 Found 373 hits with Last Name = 'silakari' and Initial = 'o'
Found 373 hits with Last Name = 'silakari' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394355
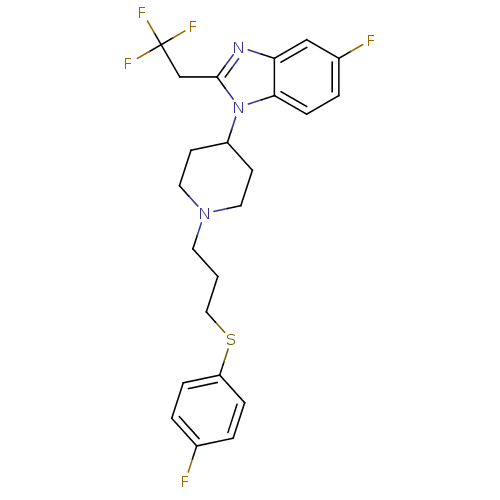
(CHEMBL2159766)Show SMILES Fc1ccc(SCCCN2CCC(CC2)n2c(CC(F)(F)F)nc3cc(F)ccc23)cc1 Show InChI InChI=1S/C23H24F5N3S/c24-16-2-5-19(6-3-16)32-13-1-10-30-11-8-18(9-12-30)31-21-7-4-17(25)14-20(21)29-22(31)15-23(26,27)28/h2-7,14,18H,1,8-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of CCR3 receptor |
Bioorg Med Chem 20: 6208-36 (2012)
Article DOI: 10.1016/j.bmc.2012.09.013
BindingDB Entry DOI: 10.7270/Q20V8DWW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394356
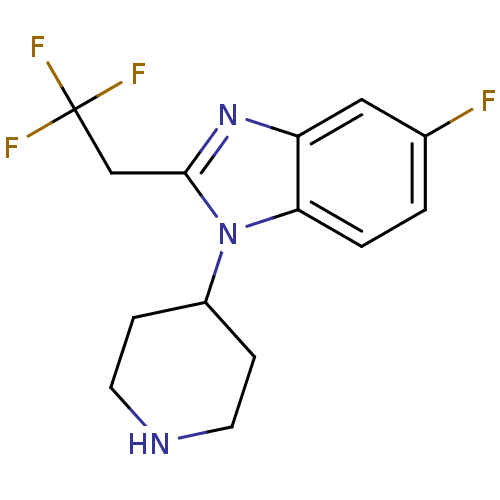
(CHEMBL2159765)Show InChI InChI=1S/C14H15F4N3/c15-9-1-2-12-11(7-9)20-13(8-14(16,17)18)21(12)10-3-5-19-6-4-10/h1-2,7,10,19H,3-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of CCR3 receptor |
Bioorg Med Chem 20: 6208-36 (2012)
Article DOI: 10.1016/j.bmc.2012.09.013
BindingDB Entry DOI: 10.7270/Q20V8DWW |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50050769
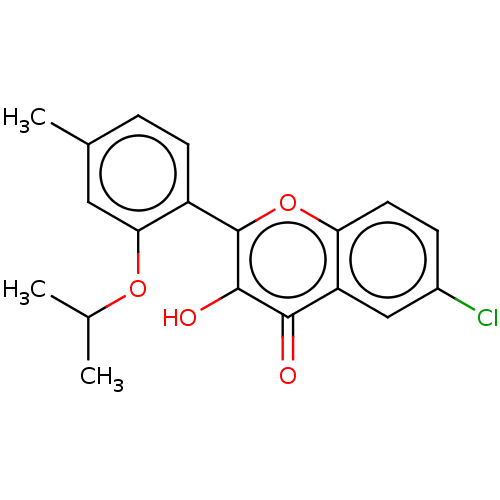
(CHEMBL3318019)Show InChI InChI=1S/C19H17ClO4/c1-10(2)23-16-8-11(3)4-6-13(16)19-18(22)17(21)14-9-12(20)5-7-15(14)24-19/h4-10,22H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50016784
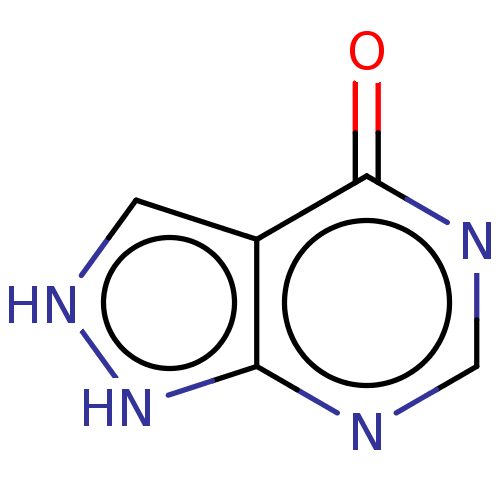
(ALLOPURINOL | BW-56-158 | BW-56158 | CHEBI:40279 |...)Show InChI InChI=1S/C5H2N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated CFTR (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10512
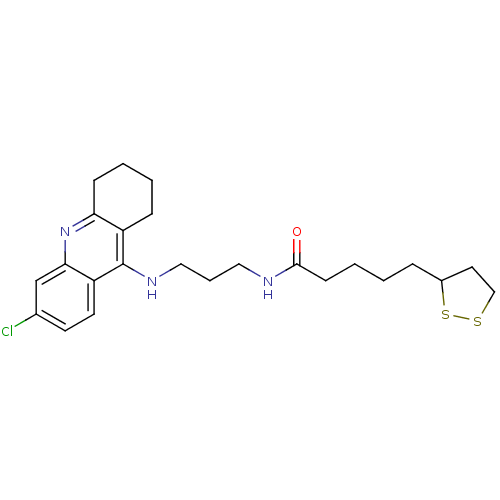
(CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...)Show SMILES Clc1ccc2c(NCCCNC(=O)CCCCC3CCSS3)c3CCCCc3nc2c1 Show InChI InChI=1S/C24H32ClN3OS2/c25-17-10-11-20-22(16-17)28-21-8-3-2-7-19(21)24(20)27-14-5-13-26-23(29)9-4-1-6-18-12-15-30-31-18/h10-11,16,18H,1-9,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 151: 62-97 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.057
BindingDB Entry DOI: 10.7270/Q2NC63V1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50613673

(CHEMBL1964259)Show SMILES OCCOCCNS(=O)(=O)c1ccc(N\C=C2/C(=O)Nc3ccc4ncsc4c23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094551
