 Found 49 hits with Last Name = 'slack' and Initial = 'k'
Found 49 hits with Last Name = 'slack' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185364
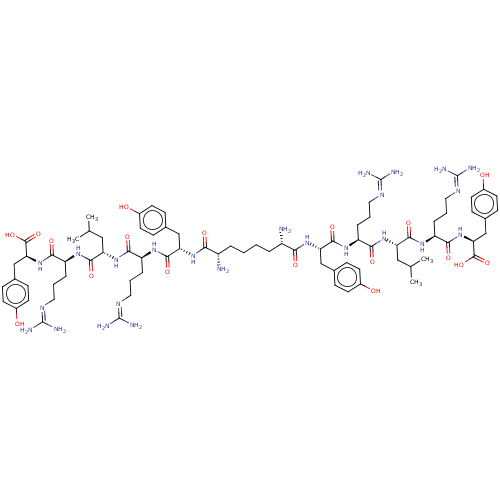
(CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C80H122N24O18/c1-43(2)37-59(71(115)95-57(15-9-35-93-79(87)88)69(113)103-63(75(119)120)41-47-21-29-51(107)30-22-47)101-67(111)55(13-7-33-91-77(83)84)97-73(117)61(39-45-17-25-49(105)26-18-45)99-65(109)53(81)11-5-6-12-54(82)66(110)100-62(40-46-19-27-50(106)28-20-46)74(118)98-56(14-8-34-92-78(85)86)68(112)102-60(38-44(3)4)72(116)96-58(16-10-36-94-80(89)90)70(114)104-64(76(121)122)42-48-23-31-52(108)32-24-48/h17-32,43-44,53-64,105-108H,5-16,33-42,81-82H2,1-4H3,(H,95,115)(H,96,116)(H,97,117)(H,98,118)(H,99,109)(H,100,110)(H,101,111)(H,102,112)(H,103,113)(H,104,114)(H,119,120)(H,121,122)(H4,83,84,91)(H4,85,86,92)(H4,87,88,93)(H4,89,90,94)/t53?,54?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]hPP from human NPY4 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185366
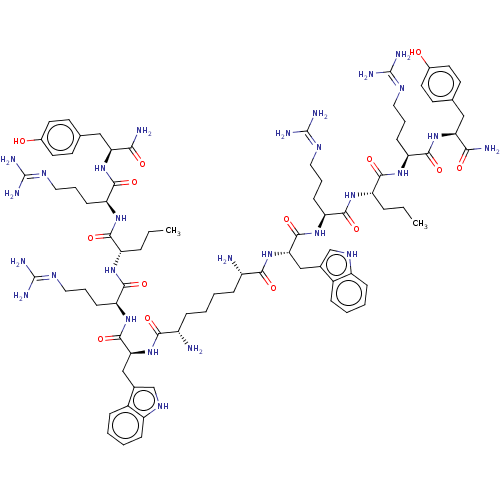
(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185367
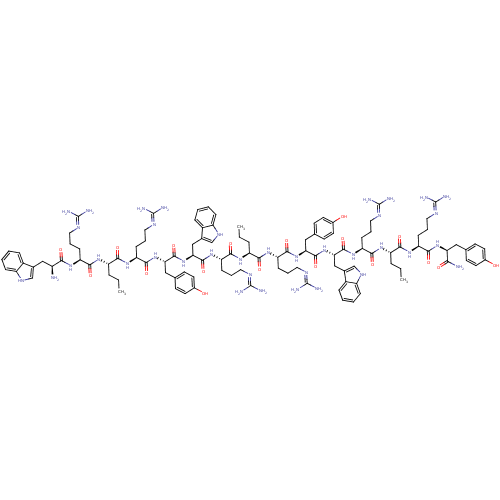
(CHEMBL413871 | H-[Trp-Arg-Nva-Arg-Tyr]3-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:154.161,136.143,125.132,88.92,99.103,81.85,56.58,70.74,33.34,3.3,18.18,7.14,wD:143.150,111.116,44.45,(19.56,-19.66,;18.24,-18.88,;16.9,-19.64,;15.57,-18.87,;15.58,-17.32,;14.25,-16.54,;12.91,-17.3,;14.26,-15.01,;15.6,-14.25,;15.61,-12.71,;16.95,-11.95,;16.96,-10.41,;18.3,-9.65,;19.63,-10.43,;18.31,-8.11,;12.93,-14.23,;11.59,-14.99,;11.58,-16.53,;10.27,-14.2,;8.93,-14.96,;10.28,-12.67,;11.62,-11.91,;13.02,-12.54,;14.06,-11.39,;13.29,-10.05,;13.78,-8.59,;12.76,-7.43,;11.24,-7.73,;10.76,-9.2,;11.79,-10.37,;14.23,-19.63,;12.9,-18.84,;14.22,-21.16,;15.55,-21.94,;16.88,-21.18,;18.21,-21.96,;19.55,-21.2,;20.88,-21.98,;22.21,-21.22,;22.23,-19.69,;23.54,-22,;15.53,-23.48,;14.19,-24.24,;16.86,-24.26,;16.85,-25.8,;15.51,-26.56,;15.5,-28.1,;16.71,-28.8,;16.7,-30.21,;15.49,-30.9,;15.47,-32.44,;14.26,-30.19,;14.27,-28.79,;18.17,-26.58,;19.51,-25.82,;18.16,-28.11,;19.49,-28.9,;20.83,-28.14,;22.15,-28.92,;22.3,-30.46,;23.82,-30.79,;24.6,-29.45,;26.12,-29.14,;26.6,-27.67,;25.58,-26.51,;24.06,-26.82,;23.57,-28.29,;19.47,-30.44,;18.14,-31.2,;20.8,-31.21,;20.79,-32.75,;22.12,-33.54,;23.