
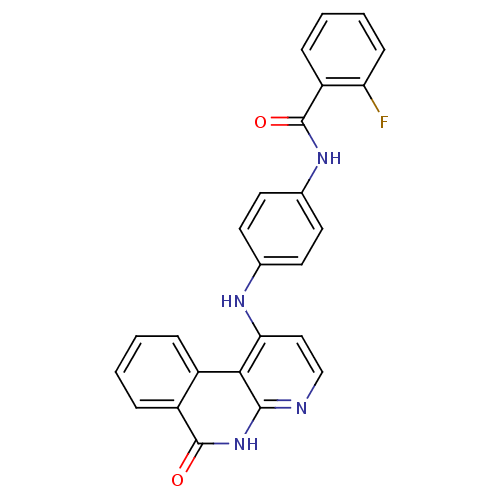
(CHEMBL2380823)Show SMILES Fc1ccccc1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H17FN4O2/c26-20-8-4-3-7-19(20)25(32)29-16-11-9-15(10-12-16)28-21-13-14-27-23-22(21)17-5-1-2-6-18(17)24(31)30-23/h1-14H,(H,29,32)(H2,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433639
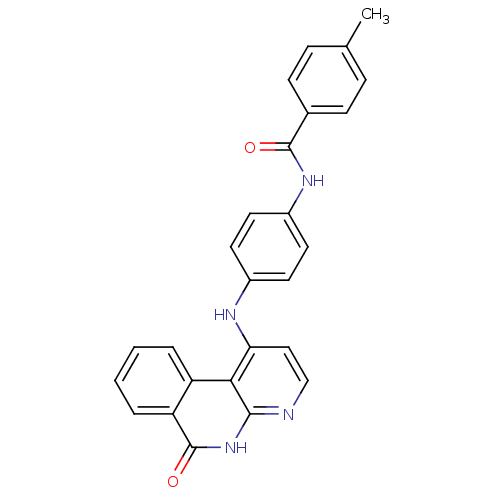
(CHEMBL2380826)Show SMILES Cc1ccc(cc1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H20N4O2/c1-16-6-8-17(9-7-16)25(31)29-19-12-10-18(11-13-19)28-22-14-15-27-24-23(22)20-4-2-3-5-21(20)26(32)30-24/h2-15H,1H3,(H,29,31)(H2,27,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291413

(1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC2CC3(C2)CN(C3)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-21(31)30-14-25(15-30)12-18(13-25)29-24-22(23(26)27-16-28-24)17-8-10-20(11-9-17)32-19-6-4-3-5-7-19/h2-11,16,18H,1,12-15H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291513
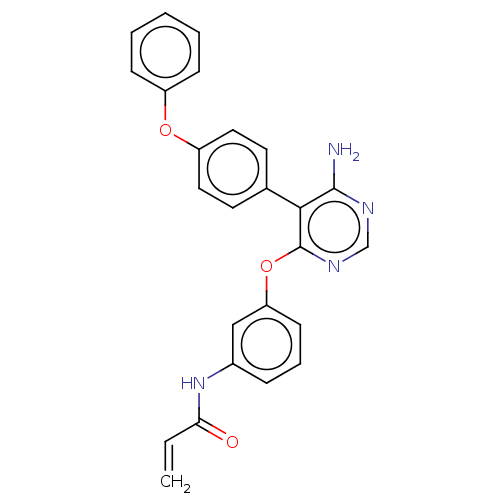
(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)o...)Show SMILES Nc1ncnc(Oc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H20N4O3/c1-2-22(30)29-18-7-6-10-21(15-18)32-25-23(24(26)27-16-28-25)17-11-13-20(14-12-17)31-19-8-4-3-5-9-19/h2-16H,1H2,(H,29,30)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433639

(CHEMBL2380826)Show SMILES Cc1ccc(cc1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H20N4O2/c1-16-6-8-17(9-7-16)25(31)29-19-12-10-18(11-13-19)28-22-14-15-27-24-23(22)20-4-2-3-5-21(20)26(32)30-24/h2-15H,1H3,(H,29,31)(H2,27,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433631