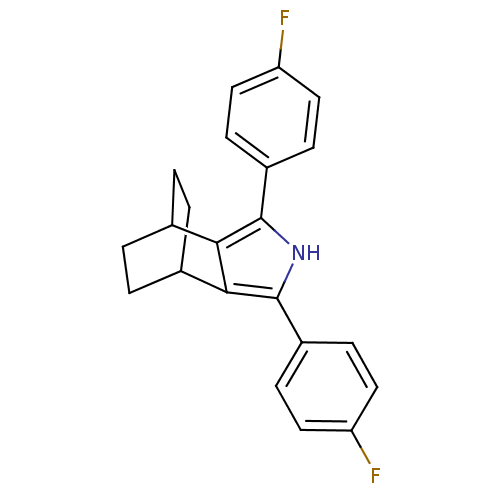
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.2.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(CC3)c12)-c1ccc(F)cc1 |(21.01,-36.41,;22.48,-35.94,;22.8,-34.43,;24.27,-33.96,;25.41,-35,;25.09,-36.5,;23.63,-36.98,;26.88,-34.53,;28.12,-35.45,;29.38,-34.55,;28.9,-33.08,;29.67,-31.77,;28.93,-30.43,;27.39,-30.42,;26.61,-31.74,;27.93,-30.96,;28.33,-32.53,;27.36,-33.07,;30.84,-35.03,;31.15,-36.54,;32.61,-37.02,;33.76,-36,;35.23,-36.48,;33.44,-34.48,;31.98,-34,)| Show InChI InChI=1S/C22H19F2N/c23-17-9-5-15(6-10-17)21-19-13-1-2-14(4-3-13)20(19)22(25-21)16-7-11-18(24)12-8-16/h5-14,25H,1-4H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.601 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094542
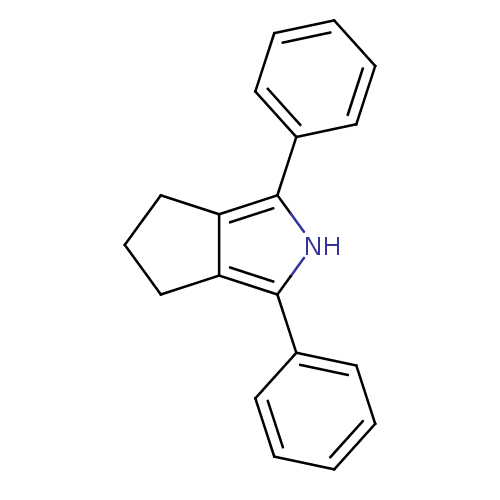
(1,3-Diphenyl-2,4,5,6-tetrahydro-cyclopenta[c]pyrro...)Show InChI InChI=1S/C19H17N/c1-3-8-14(9-4-1)18-16-12-7-13-17(16)19(20-18)15-10-5-2-6-11-15/h1-6,8-11,20H,7,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.701 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094539
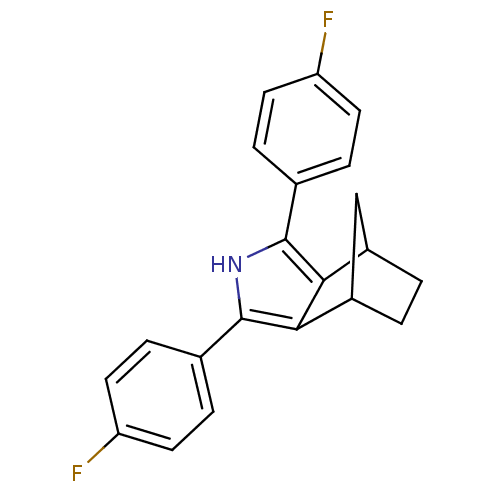
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(C3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C21H17F2N/c22-16-7-3-12(4-8-16)20-18-14-1-2-15(11-14)19(18)21(24-20)13-5-9-17(23)10-6-13/h3-10,14-15,24H,1-2,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50462398
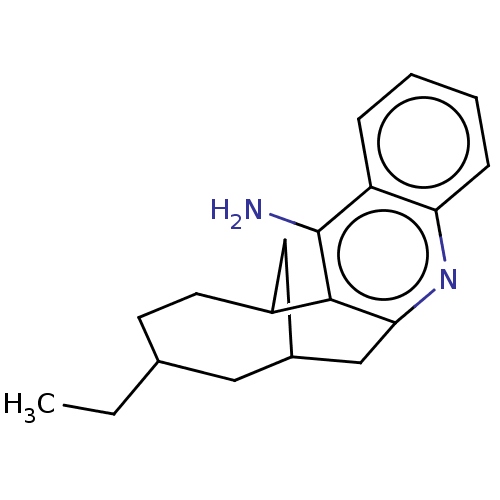
(CHEMBL4249848)Show SMILES CCC1CCC2CC(Cc3nc4ccccc4c(N)c23)C1 |TLB:17:19:6:4.3.2.20,THB:1:2:6:9.19.8| Show InChI InChI=1S/C19H24N2/c1-2-12-7-8-14-10-13(9-12)11-17-18(14)19(20)15-5-3-4-6-16(15)21-17/h3-6,12-14H,2,7-11H2,1H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AchE using acetylthiocholine iodide as substrate after 15 mins by spectrophotometric analysis |
Eur J Med Chem 151: 62-97 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.057
BindingDB Entry DOI: 10.7270/Q2NC63V1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50462402
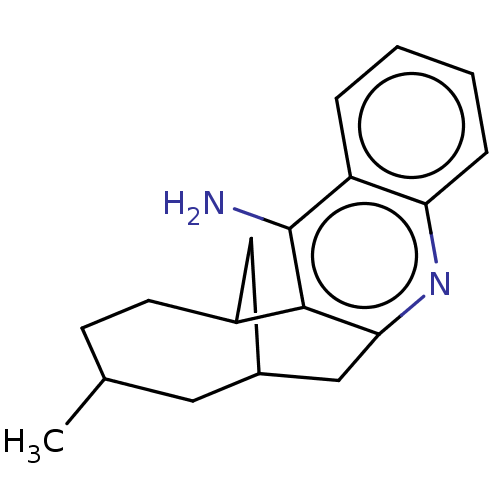
(CHEMBL4249379)Show SMILES CC1CCC2CC(Cc3nc4ccccc4c(N)c23)C1 |TLB:16:18:5:3.2.1.19,THB:0:1:5:8.18.7| Show InChI InChI=1S/C18H22N2/c1-11-6-7-13-9-12(8-11)10-16-17(13)18(19)14-4-2-3-5-15(14)20-16/h2-5,11-13H,6-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AchE using acetylthiocholine iodide as substrate after 15 mins by spectrophotometric analysis |