45,-32.78,;24.78,-33.55,;26.12,-32.79,;27.45,-33.57,;27.44,-35.11,;28.79,-32.81,;19.45,-33.51,;18.12,-32.73,;19.44,-35.06,;18.1,-35.81,;18.09,-37.35,;19.42,-38.13,;19.41,-39.67,;16.77,-35.03,;16.78,-33.49,;15.43,-35.79,;15.42,-37.33,;16.75,-38.11,;16.74,-39.65,;18.07,-40.43,;18.06,-41.97,;19.39,-42.75,;20.72,-41.99,;19.37,-44.29,;14.08,-38.09,;12.76,-37.31,;14.08,-39.63,;12.74,-40.39,;12.73,-41.93,;14.05,-42.71,;15.39,-41.95,;16.72,-42.73,;16.71,-44.26,;18.04,-45.04,;15.37,-45.02,;14.05,-44.24,;11.41,-39.61,;11.42,-38.07,;10.07,-40.37,;8.74,-39.59,;8.84,-38.05,;7.56,-37.19,;6.12,-37.73,;5.16,-36.53,;6.01,-35.24,;5.61,-33.74,;6.71,-32.66,;8.21,-33.06,;8.6,-34.57,;7.5,-35.65,;7.4,-40.35,;7.39,-41.89,;6.08,-39.57,;4.74,-40.33,;4.73,-41.87,;6.05,-42.65,;6.04,-44.18,;7.37,-44.97,;8.71,-44.21,;8.72,-42.66,;10.04,-44.99,;3.41,-39.55,;3.42,-38.01,;2.07,-40.31,;.75,-39.53,;-.59,-40.29,;-1.92,-39.51,;-3.26,-40.27,;.76,-37.99,;-.57,-37.21,;2.1,-37.23,;2.11,-35.69,;3.45,-34.93,;3.46,-33.39,;4.8,-32.63,;4.81,-31.09,;6.15,-30.33,;7.48,-31.11,;6.16,-28.8,;.78,-34.91,;-.55,-35.67,;.8,-33.37,;-.53,-32.59,;-1.87,-33.35,;-3.2,-32.58,;-3.18,-31.03,;-4.52,-30.26,;-5.85,-31.02,;-7.17,-30.24,;-5.86,-32.56,;-4.53,-33.33,;-.52,-31.05,;-1.85,-30.27,;.82,-30.3,)| Show InChI InChI=1S/C111H159N37O18/c1-4-19-77(136-96(157)80(28-13-46-126-106(114)115)135-92(153)73(112)55-64-58-132-74-25-10-7-22-70(64)74)94(155)140-84(32-17-50-130-110(122)123)100(161)145-87(53-62-36-42-68(150)43-37-62)102(163)148-90(57-66-60-134-76-27-12-9-24-72(66)76)105(166)143-82(30-15-48-128-108(118)119)98(159)138-79(21-6-3)95(156)141-85(33-18-51-131-111(124)125)101(162)146-88(54-63-38-44-69(151)45-39-63)103(164)147-89(56-65-59-133-75-26-11-8-23-71(65)75)104(165)142-81(29-14-47-127-107(116)117)97(158)137-78(20-5-2)93(154)139-83(31-16-49-129-109(120)121)99(160)144-86(91(113)152)52-61-34-40-67(149)41-35-61/h7-12,22-27,34-45,58-60,73,77-90,132-134,149-151H,4-6,13-21,28-33,46-57,112H2,1-3H3,(H2,113,152)(H,135,153)(H,136,157)(H,137,158)(H,138,159)(H,139,154)(H,140,155)(H,141,156)(H,142,165)(H,143,166)(H,144,160)(H,145,161)(H,146,162)(H,147,164)(H,148,163)(H4,114,115,126)(H4,116,117,127)(H4,118,119,128)(H4,120,121,129)(H4,122,123,130)(H4,124,125,131)/t73-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]hPP from human NPY4 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]hPP from human NPY4 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185365