(CHEMBL2380838)Show SMILES COc1cccc(F)c1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H19FN4O3/c1-34-21-8-4-7-19(27)23(21)26(33)30-16-11-9-15(10-12-16)29-20-13-14-28-24-22(20)17-5-2-3-6-18(17)25(32)31-24/h2-14H,1H3,(H,30,33)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
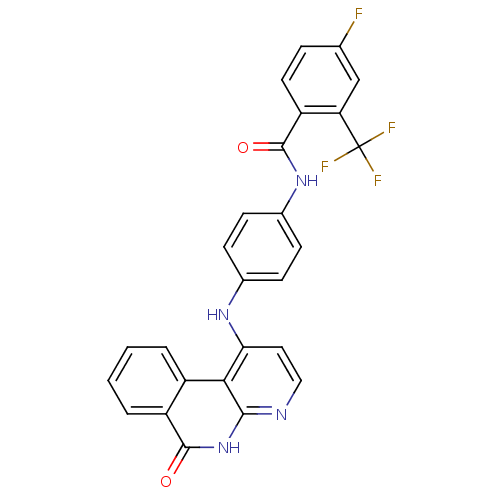
Tyrosine-protein kinase BTK
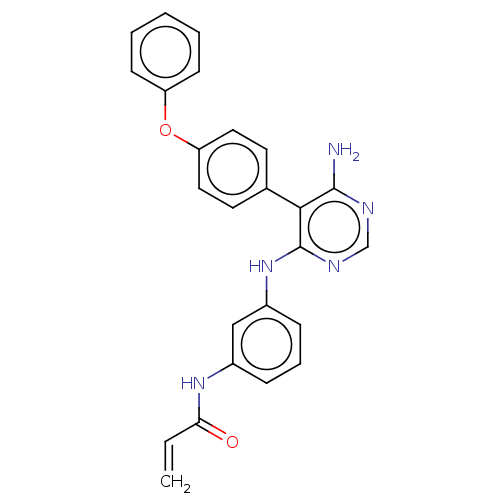
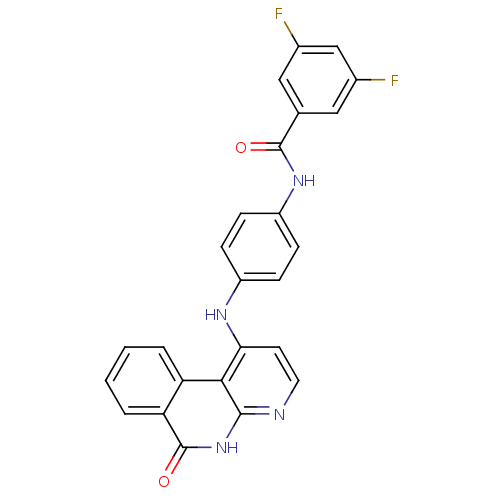
(Homo sapiens (Human)) | BDBM291573

(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433641

(CHEMBL2380824)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H17F3N4O2/c27-26(28,29)20-8-4-3-7-19(20)25(35)32-16-11-9-15(10-12-16)31-21-13-14-30-23-22(21)17-5-1-2-6-18(17)24(34)33-23/h1-14H,(H,32,35)(H2,30,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433638

(CHEMBL2380827)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H17FN4O2/c26-16-7-5-15(6-8-16)24(31)29-18-11-9-17(10-12-18)28-21-13-14-27-23-22(21)19-3-1-2-4-20(19)25(32)30-23/h1-14H,(H,29,31)(H2,27,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
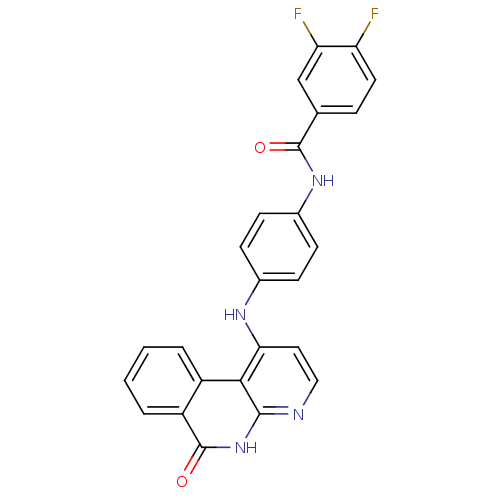
(Homo sapiens (Human)) | BDBM291573

