 Found 1132 hits with Last Name = 'singh' and Initial = 'n'
Found 1132 hits with Last Name = 'singh' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055832
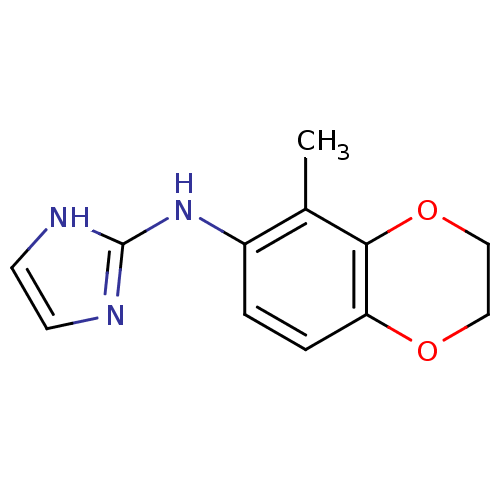
((1H-Imidazol-2-yl)-(5-methyl-2,3-dihydro-benzo[1,4...)Show InChI InChI=1S/C12H13N3O2/c1-8-9(15-12-13-4-5-14-12)2-3-10-11(8)17-7-6-16-10/h2-5H,6-7H2,1H3,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50052880

(CHEMBL49137 | Imidazolidin-2-ylidene-(5-methyl-qui...)Show SMILES [#6]-c1c(ccc2nccnc12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C12H13N5/c1-8-9(17-12-15-6-7-16-12)2-3-10-11(8)14-5-4-13-10/h2-5H,6-7H2,1H3,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM34572
(BRIMONIDINE | CHEMBL844 | MLS000069370 | SMR000058...)Show InChI InChI=1S/C11H10BrN5/c12-9-7(17-11-15-5-6-16-11)1-2-8-10(9)14-4-3-13-8/h1-4H,5-6H2,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50016897
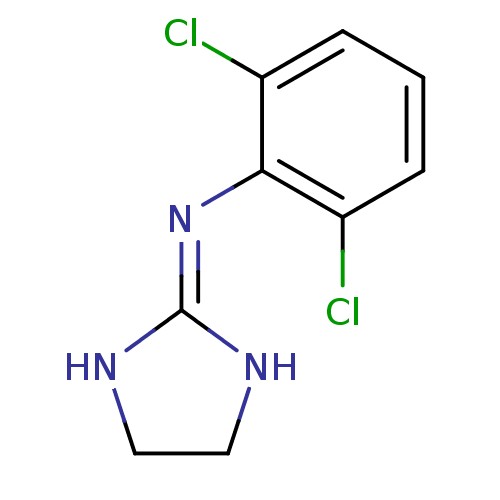
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-2A adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055835
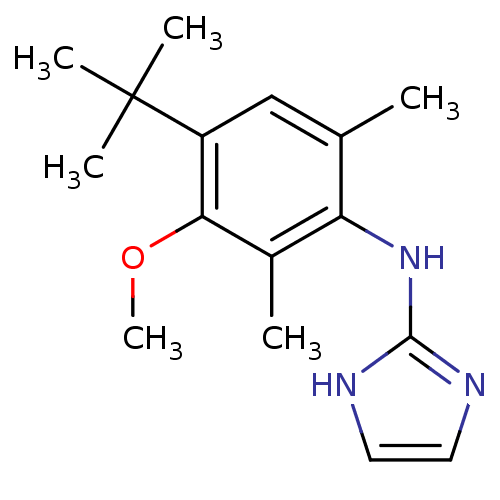
((4-tert-Butyl-3-methoxy-2,6-dimethyl-phenyl)-(1H-i...)Show InChI InChI=1S/C16H23N3O/c1-10-9-12(16(3,4)5)14(20-6)11(2)13(10)19-15-17-7-8-18-15/h7-9H,1-6H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055830

((1H-Imidazol-2-yl)-(5-methyl-3,4-dihydro-2H-benzo[...)Show InChI InChI=1S/C12H14N4O/c1-8-9(16-12-14-4-5-15-12)2-3-10-11(8)13-6-7-17-10/h2-5,13H,6-7H2,1H3,(H2,14,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50016897

(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for rat Alpha-2B adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Nischarin
(Homo sapiens (Human)) | BDBM50016897