Eur J Med Chem 151: 62-97 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.057
BindingDB Entry DOI: 10.7270/Q2NC63V1 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126727
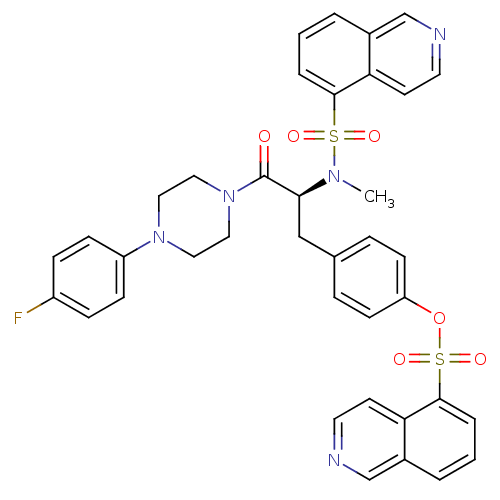
((S)-4-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-(N-m...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccc(F)cc1)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C38H34FN5O6S2/c1-42(51(46,47)36-6-2-4-28-25-40-18-16-33(28)36)35(38(45)44-22-20-43(21-23-44)31-12-10-30(39)11-13-31)24-27-8-14-32(15-9-27)50-52(48,49)37-7-3-5-29-26-41-19-17-34(29)37/h2-19,25-26,35H,20-24H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094540
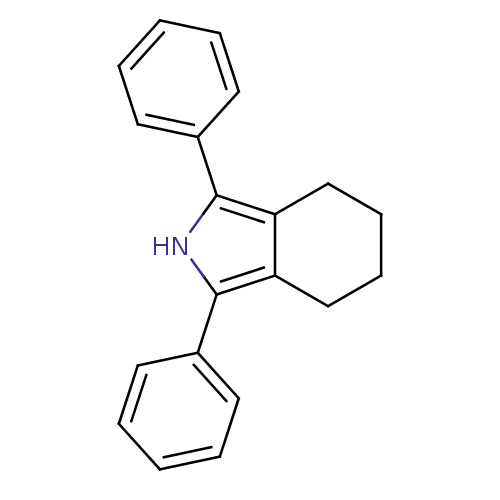
(1,3-diphenyl-4,5,6,7-tetrahydro-2H-isoindole | CHE...)Show InChI InChI=1S/C20H19N/c1-3-9-15(10-4-1)19-17-13-7-8-14-18(17)20(21-19)16-11-5-2-6-12-16/h1-6,9-12,21H,7-8,13-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Reversible inhibition of NH2-terminal 6His-tagged FAK catalytic domain (410 to 689 residues) (unknown origin) expressed in Sf9 insect cells using p(G... |
Eur J Med Chem 134: 159-184 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.003
BindingDB Entry DOI: 10.7270/Q23X88SH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094546
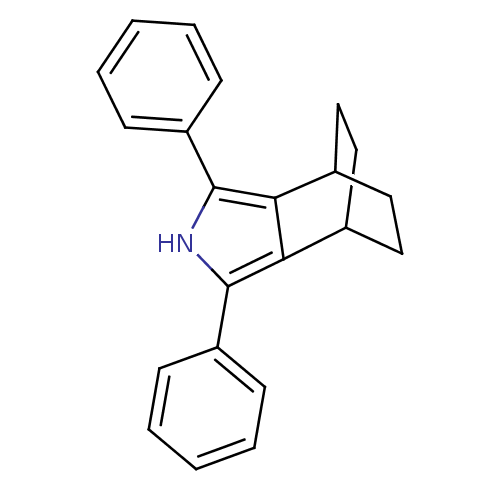
(3,5-Diphenyl-4-aza-tricyclo[5.2.2.0*2,6*]undeca-2,...)Show SMILES C1CC2CCC1c1c([nH]c(c21)-c1ccccc1)-c1ccccc1 |(14.74,-30.53,;13.2,-30.52,;12.42,-31.84,;13.75,-31.06,;14.15,-32.63,;15.49,-31.87,;14.71,-33.19,;15.19,-34.65,;13.94,-35.55,;12.69,-34.64,;13.18,-33.17,;11.23,-35.11,;10.08,-34.06,;8.61,-34.53,;8.28,-36.04,;9.44,-37.08,;10.9,-36.61,;16.65,-35.13,;16.96,-36.64,;18.43,-37.13,;19.58,-36.1,;19.26,-34.58,;17.8,-34.11,)| Show InChI InChI=1S/C22H21N/c1-3-7-17(8-4-1)21-19-15-11-13-16(14-12-15)20(19)22(23-21)18-9-5-2-6-10-18/h1-10,15-16,23H,11-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094534
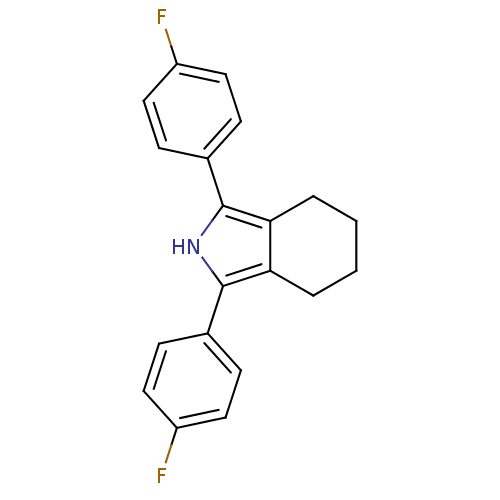
(1,3-Bis-(4-fluoro-phenyl)-4,5,6,7-tetrahydro-2H-is...)Show InChI InChI=1S/C20H17F2N/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)20(23-19)14-7-11-16(22)12-8-14/h5-12,23H,1-4H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094532
