 Found 1855 hits with Last Name = 'singh' and Initial = 'k'
Found 1855 hits with Last Name = 'singh' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527524

(CHEMBL4566742)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CCC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H32N4O6/c1-36-21-8-7-17(15-22(21)37-2)26-19-5-3-4-6-20(19)27(35)31(28-26)18-11-13-29(14-12-18)25(34)16-30-23(32)9-10-24(30)33/h3-4,7-8,15,18-20H,5-6,9-14,16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527552
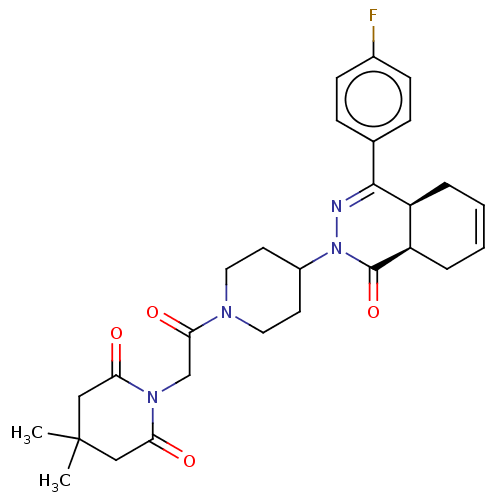
(CHEMBL4435111)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(F)cc1 |r,c:3,9| Show InChI InChI=1S/C28H33FN4O4/c1-28(2)15-23(34)32(24(35)16-28)17-25(36)31-13-11-20(12-14-31)33-27(37)22-6-4-3-5-21(22)26(30-33)18-7-9-19(29)10-8-18/h3-4,7-10,20-22H,5-6,11-17H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527532
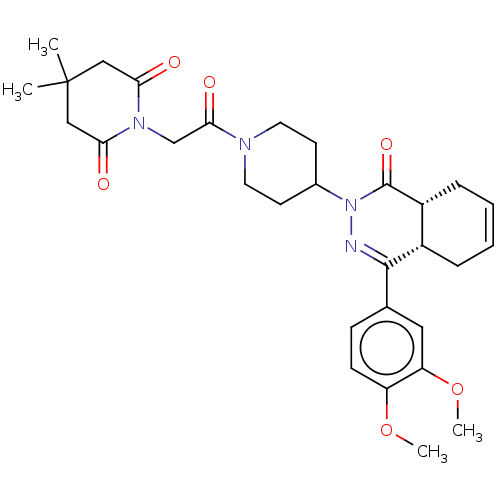
(CHEMBL4441748)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H38N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h5-6,9-10,15,20-22H,7-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527537
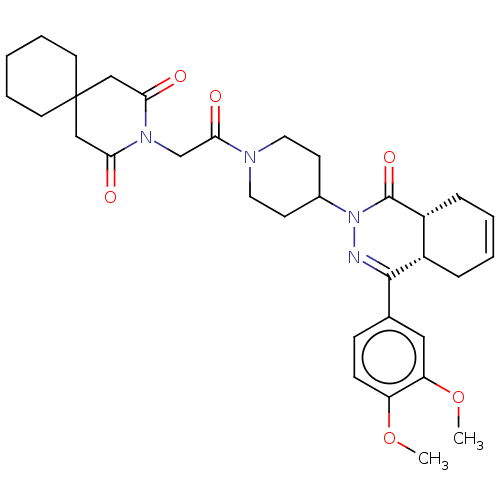
(CHEMBL4453005)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C33H42N4O6/c1-42-26-11-10-22(18-27(26)43-2)31-24-8-4-5-9-25(24)32(41)37(34-31)23-12-16-35(17-13-23)30(40)21-36-28(38)19-33(20-29(36)39)14-6-3-7-15-33/h4-5,10-11,18,23-25H,3,6-9,12-17,19-21H2,1-2H3/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527531
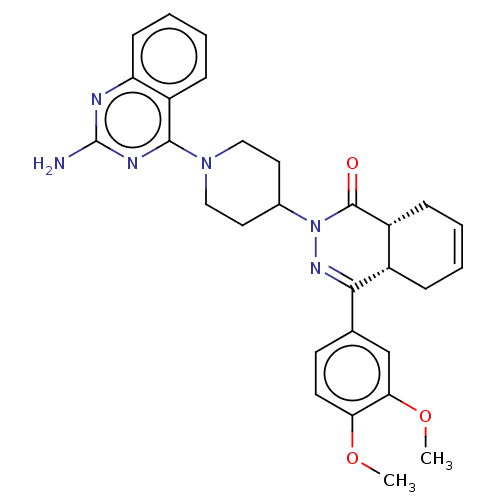
(CHEMBL4524402)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H32N6O3/c1-37-24-12-11-18(17-25(24)38-2)26-20-7-3-4-8-21(20)28(36)35(33-26)19-13-15-34(16-14-19)27-22-9-5-6-10-23(22)31-29(30)32-27/h3-6,9-12,17,19-21H,7-8,13-16H2,1-2H3,(H2,30,31,32)/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527528
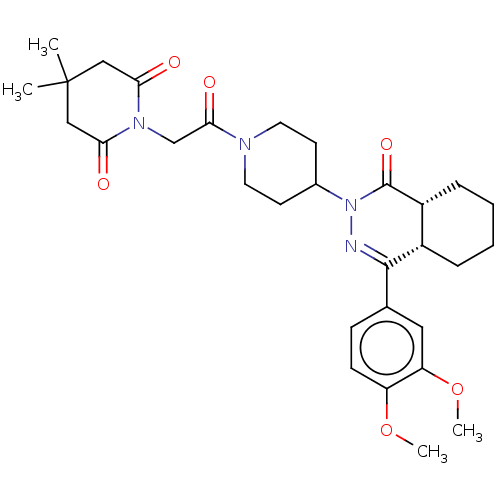
(CHEMBL4441871)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C30H40N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h9-10,15,20-22H,5-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527550