(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
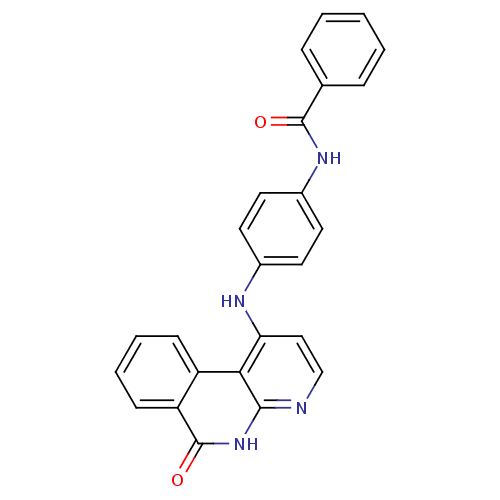
(Homo sapiens (Human)) | BDBM50433629

(CHEMBL2380845)Show SMILES O=C(Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1)c1ccccc1 Show InChI InChI=1S/C25H18N4O2/c30-24(16-6-2-1-3-7-16)28-18-12-10-17(11-13-18)27-21-14-15-26-23-22(21)19-8-4-5-9-20(19)25(31)29-23/h1-15H,(H,28,30)(H2,26,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291515
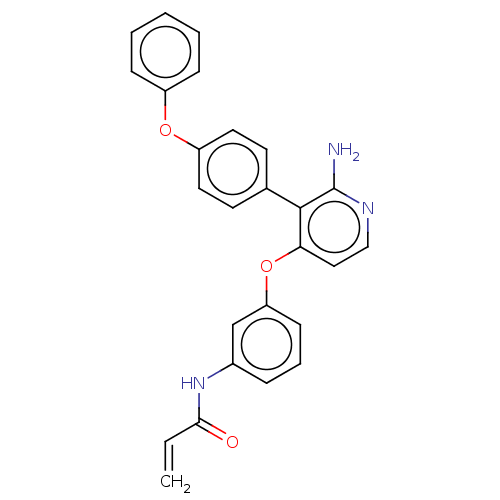
(N-(3-((2-amino-3-(4-phenoxyphenyl)pyridin-4-yl)oxy...)Show SMILES Nc1nccc(Oc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H21N3O3/c1-2-24(30)29-19-7-6-10-22(17-19)32-23-15-16-28-26(27)25(23)18-11-13-21(14-12-18)31-20-8-4-3-5-9-20/h2-17H,1H2,(H2,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433634

(CHEMBL2380831)Show SMILES Fc1cccc(F)c1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-18-6-3-7-19(27)22(18)25(33)30-15-10-8-14(9-11-15)29-20-12-13-28-23-21(20)16-4-1-2-5-17(16)24(32)31-23/h1-13H,(H,30,33)(H2,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291425
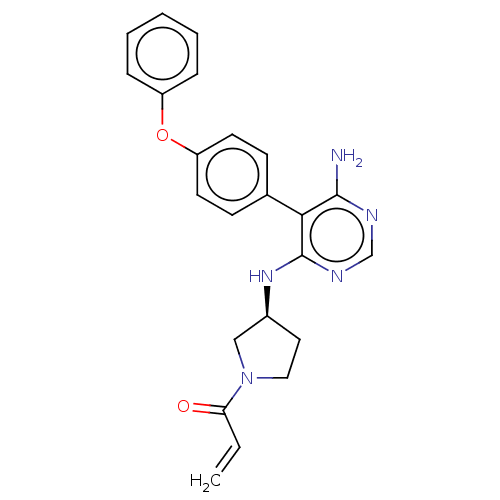
((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...)Show SMILES Nc1ncnc(N[C@H]2CCN(C2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H23N5O2/c1-2-20(29)28-13-12-17(14-28)27-23-21(22(24)25-15-26-23)16-8-10-19(11-9-16)30-18-6-4-3-5-7-18/h2-11,15,17H,1,12-14H2,(H3,24,25,26,27)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
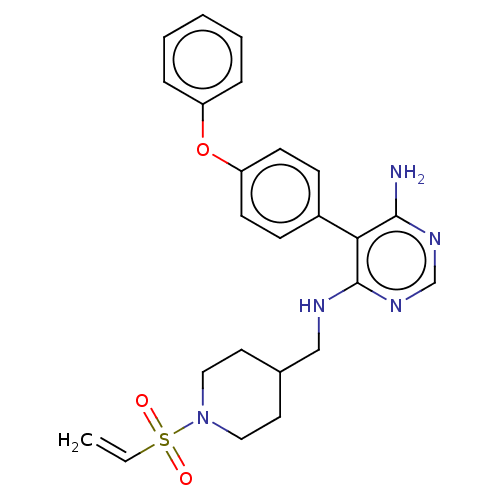
(Homo sapiens (Human)) | BDBM291625