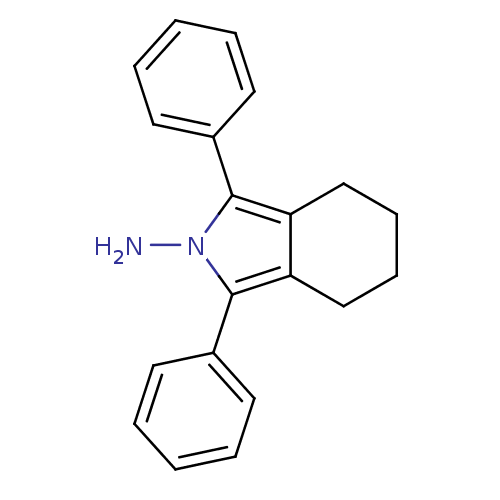
(1,3-Diphenyl-4,5,6,7-tetrahydro-isoindol-2-ylamine...)Show InChI InChI=1S/C20H20N2/c21-22-19(15-9-3-1-4-10-15)17-13-7-8-14-18(17)20(22)16-11-5-2-6-12-16/h1-6,9-12H,7-8,13-14,21H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10112
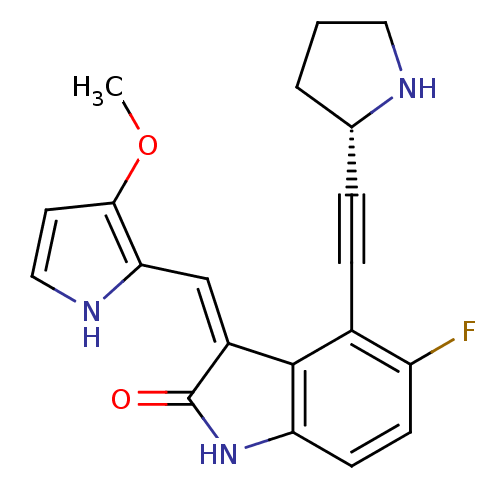
((3Z)-5-fluoro-3-[(3-methoxy-1H-pyrrol-2-yl)methyli...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H]3CCCN3)c12 |r| Show InChI InChI=1S/C20H18FN3O2/c1-26-18-8-10-23-17(18)11-14-19-13(5-4-12-3-2-9-22-12)15(21)6-7-16(19)24-20(14)25/h6-8,10-12,22-23H,2-3,9H2,1H3,(H,24,25)/b14-11-/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094558
(3,5-Diphenyl-4-aza-tricyclo[5.2.1.0*2,6*]deca-2,5-...)Show InChI InChI=1S/C21H19N/c1-3-7-14(8-4-1)20-18-16-11-12-17(13-16)19(18)21(22-20)15-9-5-2-6-10-15/h1-10,16-17,22H,11-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094548
(1,3-Bis-(4-fluoro-phenyl)-2,4,5,6-tetrahydro-cyclo...)Show InChI InChI=1S/C19H15F2N/c20-14-8-4-12(5-9-14)18-16-2-1-3-17(16)19(22-18)13-6-10-15(21)11-7-13/h4-11,22H,1-3H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10109
((3Z)-4-[(3R,4S,5R)-4-amino-3,5-dihydroxyhex-1-yn-1...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H](O)[C@@H](N)[C@@H](C)O)c12 |r| Show InChI InChI=1S/C20H20FN3O4/c1-10(25)19(22)16(26)6-3-11-13(21)4-5-14-18(11)12(20(27)24-14)9-15-17(28-2)7-8-23-15/h4-5,7-10,16,19,23,25-26H,22H2,1-2H3,(H,24,27)/b12-9-/t10-,16-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094528
(5,6-Dimethyl-1,3-diphenyl-4,5,6,7-tetrahydro-2H-is...)Show InChI InChI=1S/C22H23N/c1-15-13-19-20(14-16(15)2)22(18-11-7-4-8-12-18)23-21(19)17-9-5-3-6-10-17/h3-12,15-16,23H,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094535
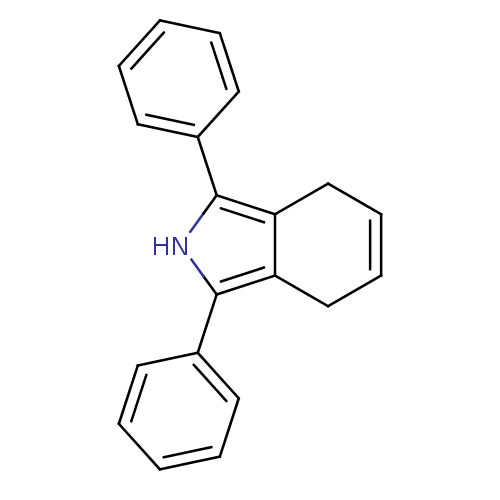
(1,3-diphenyl-4,7-dihydro-2H-isoindole | CHEMBL1408...)Show SMILES C1C=CCc2c1c([nH]c2-c1ccccc1)-c1ccccc1 |c:1| Show InChI InChI=1S/C20H17N/c1-3-9-15(10-4-1)19-17-13-7-8-14-18(17)20(21-19)16-11-5-2-6-12-16/h1-12,21H,13-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094561
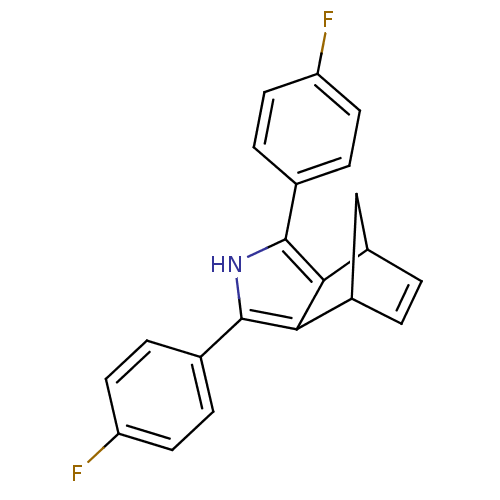