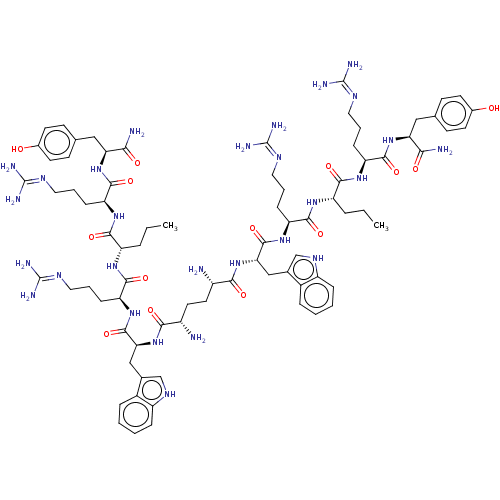
(Adp[-Trp-Arg-Nva-Arg-Tyr-NH2]2 | CHEMBL2371909 | C...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:99.104,3.3,18.18,73.77,66.69,41.43,wD:110.115,7.7,84.88,55.59,32.35,36.39,(.45,-20.37,;.5,-18.83,;-.81,-18.01,;-.76,-16.47,;.6,-15.75,;1.91,-16.56,;1.86,-18.1,;3.26,-15.83,;3.31,-14.29,;2.01,-13.48,;2.06,-11.94,;.74,-11.13,;.79,-9.59,;2.16,-8.86,;-.51,-8.78,;4.57,-16.65,;5.93,-15.92,;5.98,-14.38,;7.24,-16.73,;7.19,-18.27,;5.83,-19,;4.44,-18.35,;3.38,-19.47,;4.11,-20.83,;3.6,-22.29,;4.61,-23.46,;6.13,-23.19,;6.64,-21.73,;5.64,-20.55,;8.6,-16,;8.56,-14.47,;7.22,-13.75,;9.86,-13.67,;11.2,-14.39,;9.82,-12.14,;11.11,-11.35,;11.07,-9.82,;9.73,-9.1,;12.37,-9.03,;12.33,-7.5,;13.71,-9.75,;15.05,-8.98,;15.05,-7.44,;16.38,-6.67,;17.79,-7.28,;18.82,-6.12,;18.04,-4.78,;18.5,-3.31,;17.46,-2.17,;15.95,-2.49,;15.48,-3.97,;16.52,-5.12,;16.38,-9.75,;16.38,-11.29,;17.72,-8.98,;19.05,-9.75,;19.05,-11.29,;20.38,-12.06,;20.38,-13.6,;21.72,-14.37,;21.72,-15.91,;20.38,-16.68,;23.05,-16.68,;20.38,-8.98,;20.38,-7.44,;21.72,-9.75,;23.05,-8.98,;23.05,-7.44,;21.72,-6.67,;21.72,-5.13,;24.39,-9.75,;24.39,-11.29,;25.72,-8.98,;25.72,-7.44,;24.39,-6.67,;24.39,-5.13,;23.05,-4.36,;23.05,-2.82,;21.72,-2.05,;20.38,-2.82,;21.72,-.51,;27.05,-6.67,;28.39,-7.44,;27.05,-5.13,;28.39,-4.36,;29.72,-5.13,;31.05,-4.36,;31.05,-2.96,;32.27,-2.26,;33.47,-2.96,;34.81,-2.19,;33.47,-4.36,;32.26,-5.06,;28.39,-2.82,;29.72,-2.05,;27.05,-2.05,;-2.07,-15.66,;-2.02,-14.12,;-3.43,-16.39,;-3.48,-17.93,;-2.17,-18.74,;-2.22,-20.28,;-.91,-21.09,;-.96,-22.63,;.35,-23.44,;1.71,-22.72,;.3,-24.98,;-4.83,-18.66,;-6.14,-17.84,;-4.88,-20.19,;-6.24,-20.92,;-7.55,-20.11,;-8.91,-20.84,;-8.95,-22.24,;-10.19,-22.9,;-11.37,-22.16,;-12.74,-22.89,;-11.34,-20.76,;-10.1,-20.1,;-6.29,-22.46,;-7.65,-23.19,;-4.98,-23.27,)| Show InChI InChI=1S/C80H118N28O14/c1-3-13-55(69(115)101-59(21-11-35-95-79(89)90)73(119)105-61(65(83)111)37-43-23-27-47(109)28-24-43)99-71(117)57(19-9-33-93-77(85)86)103-75(121)63(39-45-41-97-53-17-7-5-15-49(45)53)107-67(113)51(81)31-32-52(82)68(114)108-64(40-46-42-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-34-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-36-96-80(91)92)74(120)106-62(66(84)112)38-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,41-42,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-40,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51?,52?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185369