(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-clonidine from bovine imidazoline receptor I-1 |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182163
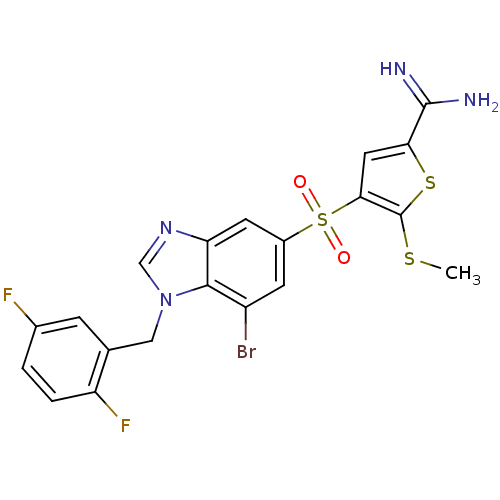
(4-[7-bromo-1-(2,5-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(F)ccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)26-9-27(18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055827
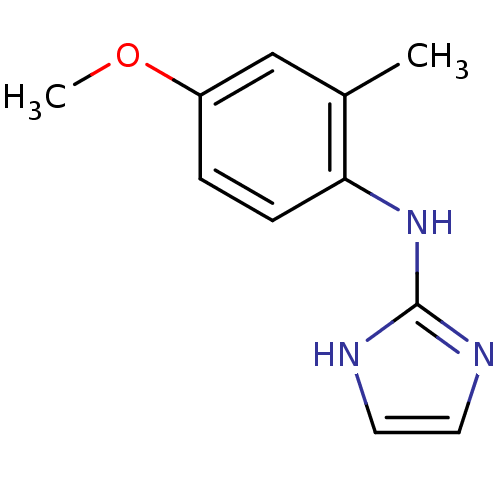
((1H-Imidazol-2-yl)-(4-methoxy-2-methyl-phenyl)-ami...)Show InChI InChI=1S/C11H13N3O/c1-8-7-9(15-2)3-4-10(8)14-11-12-5-6-13-11/h3-7H,1-2H3,(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233679
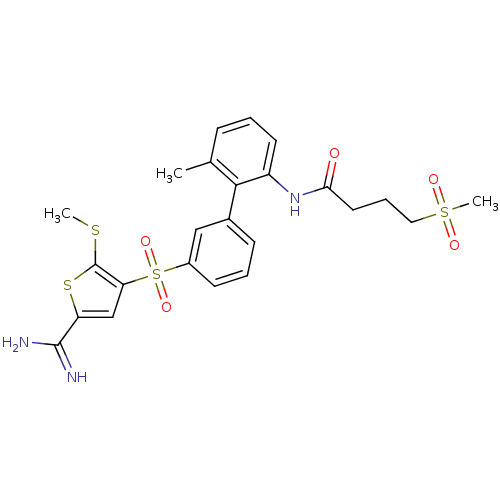
(CHEMBL399284 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C24H27N3O5S4/c1-15-7-4-10-18(27-21(28)11-6-12-35(3,29)30)22(15)16-8-5-9-17(13-16)36(31,32)20-14-19(23(25)26)34-24(20)33-2/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50052880

(CHEMBL49137 | Imidazolidin-2-ylidene-(5-methyl-qui...)Show SMILES [#6]-c1c(ccc2nccnc12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C12H13N5/c1-8-9(17-12-15-6-7-16-12)2-3-10-11(8)14-5-4-13-10/h2-5H,6-7H2,1H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from rat Alpha-2B adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50055832

((1H-Imidazol-2-yl)-(5-methyl-2,3-dihydro-benzo[1,4...)Show InChI InChI=1S/C12H13N3O2/c1-8-9(15-12-13-4-5-14-12)2-3-10-11(8)17-7-6-16-10/h2-5H,6-7H2,1H3,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2C adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233691