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CC(C=C3)c12)-c1ccc(F)cc1 |c:15| Show InChI InChI=1S/C21H15F2N/c22-16-7-3-12(4-8-16)20-18-14-1-2-15(11-14)19(18)21(24-20)13-5-9-17(23)10-6-13/h1-10,14-15,24H,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50094541
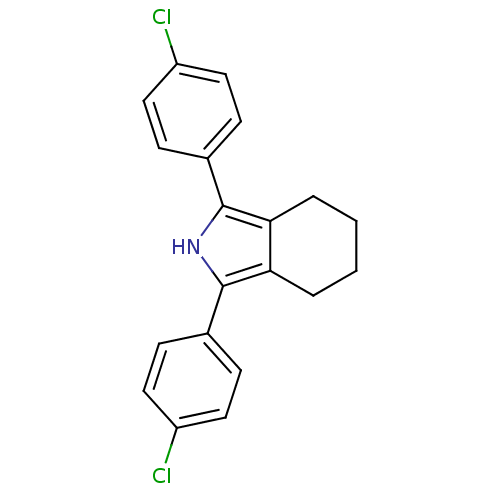
(1,3-Bis-(4-chloro-phenyl)-4,5,6,7-tetrahydro-2H-is...)Show SMILES Clc1ccc(cc1)-c1[nH]c(c2CCCCc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H17Cl2N/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)20(23-19)14-7-11-16(22)12-8-14/h5-12,23H,1-4H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr HS Gour University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage COX2 |
Eur J Med Chem 43: 1559-69 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.028
BindingDB Entry DOI: 10.7270/Q2M909WP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of spleen tyrosine kinase (unknown origin) |
Eur J Med Chem 67: 434-46 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.070
BindingDB Entry DOI: 10.7270/Q2Z039JB |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50207475
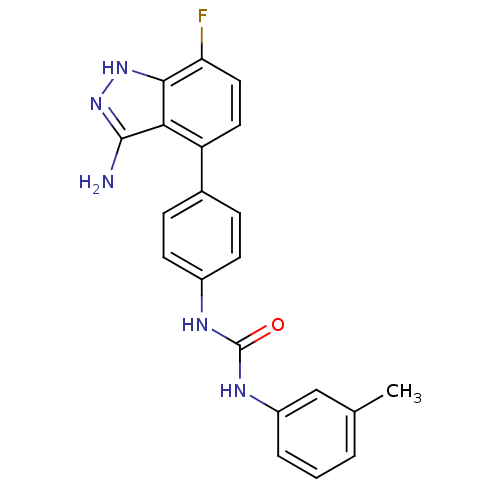
(1-(4-(3-amino-7-fluoro-1H-indazol-4-yl)phenyl)-3-m...)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2ccc(F)c3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-3-2-4-15(11-12)25-21(28)24-14-7-5-13(6-8-14)16-9-10-17(22)19-18(16)20(23)27-26-19/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit by HTRF method |
Eur J Med Chem 45: 393-404 (2010)
Article DOI: 10.1016/j.ejmech.2009.09.013
BindingDB Entry DOI: 10.7270/Q2RX9C6D |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50404086
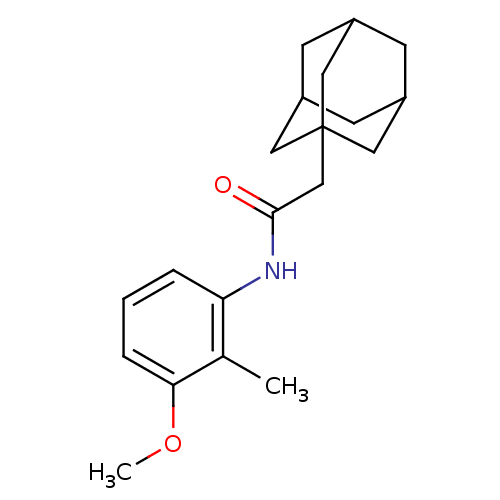
(CHEMBL341226)Show SMILES COc1cccc(NC(=O)CC23CC4CC(CC(C4)C2)C3)c1C |TLB:20:11:18:15.14.16,THB:19:11:14:17.18.16,19:17:11.12.20:14,20:15:11.12.19:18| Show InChI InChI=1S/C20H27NO2/c1-13-17(4-3-5-18(13)23-2)21-19(22)12-20-9-14-6-15(10-20)8-16(7-14)11-20/h3-5,14-16H,6-12H2,1-2H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126702
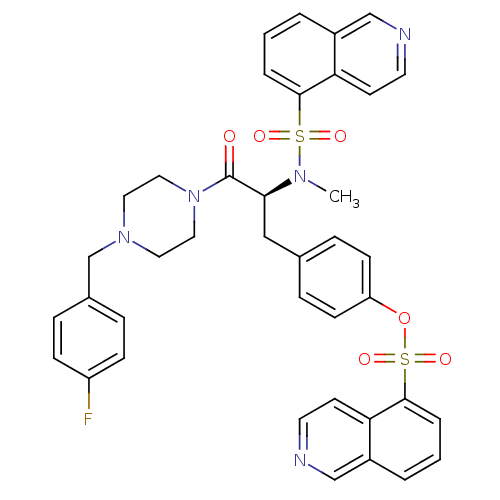