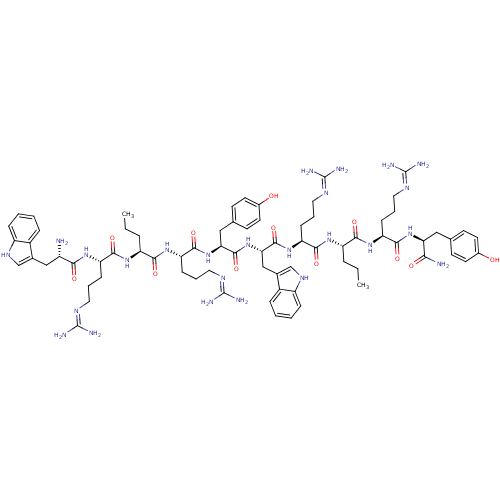
(CHEMBL408891 | H-[Trp-Arg-Nva-Arg-Tyr]2-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:88.92,56.58,wD:99.103,81.85,70.74,33.34,44.45,3.3,18.18,7.14,(1.08,-23.2,;1.08,-21.66,;2.42,-20.89,;2.42,-19.35,;1.08,-18.58,;1.08,-17.04,;2.42,-16.27,;-.25,-16.27,;-1.58,-17.04,;-2.92,-16.27,;-4.25,-17.04,;-5.59,-16.27,;-6.92,-17.04,;-6.92,-18.58,;-8.25,-16.27,;-.25,-14.73,;1.08,-13.96,;2.42,-14.73,;1.08,-12.42,;2.42,-11.65,;-.25,-11.65,;-1.58,-12.42,;-1.74,-13.96,;-3.25,-14.29,;-4.03,-12.96,;-5.54,-12.63,;-6.02,-11.17,;-4.99,-10.02,;-3.48,-10.33,;-2.99,-11.79,;3.75,-18.58,;3.75,-17.04,;5.08,-19.35,;5.08,-20.89,;3.75,-21.66,;3.75,-23.2,;2.42,-23.97,;2.42,-25.51,;1.08,-26.28,;-.25,-25.51,;1.08,-27.82,;6.42,-21.66,;7.75,-20.89,;6.42,-23.2,;7.75,-23.97,;7.75,-25.51,;6.42,-26.28,;5.21,-25.59,;4,-26.3,;4.01,-27.69,;2.67,-28.46,;5.22,-28.39,;6.42,-27.68,;9.09,-23.2,;9.09,-21.66,;10.42,-23.97,;11.75,-23.2,;11.75,-21.66,;13.09,-20.89,;14.49,-21.5,;15.53,-20.35,;14.76,-19.01,;15.23,-17.54,;14.19,-16.39,;12.67,-16.71,;12.2,-18.19,;13.24,-19.34,;13.09,-23.97,;13.09,-25.51,;14.42,-23.2,;15.76,-23.97,;15.76,-25.51,;14.42,-26.28,;14.42,-27.82,;13.09,-28.59,;11.75,-27.82,;11.75,-26.28,;10.42,-28.59,;17.09,-23.2,;17.09,-21.66,;18.42,-23.97,;19.76,-23.2,;21.09,-23.97,;22.43,-23.2,;23.76,-23.97,;19.76,-21.66,;21.09,-20.89,;18.42,-20.89,;18.42,-19.35,;17.09,-18.58,;17.09,-17.04,;15.76,-16.27,;15.76,-14.73,;14.42,-13.96,;13.09,-14.73,;14.42,-12.42,;19.76,-18.58,;21.09,-19.35,;19.76,-17.04,;21.09,-16.27,;22.43,-17.04,;23.76,-16.27,;23.75,-14.86,;24.96,-14.16,;26.17,-14.86,;27.51,-14.09,;26.18,-16.26,;24.97,-16.96,;21.09,-14.73,;22.43,-13.96,;19.76,-13.96,)| Show InChI InChI=1S/C74H107N25O12/c1-3-13-52(92-65(106)54(19-9-31-85-71(77)78)91-62(103)49(75)37-43-39-89-50-17-7-5-15-47(43)50)64(105)95-57(22-12-34-88-74(83)84)68(109)98-59(36-42-25-29-46(101)30-26-42)69(110)99-60(38-44-40-90-51-18-8-6-16-48(44)51)70(111)96-55(20-10-32-86-72(79)80)66(107)93-53(14-4-2)63(104)94-56(21-11-33-87-73(81)82)67(108)97-58(61(76)102)35-41-23-27-45(100)28-24-41/h5-8,15-18,23-30,39-40,49,52-60,89-90,100-101H,3-4,9-14,19-22,31-38,75H2,1-2H3,(H2,76,102)(H,91,103)(H,92,106)(H,93,107)(H,94,104)(H,95,105)(H,96,111)(H,97,108)(H,98,109)(H,99,110)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t49-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]hPP from human NPY4 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185364

(CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C80H122N24O18/c1-43(2)37-59(71(115)95-57(15-9-35-93-79(87)88)69(113)103-63(75(119)120)41-47-21-29-51(107)30-22-47)101-67(111)55(13-7-33-91-77(83)84)97-73(117)61(39-45-17-25-49(105)26-18-45)99-65(109)53(81)11-5-6-12-54(82)66(110)100-62(40-46-19-27-50(106)28-20-46)74(118)98-56(14-8-34-92-78(85)86)68(112)102-60(38-44(3)4)72(116)96-58(16-10-36-94-80(89)90)70(114)104-64(76(121)122)42-48-23-31-52(108)32-24-48/h17-32,43-44,53-64,105-108H,5-16,33-42,81-82H2,1-4H3,(H,95,115)(H,96,116)(H,97,117)(H,98,118)(H,99,109)(H,100,110)(H,101,111)(H,102,112)(H,103,113)(H,104,114)(H,119,120)(H,121,122)(H4,83,84,91)(H4,85,86,92)(H4,87,88,93)(H4,89,90,94)/t53?,54?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50049744
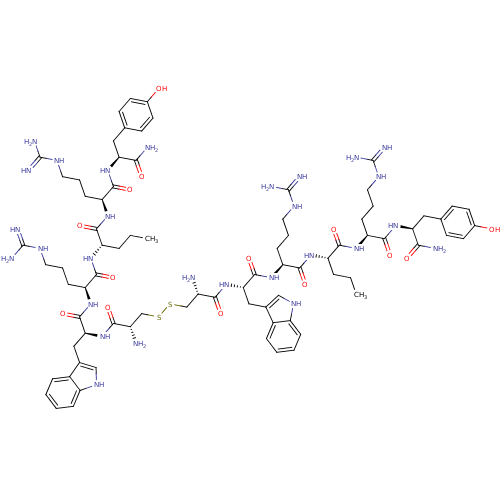
(CHEMBL438548 | NPY-analogs | [Cys-Trp-Arg-Nva-Arg-...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CSSC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C80H118N28O14S2/c1-3-13-55(69(115)101-59(21-11-33-95-79(89)90)73(119)105-61(65(83)111)35-43-23-27-47(109)28-24-43)99-71(117)57(19-9-31-93-77(85)86)103-75(121)63(37-45-39-97-53-17-7-5-15-49(45)53)107-67(113)51(81)41-123-124-42-52(82)68(114)108-64(38-46-40-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-32-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-34-96-80(91)92)74(120)106-62(66(84)112)36-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,39-40,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-38,41-42,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51-,52-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185367

(CHEMBL413871 | H-[Trp-Arg-Nva-Arg-Tyr]3-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:154.161,136.143,125.132,88.92,99.103,81.85,56.58,70.74,33.34,3.3,18.18,7.14,wD:143.150,111.116,44.45,(19.56,-19.66,;18.24,-18.88,;16.9,-19.64,;15.57,-18.87,;15.58,-17.32,;14.25,-16.54,;12.91,-17.3,;14.26,-15.01,;15.6,-14.25,;15.61,-12.71,;16.95,-11.95,;16.96,-10.41,;18.3,-9.65,;19.63,-10.43,;18.31,-8.11,;12.93,-14.23,;11.59,-14.99,