(4-(2'-amino-6'-methyl-biphenyl-3-sulfonyl)-5-methy...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1N)C(N)=N Show InChI InChI=1S/C19H19N3O2S3/c1-11-5-3-8-14(20)17(11)12-6-4-7-13(9-12)27(23,24)16-10-15(18(21)22)26-19(16)25-2/h3-10H,20H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182160
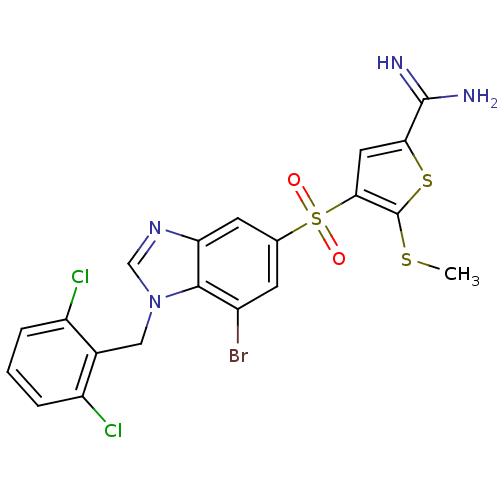
(4-[7-bromo-1-(2,6-dichloro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(Cl)cccc3Cl)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233674
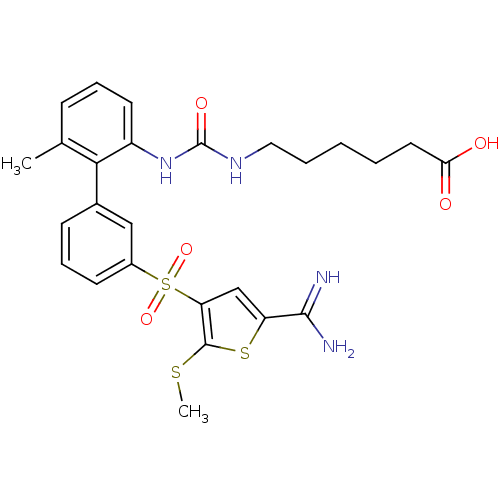
(6-{3-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophe...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCCCCC(O)=O)C(N)=N Show InChI InChI=1S/C26H30N4O5S3/c1-16-8-6-11-19(30-26(33)29-13-5-3-4-12-22(31)32)23(16)17-9-7-10-18(14-17)38(34,35)21-15-20(24(27)28)37-25(21)36-2/h6-11,14-15H,3-5,12-13H2,1-2H3,(H3,27,28)(H,31,32)(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50052880

(CHEMBL49137 | Imidazolidin-2-ylidene-(5-methyl-qui...)Show SMILES [#6]-c1c(ccc2nccnc12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C12H13N5/c1-8-9(17-12-15-6-7-16-12)2-3-10-11(8)14-5-4-13-10/h2-5H,6-7H2,1H3,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2C adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233686

(4-(2'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-3-4-9-15(12)13-7-5-8-14(10-13)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182171
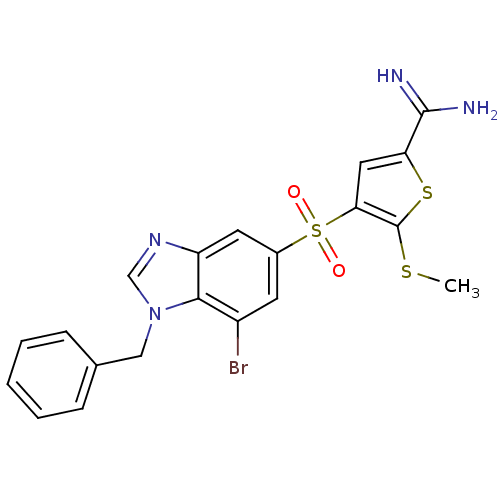
(4-(1-benzyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3ccccc3)cnc2c1)C(N)=N Show InChI InChI=1S/C20H17BrN4O2S3/c1-28-20-17(9-16(29-20)19(22)23)30(26,27)13-7-14(21)18-15(8-13)24-11-25(18)10-12-5-3-2-4-6-12/h2-9,11H,10H2,1H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182170
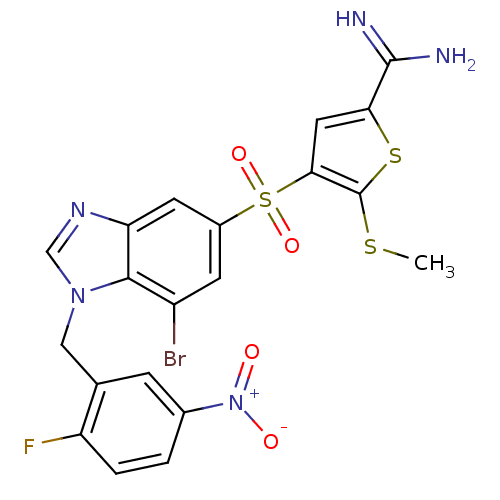
(4-[7-bromo-1-(2-fluoro-5-nitro-benzyl)-1H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(ccc3F)[N+]([O-])=O)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)25-9-26(18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50016897

(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-2C adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055829
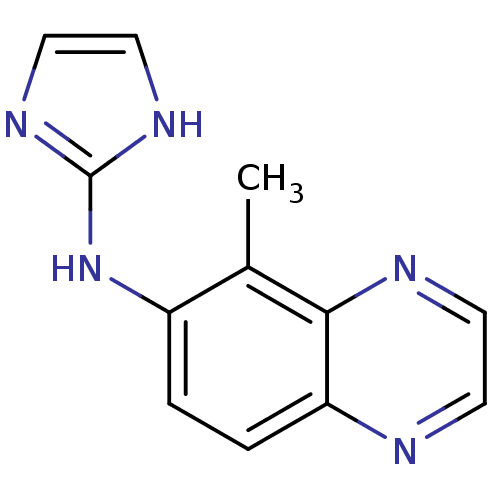
((1H-Imidazol-2-yl)-(5-methyl-quinoxalin-6-yl)-amin...)Show InChI InChI=1S/C12H11N5/c1-8-9(17-12-15-6-7-16-12)2-3-10-11(8)14-5-4-13-10/h2-7H,1H3,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055837