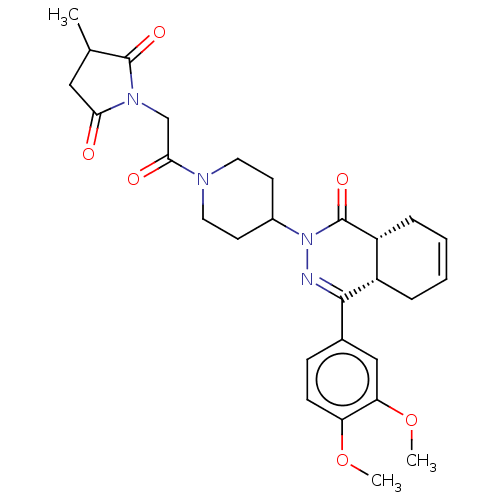
(CHEMBL4461456)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-17-14-24(33)31(27(17)35)16-25(34)30-12-10-19(11-13-30)32-28(36)21-7-5-4-6-20(21)26(29-32)18-8-9-22(37-2)23(15-18)38-3/h4-5,8-9,15,17,19-21H,6-7,10-14,16H2,1-3H3/t17?,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527535
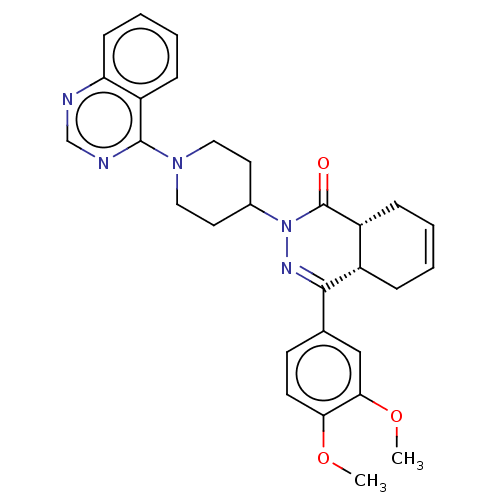
(CHEMBL4589927)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H31N5O3/c1-36-25-12-11-19(17-26(25)37-2)27-21-7-3-4-8-22(21)29(35)34(32-27)20-13-15-33(16-14-20)28-23-9-5-6-10-24(23)30-18-31-28/h3-6,9-12,17-18,20-22H,7-8,13-16H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527533
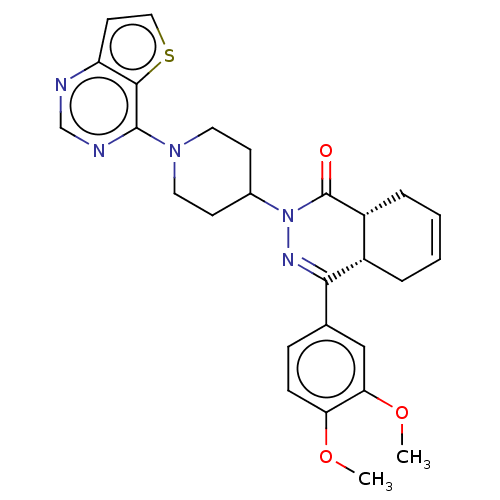
(CHEMBL4592553)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H29N5O3S/c1-34-22-8-7-17(15-23(22)35-2)24-19-5-3-4-6-20(19)27(33)32(30-24)18-9-12-31(13-10-18)26-25-21(11-14-36-25)28-16-29-26/h3-4,7-8,11,14-16,18-20H,5-6,9-10,12-13H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527555
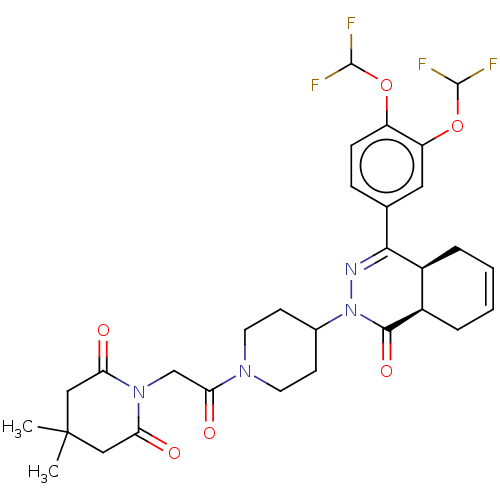
(CHEMBL4530777)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC(F)F)c(OC(F)F)c1 |r,c:3,9| Show InChI InChI=1S/C30H34F4N4O6/c1-30(2)14-23(39)37(24(40)15-30)16-25(41)36-11-9-18(10-12-36)38-27(42)20-6-4-3-5-19(20)26(35-38)17-7-8-21(43-28(31)32)22(13-17)44-29(33)34/h3-4,7-8,13,18-20,28-29H,5-6,9-12,14-16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527560
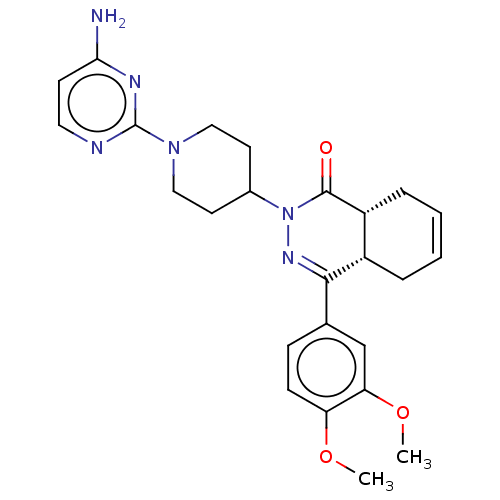
(CHEMBL4444578)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nccc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)25-27-12-9-22(26)28-25/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527567
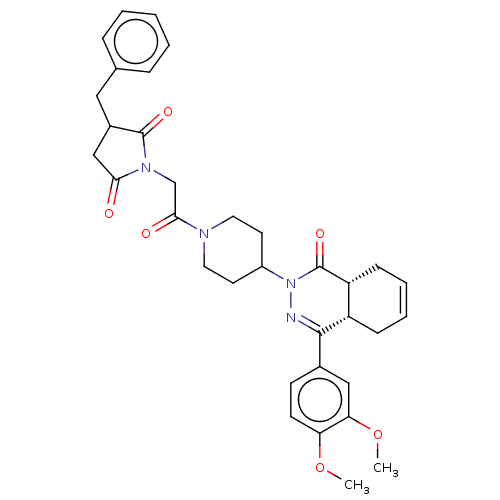
(CHEMBL4473426)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(Cc3ccccc3)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C34H38N4O6/c1-43-28-13-12-23(19-29(28)44-2)32-26-10-6-7-11-27(26)34(42)38(35-32)25-14-16-36(17-15-25)31(40)21-37-30(39)20-24(33(37)41)18-22-8-4-3-5-9-22/h3-9,12-13,19,24-27H,10-11,14-18,20-21H2,1-2H3/t24?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527554