(CHEMBL282807 | Isoquinoline-5-sulfonic acid 4-{(S)...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(Cc2ccc(F)cc2)CC1)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C39H36FN5O6S2/c1-43(52(47,48)37-6-2-4-30-25-41-18-16-34(30)37)36(39(46)45-22-20-44(21-23-45)27-29-8-12-32(40)13-9-29)24-28-10-14-33(15-11-28)51-53(49,50)38-7-3-5-31-26-42-19-17-35(31)38/h2-19,25-26,36H,20-24,27H2,1H3/t36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126713
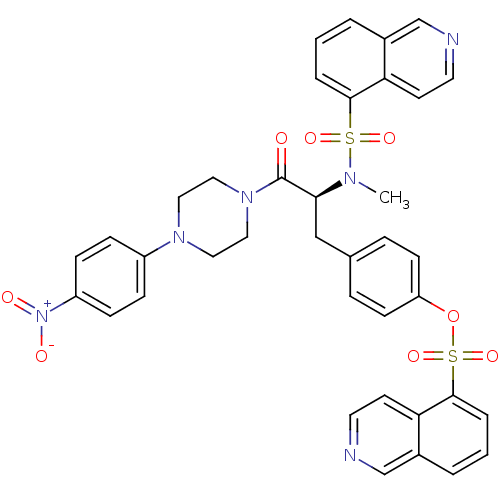
(CHEMBL282173 | Isoquinoline-5-sulfonic acid 4-{(S)...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccc(cc1)[N+]([O-])=O)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C38H34N6O8S2/c1-41(53(48,49)36-6-2-4-28-25-39-18-16-33(28)36)35(38(45)43-22-20-42(21-23-43)30-10-12-31(13-11-30)44(46)47)24-27-8-14-32(15-9-27)52-54(50,51)37-7-3-5-29-26-40-19-17-34(29)37/h2-19,25-26,35H,20-24H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451951

(CHEMBL4217941)Show SMILES COc1cc(cc(OC)c1OC)-c1cc(=O)c2ccc(OCCN3CCC(C)CC3)cc2o1 Show InChI InChI=1S/C26H31NO6/c1-17-7-9-27(10-8-17)11-12-32-19-5-6-20-21(28)16-22(33-23(20)15-19)18-13-24(29-2)26(31-4)25(14-18)30-3/h5-6,13-17H,7-12H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM8826
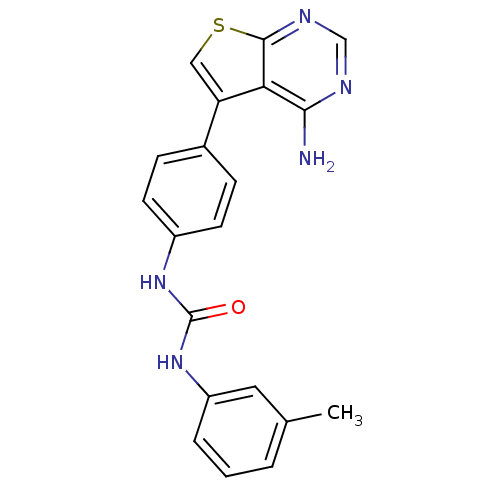
(3-(4-{4-aminothieno[2,3-d]pyrimidin-5-yl}phenyl)-1...)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3ncnc(N)c23)c1 Show InChI InChI=1S/C20H17N5OS/c1-12-3-2-4-15(9-12)25-20(26)24-14-7-5-13(6-8-14)16-10-27-19-17(16)18(21)22-11-23-19/h2-11H,1H3,(H2,21,22,23)(H2,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit by HTRF method |
Eur J Med Chem 45: 393-404 (2010)
Article DOI: 10.1016/j.ejmech.2009.09.013
BindingDB Entry DOI: 10.7270/Q2RX9C6D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451934
(CHEMBL4207594)Show SMILES COc1cc(cc(OC)c1OC)-c1cc(=O)c2ccc(OCCCN3CCC(C)CC3)cc2o1 Show InChI InChI=1S/C27H33NO6/c1-18-8-11-28(12-9-18)10-5-13-33-20-6-7-21-22(29)17-23(34-24(21)16-20)19-14-25(30-2)27(32-4)26(15-19)31-3/h6-7,14-18H,5,8-13H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451935
(CHEMBL4208825)Show SMILES COc1ccc(cc1OC)-c1cc(=O)c2ccc(OCCCN3CCC(C)CC3)cc2o1 Show InChI InChI=1S/C26H31NO5/c1-18-9-12-27(13-10-18)11-4-14-31-20-6-7-21-22(28)17-24(32-25(21)16-20)19-5-8-23(29-2)26(15-19)30-3/h5-8,15-18H,4,9-14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451931