;11.58,-16.53,;10.27,-14.2,;8.93,-14.96,;10.28,-12.67,;11.62,-11.91,;13.02,-12.54,;14.06,-11.39,;13.29,-10.05,;13.78,-8.59,;12.76,-7.43,;11.24,-7.73,;10.76,-9.2,;11.79,-10.37,;14.23,-19.63,;12.9,-18.84,;14.22,-21.16,;15.55,-21.94,;16.88,-21.18,;18.21,-21.96,;19.55,-21.2,;20.88,-21.98,;22.21,-21.22,;22.23,-19.69,;23.54,-22,;15.53,-23.48,;14.19,-24.24,;16.86,-24.26,;16.85,-25.8,;15.51,-26.56,;15.5,-28.1,;16.71,-28.8,;16.7,-30.21,;15.49,-30.9,;15.47,-32.44,;14.26,-30.19,;14.27,-28.79,;18.17,-26.58,;19.51,-25.82,;18.16,-28.11,;19.49,-28.9,;20.83,-28.14,;22.15,-28.92,;22.3,-30.46,;23.82,-30.79,;24.6,-29.45,;26.12,-29.14,;26.6,-27.67,;25.58,-26.51,;24.06,-26.82,;23.57,-28.29,;19.47,-30.44,;18.14,-31.2,;20.8,-31.21,;20.79,-32.75,;22.12,-33.54,;23.45,-32.78,;24.78,-33.55,;26.12,-32.79,;27.45,-33.57,;27.44,-35.11,;28.79,-32.81,;19.45,-33.51,;18.12,-32.73,;19.44,-35.06,;18.1,-35.81,;18.09,-37.35,;19.42,-38.13,;19.41,-39.67,;16.77,-35.03,;16.78,-33.49,;15.43,-35.79,;15.42,-37.33,;16.75,-38.11,;16.74,-39.65,;18.07,-40.43,;18.06,-41.97,;19.39,-42.75,;20.72,-41.99,;19.37,-44.29,;14.08,-38.09,;12.76,-37.31,;14.08,-39.63,;12.74,-40.39,;12.73,-41.93,;14.05,-42.71,;15.39,-41.95,;16.72,-42.73,;16.71,-44.26,;18.04,-45.04,;15.37,-45.02,;14.05,-44.24,;11.41,-39.61,;11.42,-38.07,;10.07,-40.37,;8.74,-39.59,;8.84,-38.05,;7.56,-37.19,;6.12,-37.73,;5.16,-36.53,;6.01,-35.24,;5.61,-33.74,;6.71,-32.66,;8.21,-33.06,;8.6,-34.57,;7.5,-35.65,;7.4,-40.35,;7.39,-41.89,;6.08,-39.57,;4.74,-40.33,;4.73,-41.87,;6.05,-42.65,;6.04,-44.18,;7.37,-44.97,;8.71,-44.21,;8.72,-42.66,;10.04,-44.99,;3.41,-39.55,;3.42,-38.01,;2.07,-40.31,;.75,-39.53,;-.59,-40.29,;-1.92,-39.51,;-3.26,-40.27,;.76,-37.99,;-.57,-37.21,;2.1,-37.23,;2.11,-35.69,;3.45,-34.93,;3.46,-33.39,;4.8,-32.63,;4.81,-31.09,;6.15,-30.33,;7.48,-31.11,;6.16,-28.8,;.78,-34.91,;-.55,-35.67,;.8,-33.37,;-.53,-32.59,;-1.87,-33.35,;-3.2,-32.58,;-3.18,-31.03,;-4.52,-30.26,;-5.85,-31.02,;-7.17,-30.24,;-5.86,-32.56,;-4.53,-33.33,;-.52,-31.05,;-1.85,-30.27,;.82,-30.3,)| Show InChI