((5-tert-Butyl-4-methoxy-2-methyl-phenyl)-(1H-imida...)Show InChI InChI=1S/C15H21N3O/c1-10-8-13(19-5)11(15(2,3)4)9-12(10)18-14-16-6-7-17-14/h6-9H,1-5H3,(H2,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233692

(4-[3-(6-methyl-pyridin-2-yl)-benzenesulfonyl]-5-me...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)n1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-11-5-3-8-14(21-11)12-6-4-7-13(9-12)26(22,23)16-10-15(17(19)20)25-18(16)24-2/h3-10H,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233688

(4-(2'-chloro-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(N)=N Show InChI InChI=1S/C18H15ClN2O2S3/c1-24-18-16(10-15(25-18)17(20)21)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)19/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182159
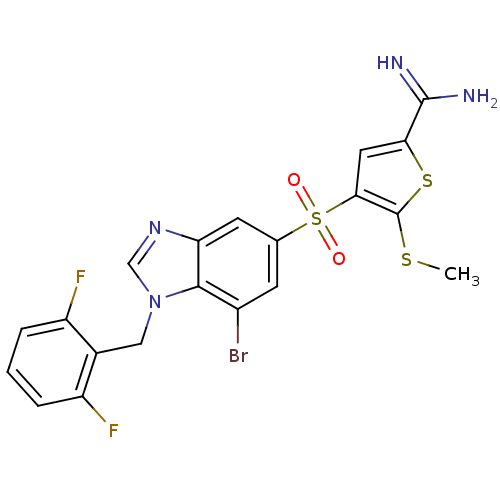
(4-[7-bromo-1-(2,6-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Nischarin
(Homo sapiens (Human)) | BDBM50050094
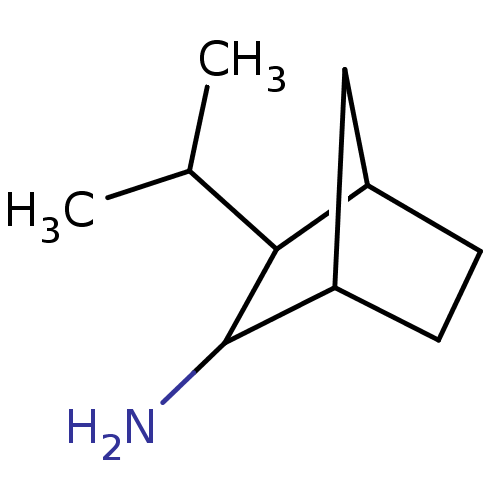
(3-Isopropyl-bicyclo[2.2.1]hept-2-ylamine | CHEMBL4...)Show SMILES CC(C)C1C2CCC(C2)C1N |TLB:10:9:8:5.6,THB:1:3:8:5.6| Show InChI InChI=1S/C10H19N/c1-6(2)9-7-3-4-8(5-7)10(9)11/h6-10H,3-5,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-clonidine from bovine imidazoline receptor I-1 |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233694

(5-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCCC(O)=O)C(N)=N Show InChI InChI=1S/C25H27N3O5S3/c1-15-7-5-10-18(28-21(29)11-3-4-12-22(30)31)23(15)16-8-6-9-17(13-16)36(32,33)20-14-19(24(26)27)35-25(20)34-2/h5-10,13-14H,3-4,11-12H2,1-2H3,(H3,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099934
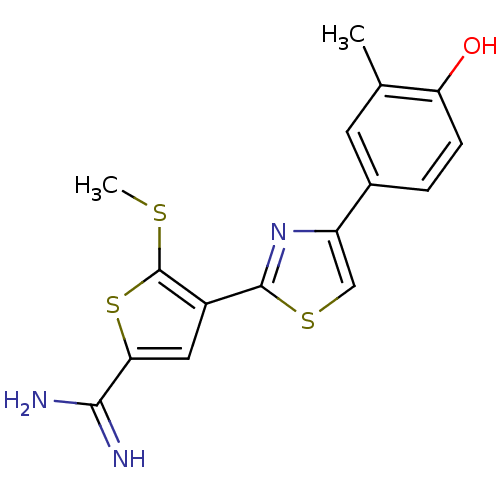
(4-[4-(4-Hydroxy-3-methyl-phenyl)-thiazol-2-yl]-5-m...)Show InChI InChI=1S/C16H15N3OS3/c1-8-5-9(3-4-12(8)20)11-7-22-15(19-11)10-6-13(14(17)18)23-16(10)21-2/h3-7,20H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM34572