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2(O)CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O3/c1-2-21(31)30-14-12-25(32,13-15-30)16-27-24-22(23(26)28-17-29-24)18-8-10-20(11-9-18)33-19-6-4-3-5-7-19/h2-11,17,32H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50433637
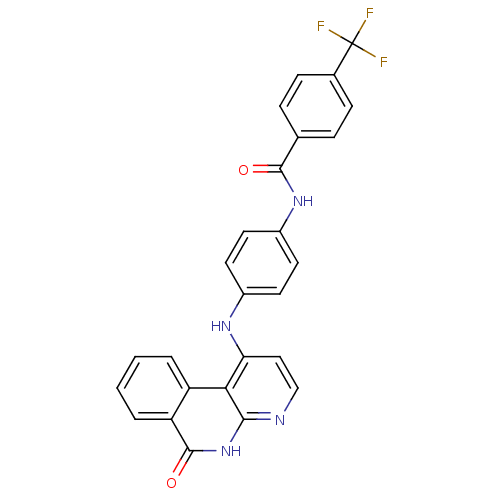
(CHEMBL2380828)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H17F3N4O2/c27-26(28,29)16-7-5-15(6-8-16)24(34)32-18-11-9-17(10-12-18)31-21-13-14-30-23-22(21)19-3-1-2-4-20(19)25(35)33-23/h1-14H,(H,32,34)(H2,30,31,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-B (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291549

((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...)Show SMILES Nc1ncnc(N[C@H]2CCCN(C2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C24H25N5O2/c1-2-21(30)29-14-6-7-18(15-29)28-24-22(23(25)26-16-27-24)17-10-12-20(13-11-17)31-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H3,25,26,27,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433643

(CHEMBL2380839)Show SMILES COc1ccccc1C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H20N4O3/c1-33-22-9-5-4-8-20(22)26(32)29-17-12-10-16(11-13-17)28-21-14-15-27-24-23(21)18-6-2-3-7-19(18)25(31)30-24/h2-15H,1H3,(H,29,32)(H2,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433633

(CHEMBL2380832)Show SMILES Fc1ccc(cc1F)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-19-10-5-14(13-20(19)27)24(32)30-16-8-6-15(7-9-16)29-21-11-12-28-23-22(21)17-3-1-2-4-18(17)25(33)31-23/h1-13H,(H,30,32)(H2,28,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433652

(CHEMBL2380835)Show SMILES Fc1c(cccc1C(F)(F)F)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C26H16F4N4O2/c27-22-18(6-3-7-19(22)26(28,29)30)25(36)33-15-10-8-14(9-11-15)32-20-12-13-31-23-21(20)16-4-1-2-5-17(16)24(35)34-23/h1-13H,(H,33,36)(H2,31,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433640

(CHEMBL2380825)Show SMILES Fc1cccc(c1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H17FN4O2/c26-16-5-3-4-15(14-16)24(31)29-18-10-8-17(9-11-18)28-21-12-13-27-23-22(21)19-6-1-2-7-20(19)25(32)30-23/h1-14H,(H,29,31)(H2,27,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50433632

(CHEMBL2380833)Show SMILES Fc1cc(F)cc(c1)C(=O)Nc1ccc(Nc2ccnc3[nH]c(=O)c4ccccc4c23)cc1 Show InChI InChI=1S/C25H16F2N4O2/c26-15-11-14(12-16(27)13-15)24(32)30-18-7-5-17(6-8-18)29-21-9-10-28-23-22(21)19-3-1-2-4-20(19)25(33)31-23/h1-13H,(H,30,32)(H2,28,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
Bioorg Med Chem Lett 23: 3081-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.008
BindingDB Entry DOI: 10.7270/Q2QN686Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291512

(N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...)Show SMILES Nc1ncnc(Nc2ccc(Oc3ccccc3)cc2)c1-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-19-8-6-7-17(15-19)23-24(26)27-16-28-25(23)30-18-11-13-21(14-12-18)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data