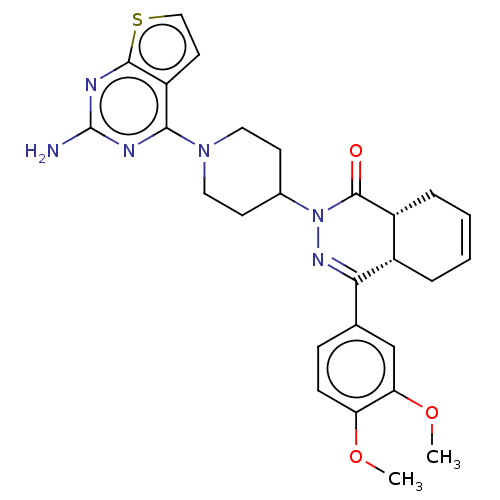
(CHEMBL4460623)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527559
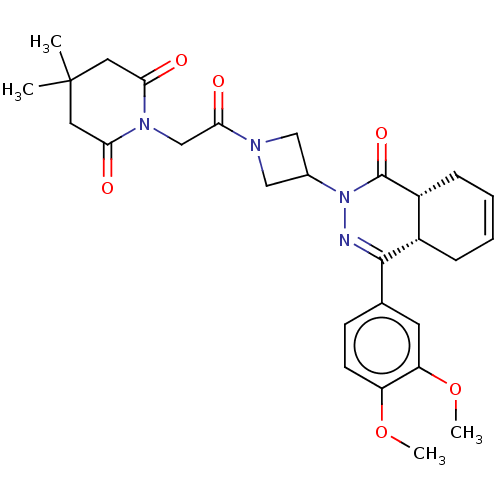
(CHEMBL4591859)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-28(2)12-23(33)31(24(34)13-28)16-25(35)30-14-18(15-30)32-27(36)20-8-6-5-7-19(20)26(29-32)17-9-10-21(37-3)22(11-17)38-4/h5-6,9-11,18-20H,7-8,12-16H2,1-4H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527525
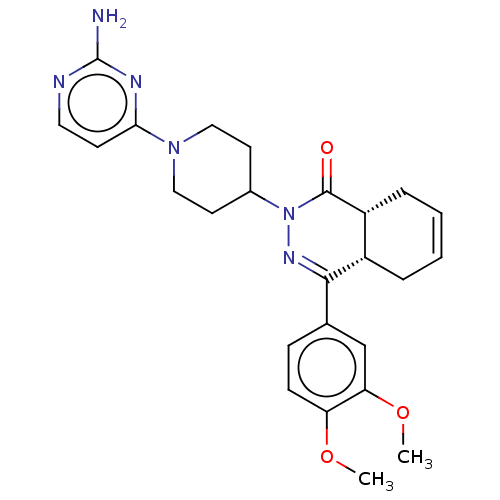
(CHEMBL4522249)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ccnc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)22-9-12-27-25(26)28-22/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527529
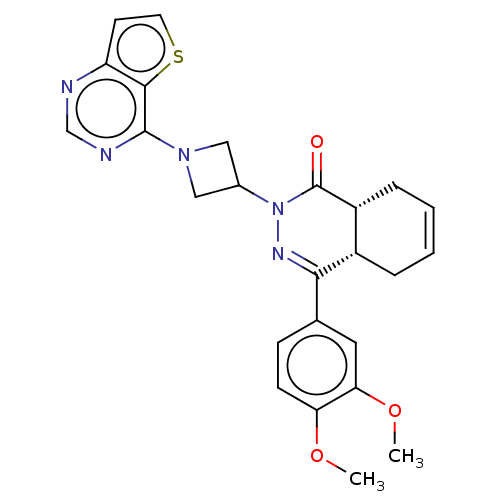
(CHEMBL4454600)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H25N5O3S/c1-32-20-8-7-15(11-21(20)33-2)22-17-5-3-4-6-18(17)25(31)30(28-22)16-12-29(13-16)24-23-19(9-10-34-23)26-14-27-24/h3-4,7-11,14,16-18H,5-6,12-13H2,1-2H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50427452
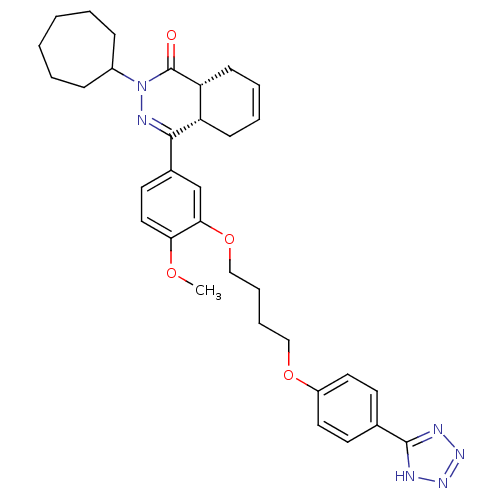
(CHEMBL2326941)Show SMILES COc1ccc(cc1OCCCCOc1ccc(cc1)-c1nnn[nH]1)C1=NN(C2CCCCCC2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:43,t:28| Show InChI InChI=1S/C33H40N6O4/c1-41-29-19-16-24(31-27-12-6-7-13-28(27)33(40)39(36-31)25-10-4-2-3-5-11-25)22-30(29)43-21-9-8-20-42-26-17-14-23(15-18-26)32-34-37-38-35-32/h6-7,14-19,22,25,27-28H,2-5,8-13,20-21H2,1H3,(H,34,35,37,38)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527527
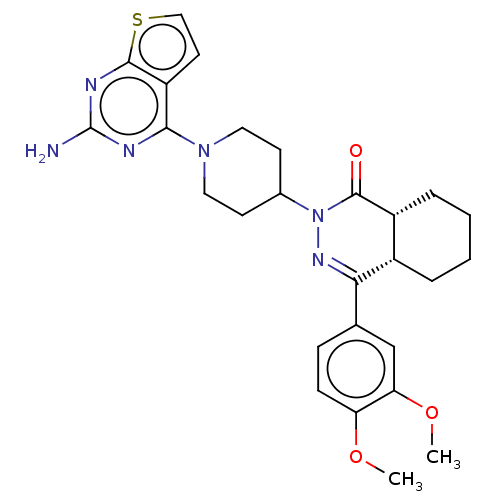
(CHEMBL4466777)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C27H32N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h7-8,11,14-15,17-19H,3-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527530
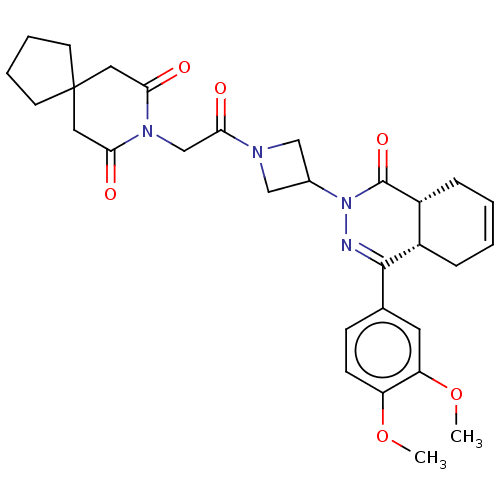