(BRIMONIDINE | CHEMBL844 | MLS000069370 | SMR000058...)Show InChI InChI=1S/C11H10BrN5/c12-9-7(17-11-15-5-6-16-11)1-2-8-10(9)14-4-3-13-8/h1-4H,5-6H2,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2C adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099928
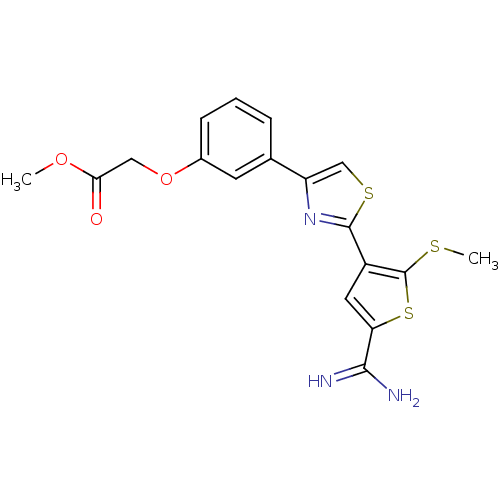
(CHEMBL28952 | {3-[2-(5-Carbamimidoyl-2-methylsulfa...)Show SMILES COC(=O)COc1cccc(c1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C18H17N3O3S3/c1-23-15(22)8-24-11-5-3-4-10(6-11)13-9-26-17(21-13)12-7-14(16(19)20)27-18(12)25-2/h3-7,9H,8H2,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50099923

(CHEMBL29037 | N-{3-[2-(5-Carbamimidoyl-2-methylsul...)Show SMILES CSc1sc(cc1-c1nc(cs1)-c1cccc(NC(=O)c2ccc(F)cc2)c1)C(N)=N Show InChI InChI=1S/C22H17FN4OS3/c1-29-22-16(10-18(31-22)19(24)25)21-27-17(11-30-21)13-3-2-4-15(9-13)26-20(28)12-5-7-14(23)8-6-12/h2-11H,1H3,(H3,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 1379-82 (2001)
BindingDB Entry DOI: 10.7270/Q24M93S1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50448214
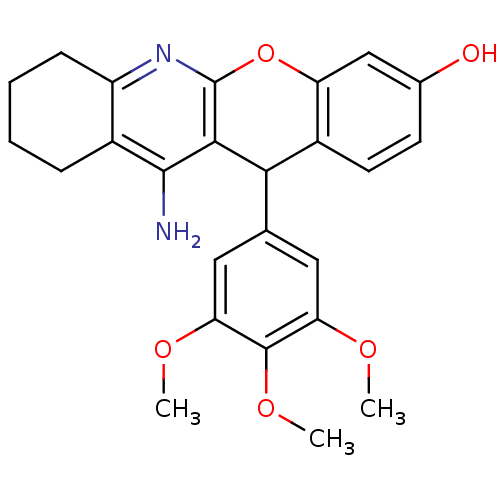
(CHEMBL3120707)Show SMILES COc1cc(cc(OC)c1OC)C1c2ccc(O)cc2Oc2nc3CCCCc3c(N)c12 Show InChI InChI=1S/C25H26N2O5/c1-29-19-10-13(11-20(30-2)24(19)31-3)21-16-9-8-14(28)12-18(16)32-25-22(21)23(26)15-6-4-5-7-17(15)27-25/h8-12,21,28H,4-7H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid (UCM)
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of electric eel AchE using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured for ... |
Eur J Med Chem 74: 491-501 (2014)
Article DOI: 10.1016/j.ejmech.2013.12.021
BindingDB Entry DOI: 10.7270/Q2G73G60 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182176
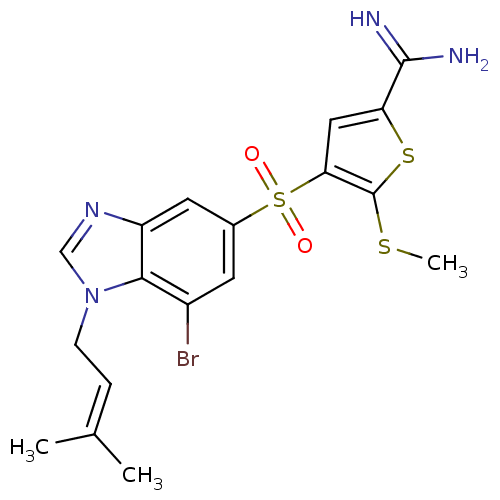
(4-[7-bromo-1-(3-methyl-but-2-enyl)-1H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2n(-[#6]\[#6]=[#6](/[#6])-[#6])cnc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-13-7-11(6-12(19)16(13)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233677