(CHEMBL4213552)Show SMILES COc1ccc(cc1OC)-c1cc(=O)c2ccc(OCCN3CCC(C)CC3)cc2o1 Show InChI InChI=1S/C25H29NO5/c1-17-8-10-26(11-9-17)12-13-30-19-5-6-20-21(27)16-23(31-24(20)15-19)18-4-7-22(28-2)25(14-18)29-3/h4-7,14-17H,8-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50258204
(2-(4-tert-butylphenyl)-4-(4-((5-ethyl-1-methyl-1H-...)Show SMILES CCc1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)nn1C Show InChI InChI=1S/C28H36N6/c1-6-23-18-22(31-32(23)5)19-33-14-16-34(17-15-33)25-9-7-8-24-26(25)30-27(29-24)20-10-12-21(13-11-20)28(2,3)4/h7-13,18H,6,14-17,19H2,1-5H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of GnRH receptor |
Bioorg Med Chem 20: 6208-36 (2012)
Article DOI: 10.1016/j.bmc.2012.09.013
BindingDB Entry DOI: 10.7270/Q20V8DWW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50366929
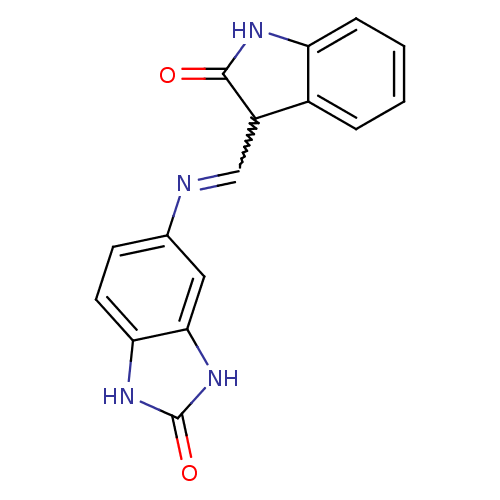
(CHEMBL1794057)Show SMILES O=C1Nc2ccccc2C1C=Nc1ccc2[nH]c(=O)[nH]c2c1 |w:10.11| Show InChI InChI=1S/C16H12N4O2/c21-15-11(10-3-1-2-4-12(10)18-15)8-17-9-5-6-13-14(7-9)20-16(22)19-13/h1-8,11H,(H,18,21)(H2,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451962
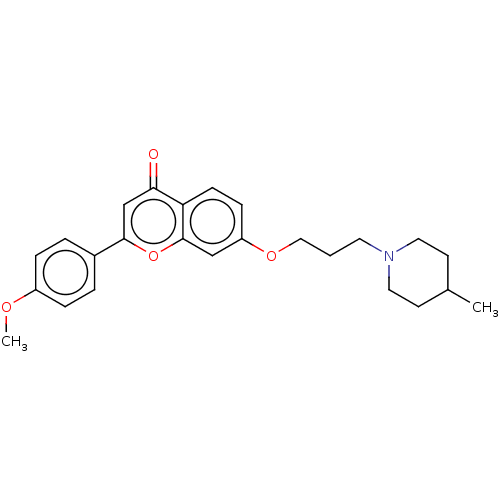
(CHEMBL4214058)Show SMILES COc1ccc(cc1)-c1cc(=O)c2ccc(OCCCN3CCC(C)CC3)cc2o1 Show InChI InChI=1S/C25H29NO4/c1-18-10-13-26(14-11-18)12-3-15-29-21-8-9-22-23(27)17-24(30-25(22)16-21)19-4-6-20(28-2)7-5-19/h4-9,16-18H,3,10-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50404085
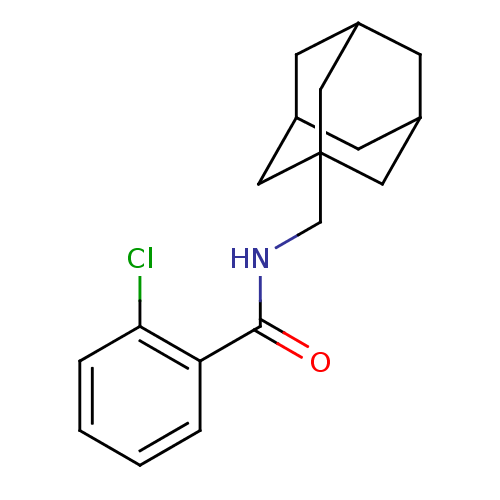
(CHEMBL336619)Show SMILES Clc1ccccc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:18:13:20:17.19.16,18:17:20:13.12.14,THB:16:17:12:15.20.14,16:15:12:17.19.18| Show InChI InChI=1S/C18H22ClNO/c19-16-4-2-1-3-15(16)17(21)20-11-18-8-12-5-13(9-18)7-14(6-12)10-18/h1-4,12-14H,5-11H2,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50613675
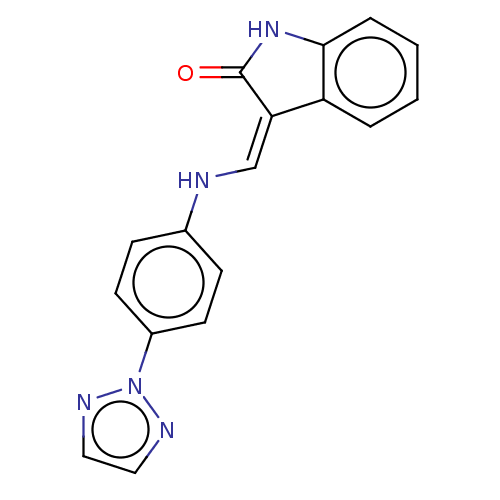
(CHEMBL5275659) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50126413
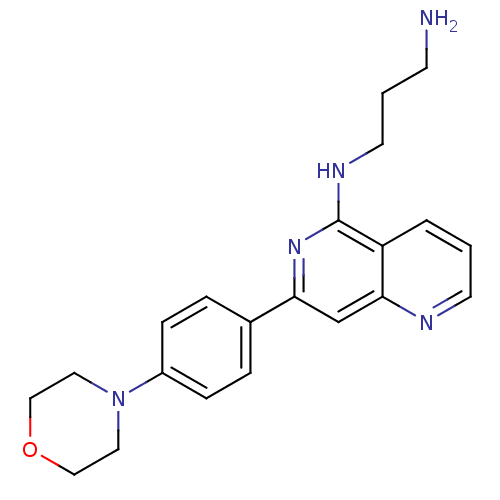
(CHEMBL287478 | N*1*-[7-(4-Morpholin-4-yl-phenyl)-[...)Show InChI InChI=1S/C21H25N5O/c22-8-2-10-24-21-18-3-1-9-23-20(18)15-19(25-21)16-4-6-17(7-5-16)26-11-13-27-14-12-26/h1,3-7,9,15H,2,8,10-14,22H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of spleen tyrosine kinase (unknown origin) |
Eur J Med Chem 67: 434-46 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.070