(CHEMBL4461705)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC3(CCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H36N4O6/c1-39-23-10-9-19(13-24(23)40-2)28-21-7-3-4-8-22(21)29(38)34(31-28)20-16-32(17-20)27(37)18-33-25(35)14-30(15-26(33)36)11-5-6-12-30/h3-4,9-10,13,20-22H,5-8,11-12,14-18H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527534
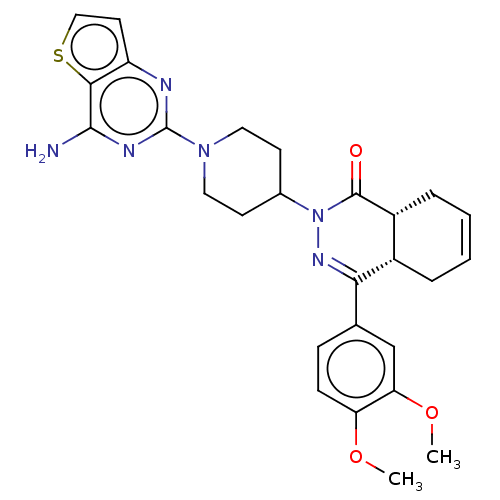
(CHEMBL4472307)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)c3sccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)27-29-20-11-14-37-24(20)25(28)30-27/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527548
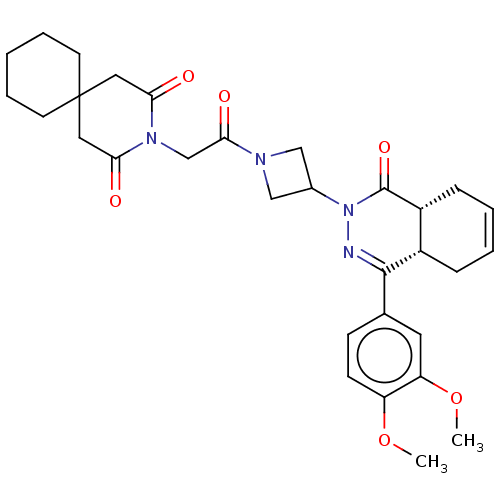
(CHEMBL4465097)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C31H38N4O6/c1-40-24-11-10-20(14-25(24)41-2)29-22-8-4-5-9-23(22)30(39)35(32-29)21-17-33(18-21)28(38)19-34-26(36)15-31(16-27(34)37)12-6-3-7-13-31/h4-5,10-11,14,21-23H,3,6-9,12-13,15-19H2,1-2H3/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527549
(CHEMBL4452707)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3[nH]cnc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C26H30N8O3/c1-36-19-8-7-15(13-20(19)37-2)21-17-5-3-4-6-18(17)25(35)34(32-21)16-9-11-33(12-10-16)24-22-23(29-14-28-22)30-26(27)31-24/h3-4,7-8,13-14,16-18H,5-6,9-12H2,1-2H3,(H3,27,28,29,30,31)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527523

(CHEMBL4461658)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H26N6O3S/c1-33-19-8-7-14(11-20(19)34-2)21-16-5-3-4-6-17(16)24(32)31(29-21)15-12-30(13-15)22-18-9-10-35-23(18)28-25(26)27-22/h3-4,7-11,15-17H,5-6,12-13H2,1-2H3,(H2,26,27,28)/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50402408
(CHEMBL2203332)Show SMILES CCCC[N+]1=C(\C=C\c2ccc(cc2)N(CC)CC)C(C)(C)c2ccccc12 |c:4| Show InChI InChI=1S/C26H35N2/c1-6-9-20-28-24-13-11-10-12-23(24)26(4,5)25(28)19-16-21-14-17-22(18-15-21)27(7-2)8-3/h10-19H,6-9,20H2,1-5H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527557