(4-(2'-hydroxymethyl-6'-methyl-biphenyl-3-sulfonyl)...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1CO)C(N)=N Show InChI InChI=1S/C20H20N2O3S3/c1-12-5-3-7-14(11-23)18(12)13-6-4-8-15(9-13)28(24,25)17-10-16(19(21)22)27-20(17)26-2/h3-10,23H,11H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM34572

(BRIMONIDINE | CHEMBL844 | MLS000069370 | SMR000058...)Show InChI InChI=1S/C11H10BrN5/c12-9-7(17-11-15-5-6-16-11)1-2-8-10(9)14-4-3-13-8/h1-4H,5-6H2,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from rat Alpha-2B adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Nischarin
(Homo sapiens (Human)) | BDBM50050093

((4-Chloro-6-methoxy-2-methyl-pyrimidin-5-yl)-imida...)Show SMILES [#6]-[#8]-c1nc(-[#6])nc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H12ClN5O/c1-5-13-7(10)6(8(14-5)16-2)15-9-11-3-4-12-9/h3-4H2,1-2H3,(H2,11,12,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-clonidine from bovine imidazoline receptor I-1 |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50109377
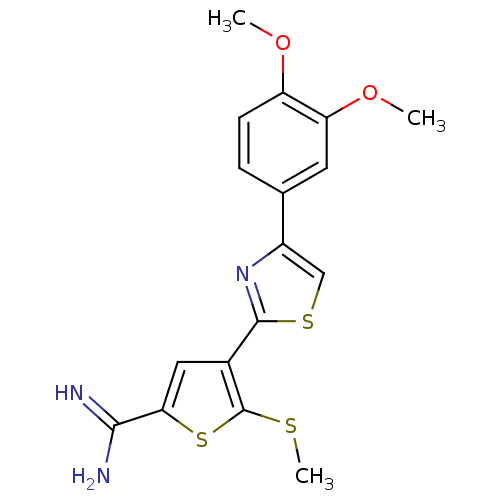
(4-[4-(3,4-Dimethoxy-phenyl)-thiazol-2-yl]-5-methyl...)Show InChI InChI=1S/C17H17N3O2S3/c1-21-12-5-4-9(6-13(12)22-2)11-8-24-16(20-11)10-7-14(15(18)19)25-17(10)23-3/h4-8H,1-3H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human kidney cell urokinase |
Bioorg Med Chem Lett 12: 491-5 (2002)
BindingDB Entry DOI: 10.7270/Q2H994H5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098163
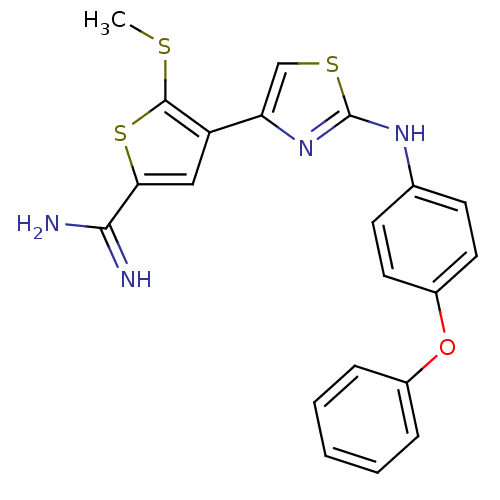
(5-Methylsulfanyl-4-[2-(4-phenoxy-phenylamino)-thia...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(Oc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H18N4OS3/c1-27-20-16(11-18(29-20)19(22)23)17-12-28-21(25-17)24-13-7-9-15(10-8-13)26-14-5-3-2-4-6-14/h2-12H,1H3,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147047
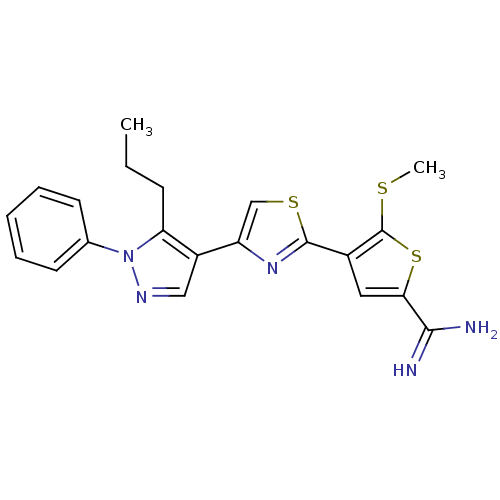
(5-Methylsulfanyl-4-[4-(1-phenyl-5-propyl-1H-pyrazo...)Show SMILES CCCc1c(cnn1-c1ccccc1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C21H21N5S3/c1-3-7-17-15(11-24-26(17)13-8-5-4-6-9-13)16-12-28-20(25-16)14-10-18(19(22)23)29-21(14)27-2/h4-6,8-12H,3,7H2,1-2H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50029051
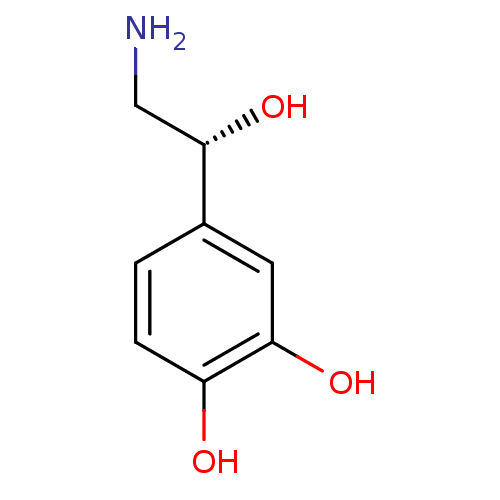
((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-2C adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233689
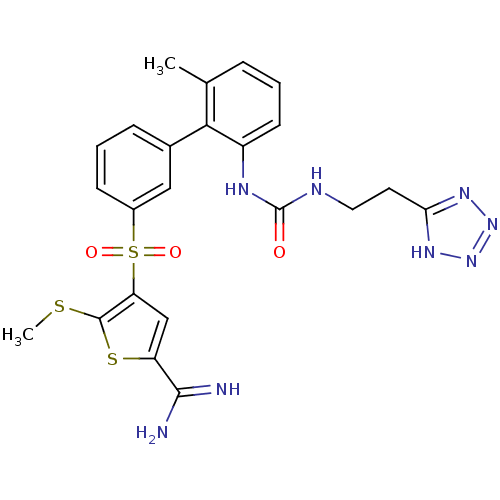
(5-methylsulfanyl-4-(6'-methyl-2'-{3-[2-(2H-tetrazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCc1nnn[nH]1)C(N)=N Show InChI InChI=1S/C23H24N8O3S3/c1-13-5-3-8-16(27-23(32)26-10-9-19-28-30-31-29-19)20(13)14-6-4-7-15(11-14)37(33,34)18-12-17(21(24)25)36-22(18)35-2/h3-8,11-12H,9-10H2,1-2H3,(H3,24,25)(H2,26,27,32)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50029051