BindingDB Entry DOI: 10.7270/Q2Z039JB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50451932
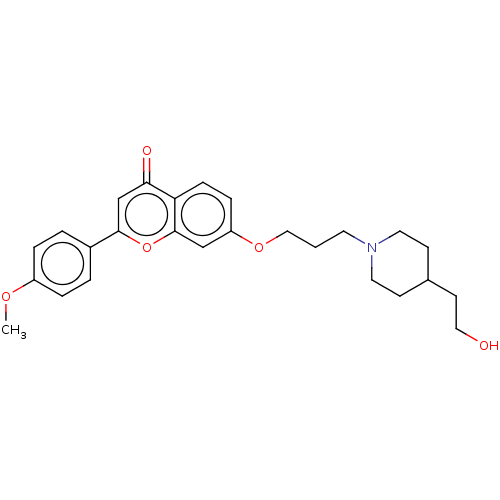
(CHEMBL4204793)Show SMILES COc1ccc(cc1)-c1cc(=O)c2ccc(OCCCN3CCC(CCO)CC3)cc2o1 Show InChI InChI=1S/C26H31NO5/c1-30-21-5-3-20(4-6-21)25-18-24(29)23-8-7-22(17-26(23)32-25)31-16-2-12-27-13-9-19(10-14-27)11-15-28/h3-8,17-19,28H,2,9-16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE using acetylthiocholine iodide as substrate by spectroscopic method |
Bioorg Med Chem 25: 6273-6285 (2017)
Article DOI: 10.1016/j.bmc.2017.09.012
BindingDB Entry DOI: 10.7270/Q22V2JP6 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50085415
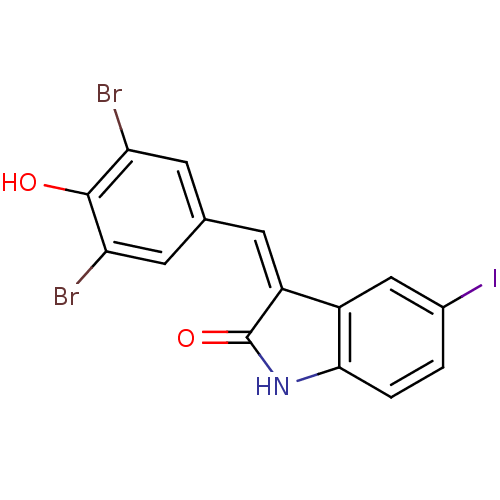
(3-(3,5-Dibromo-4-hydroxy-benzylidene)-5-iodo-1,3-d...)Show InChI InChI=1S/C15H8Br2INO2/c16-11-4-7(5-12(17)14(11)20)3-10-9-6-8(18)1-2-13(9)19-15(10)21/h1-6,20H,(H,19,21)/b10-3- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM8813
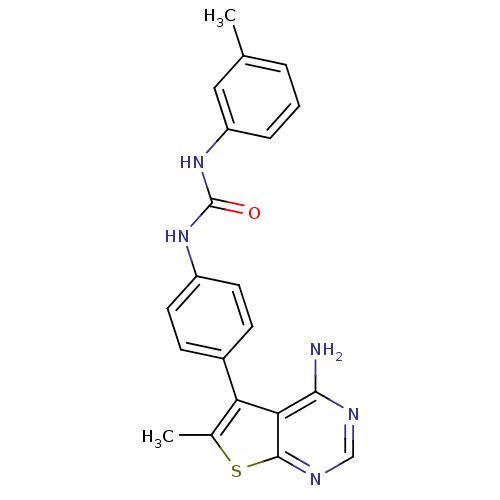
(3-(4-{4-amino-6-methylthieno[2,3-d]pyrimidin-5-yl}...)Show SMILES Cc1sc2ncnc(N)c2c1-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 Show InChI InChI=1S/C21H19N5OS/c1-12-4-3-5-16(10-12)26-21(27)25-15-8-6-14(7-9-15)17-13(2)28-20-18(17)19(22)23-11-24-20/h3-11H,1-2H3,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit by HTRF method |
Eur J Med Chem 45: 393-404 (2010)
Article DOI: 10.1016/j.ejmech.2009.09.013
BindingDB Entry DOI: 10.7270/Q2RX9C6D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50324735

((5E)-5-(QUINOXALIN-6-YLMETHYLENE)-1,3-THIAZOLIDINE...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc2nccnc2c1 |w:7.8,t:1| Show InChI InChI=1S/C12H7N3O2S/c16-11-10(18-12(17)15-11)6-7-1-2-8-9(5-7)14-4-3-13-8/h1-6H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PI3Kgamma incubated for 180 mins using [33P]gamma-ATP by scintillation proximity assay |
Bioorg Med Chem 23: 2953-74 (2015)
Article DOI: 10.1016/j.bmc.2015.03.071
BindingDB Entry DOI: 10.7270/Q2F76F9R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50411888
(CHEMBL270421)Show InChI InChI=1S/C14H11Cl2N5/c15-11-4-1-5-12(13(11)16)21-14(19-9-20-21)18-8-10-3-2-6-17-7-10/h1-7,9H,8H2,(H,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data