(CHEMBL4533993)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC(F)F)c(OC(F)F)c1 |r,c:3,9| Show InChI InChI=1S/C33H38F4N4O6/c34-31(35)46-24-9-8-20(16-25(24)47-32(36)37)29-22-6-2-3-7-23(22)30(45)41(38-29)21-10-14-39(15-11-21)28(44)19-40-26(42)17-33(18-27(40)43)12-4-1-5-13-33/h2-3,8-9,16,21-23,31-32H,1,4-7,10-15,17-19H2/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50018235

(5-Amino-2-{4-[(2,4-diamino-5-chloro-quinazolin-6-y...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)nc(N)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H24ClN7O3/c22-17-12(5-8-14-16(17)18(24)29-21(25)28-14)10-26-13-6-3-11(4-7-13)19(30)27-15(20(31)32)2-1-9-23/h3-8,15,26H,1-2,9-10,23H2,(H,27,30)(H,31,32)(H4,24,25,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50139758
((2-Naphthalen-2-yl-1-naphthalen-1-yl-2-oxo-ethyl)-...)Show SMILES OP(O)(=O)C(C(=O)c1ccc2ccccc2c1)c1cccc2ccccc12 Show InChI InChI=1S/C22H17O4P/c23-21(18-13-12-15-6-1-2-8-17(15)14-18)22(27(24,25)26)20-11-5-9-16-7-3-4-10-19(16)20/h1-14,22H,(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of chymase in human mast cells using Suc-Ala-Ala-Pro-Phe-(p-nitroanilide) as substrate for 15 mins by spectrophotometric method |
Eur J Med Chem 161: 252-276 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.018
BindingDB Entry DOI: 10.7270/Q25M690C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527566
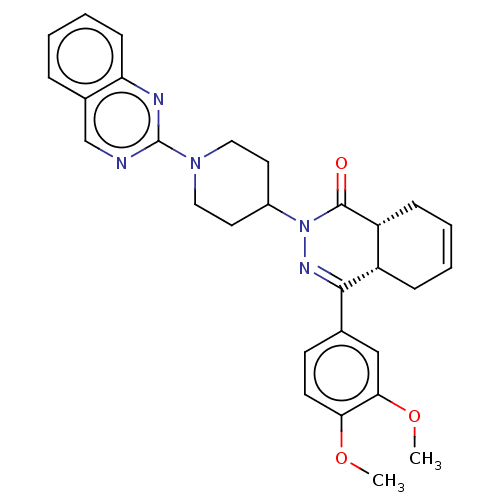
(CHEMBL4472329)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncc3ccccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H31N5O3/c1-36-25-12-11-19(17-26(25)37-2)27-22-8-4-5-9-23(22)28(35)34(32-27)21-13-15-33(16-14-21)29-30-18-20-7-3-6-10-24(20)31-29/h3-7,10-12,17-18,21-23H,8-9,13-16H2,1-2H3/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527551
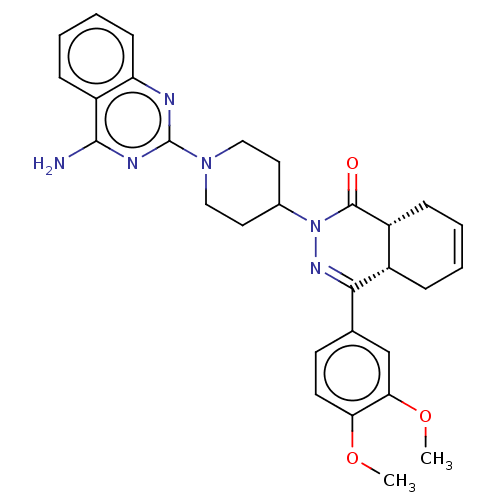
(CHEMBL4575003)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)c3ccccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H32N6O3/c1-37-24-12-11-18(17-25(24)38-2)26-20-7-3-4-8-21(20)28(36)35(33-26)19-13-15-34(16-14-19)29-31-23-10-6-5-9-22(23)27(30)32-29/h3-6,9-12,17,19-21H,7-8,13-16H2,1-2H3,(H2,30,31,32)/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527536