((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-2A adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098169
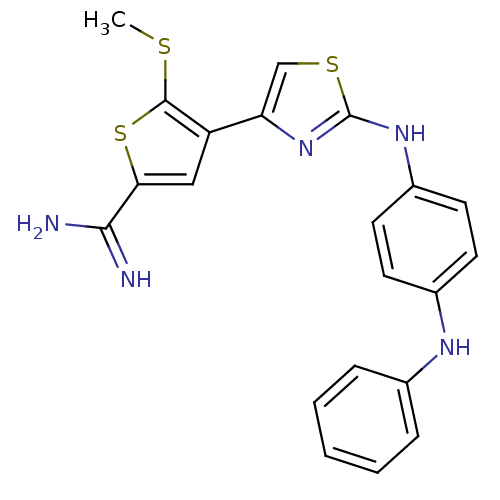
(5-Methylsulfanyl-4-[2-(4-phenylamino-phenylamino)-...)Show SMILES CSc1sc(cc1-c1csc(Nc2ccc(Nc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H19N5S3/c1-27-20-16(11-18(29-20)19(22)23)17-12-28-21(26-17)25-15-9-7-14(8-10-15)24-13-5-3-2-4-6-13/h2-12,24H,1H3,(H3,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147046
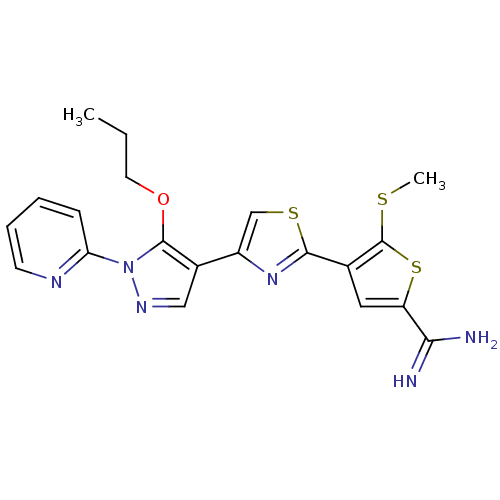
(5-Methylsulfanyl-4-[4-(5-propoxy-1-pyridin-2-yl-1H...)Show SMILES CCCOc1c(cnn1-c1ccccn1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H20N6OS3/c1-3-8-27-19-13(10-24-26(19)16-6-4-5-7-23-16)14-11-29-18(25-14)12-9-15(17(21)22)30-20(12)28-2/h4-7,9-11H,3,8H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182185
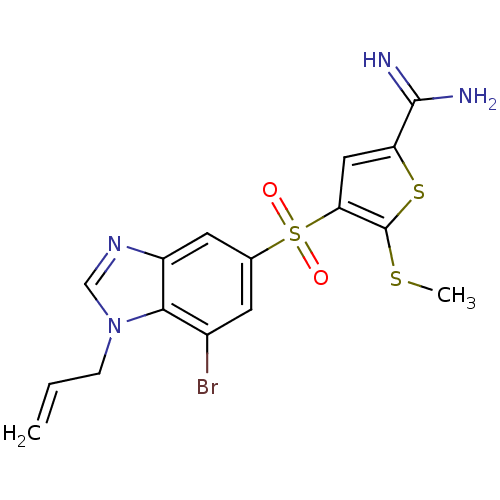
(4-(1-allyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(CC=C)cnc2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-11-6-9(5-10(17)14(11)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055831
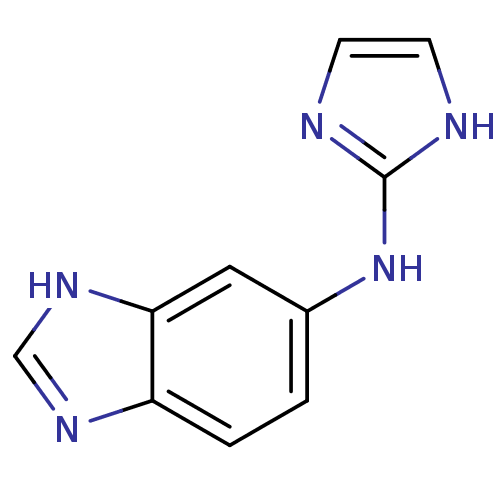
((1H-Benzoimidazol-5-yl)-(1H-imidazol-2-yl)-amine |...)Show InChI InChI=1S/C10H9N5/c1-2-8-9(14-6-13-8)5-7(1)15-10-11-3-4-12-10/h1-6H,(H,13,14)(H2,11,12,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50029051

((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for rat Alpha-2B adrenergic receptor |
J Med Chem 39: 1193-5 (1996)
Article DOI: 10.1021/jm960012o
BindingDB Entry DOI: 10.7270/Q2NZ889J |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098144
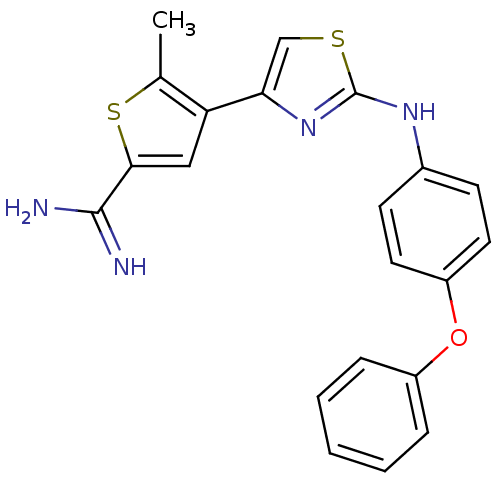
(5-Methyl-4-[2-(4-phenoxy-phenylamino)-thiazol-4-yl...)Show SMILES Cc1sc(cc1-c1csc(Nc2ccc(Oc3ccccc3)cc2)n1)C(N)=N Show InChI InChI=1S/C21H18N4OS2/c1-13-17(11-19(28-13)20(22)23)18-12-27-21(25-18)24-14-7-9-16(10-8-14)26-15-5-3-2-4-6-15/h2-12H,1H3,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 915-8 (2001)
BindingDB Entry DOI: 10.7270/Q2J67G5B |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50055832

((1H-Imidazol-2-yl)-(5-methyl-2,3-dihydro-benzo[1,4...)Show InChI InChI=1S/C12H13N3O2/c1-8-9(15-12-13-4-5-14-12)2-3-10-11(8)17-7-6-16-10/h2-5H,6-7H2,1H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from rat Alpha-2B adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data