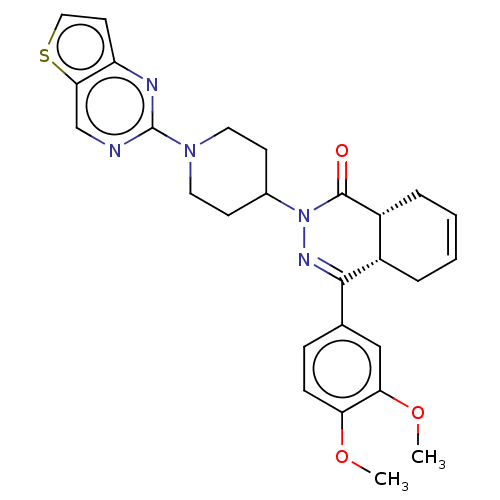
(CHEMBL4466905)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncc3sccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H29N5O3S/c1-34-22-8-7-17(15-23(22)35-2)25-19-5-3-4-6-20(19)26(33)32(30-25)18-9-12-31(13-10-18)27-28-16-24-21(29-27)11-14-36-24/h3-4,7-8,11,14-16,18-20H,5-6,9-10,12-13H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50254908
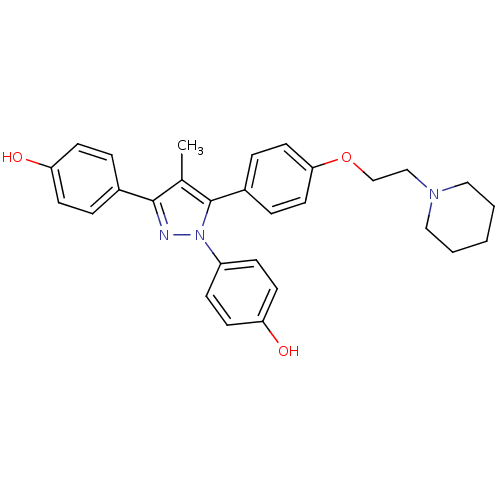
(4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...)Show SMILES Cc1c(nn(c1-c1ccc(OCCN2CCCCC2)cc1)-c1ccc(O)cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31N3O3/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31/h5-16,33-34H,2-4,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha in human MCF7 cells |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Trypanosoma brucei) | BDBM50427452

(CHEMBL2326941)Show SMILES COc1ccc(cc1OCCCCOc1ccc(cc1)-c1nnn[nH]1)C1=NN(C2CCCCCC2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:43,t:28| Show InChI InChI=1S/C33H40N6O4/c1-41-29-19-16-24(31-27-12-6-7-13-28(27)33(40)39(36-31)25-10-4-2-3-5-11-25)22-30(29)43-21-9-8-20-42-26-17-14-23(15-18-26)32-34-37-38-35-32/h6-7,14-19,22,25,27-28H,2-5,8-13,20-21H2,1H3,(H,34,35,37,38)/t27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length Trypanosoma brucei PDEB1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603512
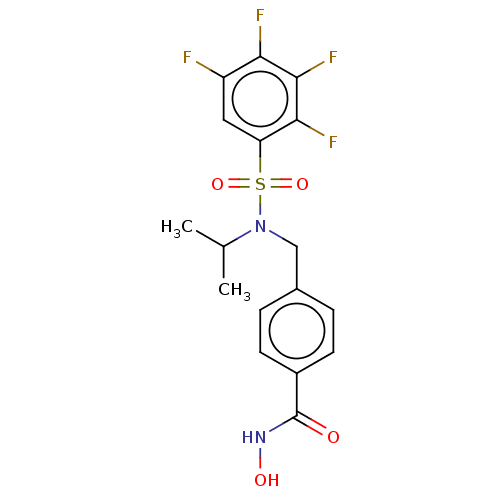
(CHEMBL5177475)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50488953
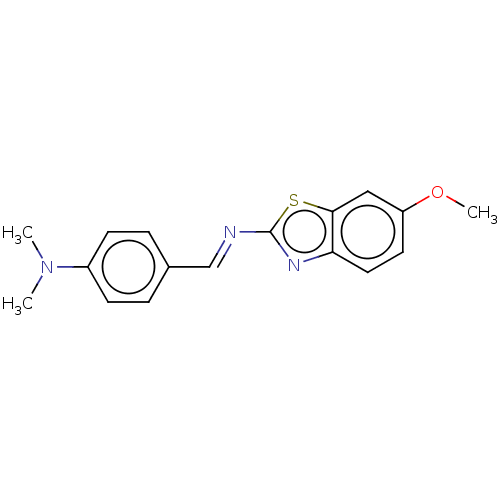
(CHEMBL2296467)Show InChI InChI=1S/C17H17N3OS/c1-20(2)13-6-4-12(5-7-13)11-18-17-19-15-9-8-14(21-3)10-16(15)22-17/h4-11H,1-3H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524365

(CHEMBL4579211)Show SMILES C(CN1CCCCC1)Oc1ccc(Nc2nccc(NCc3ccccc3)n2)cc1 Show InChI InChI=1S/C24H29N5O/c1-3-7-20(8-4-1)19-26-23-13-14-25-24(28-23)27-21-9-11-22(12-10-21)30-18-17-29-15-5-2-6-16-29/h1,3-4,7-14H,2,5-6,15-19H2,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524365

(CHEMBL4579211)Show SMILES C(CN1CCCCC1)Oc1ccc(Nc2nccc(NCc3ccccc3)n2)cc1 Show InChI InChI=1S/C24H29N5O/c1-3-7-20(8-4-1)19-26-23-13-14-25-24(28-23)27-21-9-11-22(12-10-21)30-18-17-29-15-5-2-6-16-29/h1,3-4,7-14H,2,5-6,15-19H2,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591808

(CHEMBL5207397)Show SMILES OC(=O)[C@@H]1CCCN(CC\C=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524374

(CHEMBL4453810)Show InChI InChI=1S/C18H20N4OS/c1-12-3-4-15(19-11-12)18(23)6-8-22(9-7-18)17-16-14(5-10-24-16)20-13(2)21-17/h3-5,10-11,23H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50524374

(CHEMBL4453810)Show InChI InChI=1S/C18H20N4OS/c1-12-3-4-15(19-11-12)18(23)6-8-22(9-7-18)17-16-14(5-10-24-16)20-13(2)21-17/h3-5,10-11,23H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR)
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... |
J Med Chem 62: 4638-4655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00241
BindingDB Entry DOI: 10.7270/Q23T9MN6 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50528756
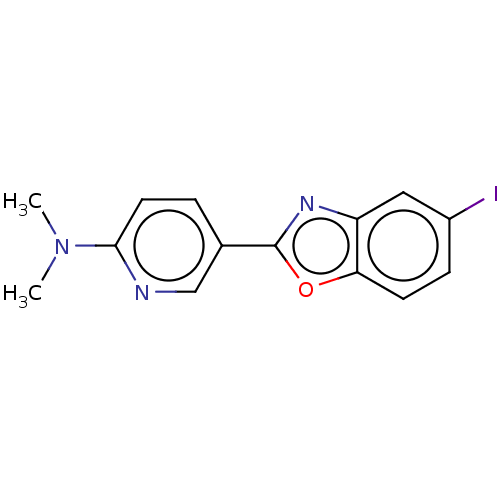
(CHEMBL4470128)Show InChI InChI=1S/C14H12IN3O/c1-18(2)13-6-3-9(8-16-13)14-17-11-7-10(15)4-5-12(11)19-14/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from human amyloid beta(1-42) aggregates incubated for 3 hrs by gamma counting analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111613
BindingDB Entry DOI: 10.7270/Q2GQ726C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50458333
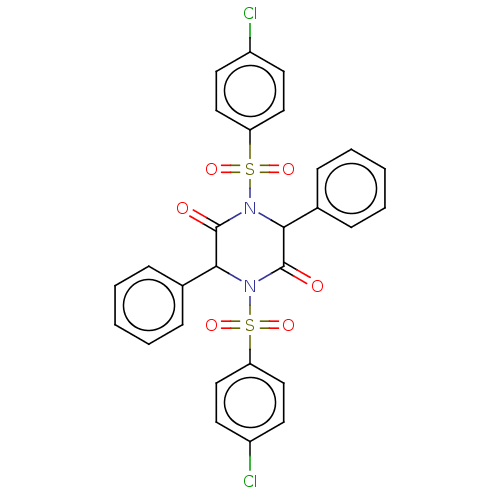
(CHEMBL4210820)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1C(C(=O)N(C(C1=O)c1ccccc1)S(=O)(=O)c1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C28H20Cl2N2O6S2/c29-21-11-15-23(16-12-21)39(35,36)31-25(19-7-3-1-4-8-19)27(33)32(26(28(31)34)20-9-5-2-6-10-20)40(37,38)24-17-13-22(30)14-18-24/h1-18,25-26H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University)
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition b... |
Eur J Med Chem 150: 87-101 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.078
BindingDB Entry DOI: 10.7270/Q2SJ1P72 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472
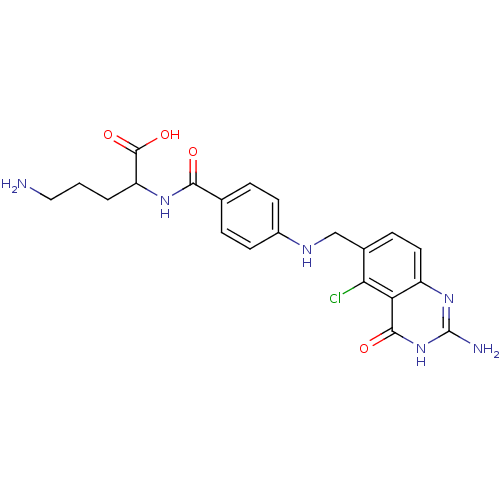
(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472

(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 32: 1559-65 (1989)
BindingDB Entry DOI: 10.7270/Q22806MJ |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003467
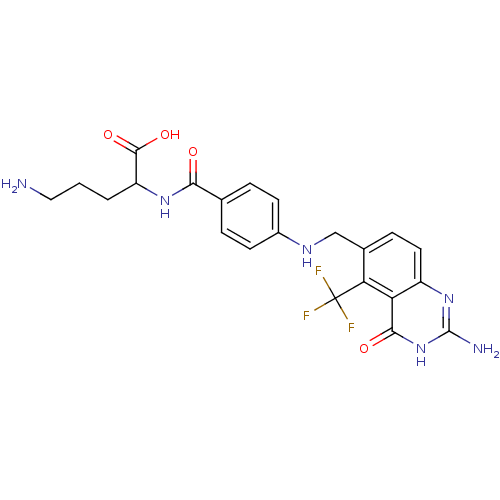
(5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2C(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C22H23F3N6O4/c23-22(24,25)17-12(5-8-14-16(17)19(33)31-21(27)30-14)10-28-13-6-3-11(4-7-13)18(32)29-15(20(34)35)2-1-9-26/h3-8,15,28H,1-2,9-10,26H2,(H,29,32)(H,34,35)(H3,27,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591807

(CHEMBL5178760)Show SMILES OC(=O)C1CCCN(CC\C=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50003471

(5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...)Show SMILES NCCCC(NC(=O)c1ccc(CNc2ccc3nc(N)[nH]c(=O)c3c2C(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C22H23F3N6O4/c23-22(24,25)17-14(8-7-13-16(17)19(33)31-21(27)30-13)28-10-11-3-5-12(6-4-11)18(32)29-15(20(34)35)2-1-9-26/h3-8,15,28H,1-2,9-10,26H2,(H,29,32)(H,34,35)(H3,27,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) |
J Med Chem 35: 4078-85 (1992)
BindingDB Entry DOI: 10.7270/Q26M35S6 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50598566
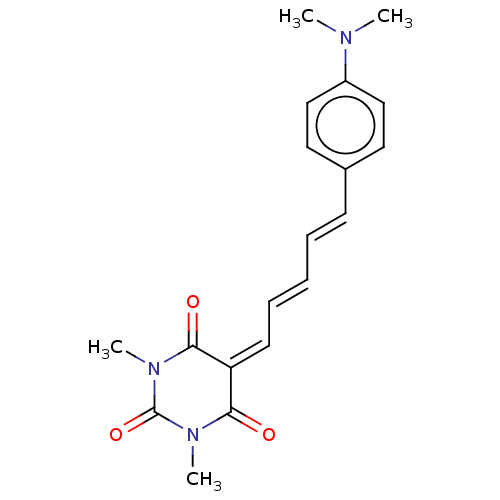
(CHEMBL5192359)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]-2/[#6](=O)-[#7](-[#6])-[#6](=O)-[#7](-[#6])-[#6]-2=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01619
BindingDB Entry DOI: 10.7270/Q2X92GCK |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591806

(CHEMBL5205864)Show SMILES Cl.OC(=O)C1CCCN(CCO\N=C(/Cc2ccc(F)cc2)c2ccc(F)cc2)C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data