InChI=1S/C111H159N37O18/c1-4-19-77(136-96(157)80(28-13-46-126-106(114)115)135-92(153)73(112)55-64-58-132-74-25-10-7-22-70(64)74)94(155)140-84(32-17-50-130-110(122)123)100(161)145-87(53-62-36-42-68(150)43-37-62)102(163)148-90(57-66-60-134-76-27-12-9-24-72(66)76)105(166)143-82(30-15-48-128-108(118)119)98(159)138-79(21-6-3)95(156)141-85(33-18-51-131-111(124)125)101(162)146-88(54-63-38-44-69(151)45-39-63)103(164)147-89(56-65-59-133-75-26-11-8-23-71(65)75)104(165)142-81(29-14-47-127-107(116)117)97(158)137-78(20-5-2)93(154)139-83(31-16-49-129-109(120)121)99(160)144-86(91(113)152)52-61-34-40-67(149)41-35-61/h7-12,22-27,34-45,58-60,73,77-90,132-134,149-151H,4-6,13-21,28-33,46-57,112H2,1-3H3,(H2,113,152)(H,135,153)(H,136,157)(H,137,158)(H,138,159)(H,139,154)(H,140,155)(H,141,156)(H,142,165)(H,143,166)(H,144,160)(H,145,161)(H,146,162)(H,147,164)(H,148,163)(H4,114,115,126)(H4,116,117,127)(H4,118,119,128)(H4,120,121,129)(H4,122,123,130)(H4,124,125,131)/t73-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185367

(CHEMBL413871 | H-[Trp-Arg-Nva-Arg-Tyr]3-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:154.161,136.143,125.132,88.92,99.103,81.85,56.58,70.74,33.34,3.3,18.18,7.14,wD:143.150,111.116,44.45,(19.56,-19.66,;18.24,-18.88,;16.9,-19.64,;15.57,-18.87,;15.58,-17.32,;14.25,-16.54,;12.91,-17.3,;14.26,-15.01,;15.6,-14.25,;15.61,-12.71,;16.95,-11.95,;16.96,-10.41,;18.3,-9.65,;19.63,-10.43,;18.31,-8.11,;12.93,-14.23,;11.59,-14.99,;11.58,-16.53,;10.27,-14.2,;8.93,-14.96,;10.28,-12.67,;11.62,-11.91,;13.02,-12.54,;14.06,-11.39,;13.29,-10.05,;13.78,-8.59,;12.76,-7.43,;11.24,-7.73,;10.76,-9.2,;11.79,-10.37,;14.23,-19.63,;12.9,-18.84,;14.22,-21.16,;15.55,-21.94,;16.88,-21.18,;18.21,-21.96,;19.55,-21.2,;20.88,-21.98,;22.21,-21.22,;22.23,-19.69,;23.54,-22,;15.53,-23.48,;14.19,-24.24,;16.86,-24.26,;16.85,-25.8,;15.51,-26.56,;15.5,-28.1,;16.71,-28.8,;16.7,-30.21,;15.49,-30.9,;15.47,-32.44,;14.26,-30.19,;14.27,-28.79,;18.17,-26.58,;19.51,-25.82,;18.16,-28.11,;19.49,-28.9,;20.83,-28.14,;22.15,-28.92,;22.3,-30.46,;23.82,-30.79,;24.6,-29.45,;26.12,-29.14,;26.6,-27.67,;25.58,-26.51,;24.06,-26.82,;23.57,-28.29,;19.47,-30.44,;18.14,-31.2,;20.8,-31.21,;20.79,-32.75,;22.12,-33.54,;23.45,-32.78,;24.78,-33.55,;26.12,-32.79,;27.45,-33.57,;27.44,-35.11,;28.79,-32.81,;19.45,-33.51,;18.12,-32.73,;19.44,-35.06,;18.1,-35.81,;18.09,-37.35,;19.42,-38.13,;19.41,-39.67,;16.77,-35.03,;16.78,-33.49,;15.43,-35.79,;15.42,-37.33,;16.75,-38.11,;16.74,-39.65,;18.07,-40.43,;18.06,-41.97,;19.39,-42.75,;20.72,-41.99,;19.37,-44.29,;14.08,-38.09,;12.76,-37.31,;14.08,-39.63,;12.74,-40.39,;12.73,-41.93,;14.05,-42.71,;15.39,-41.95,;16.72,-42.73,;16.71,-44.26,;18.04,-45.04,;15.37,-45.02,;14.05,-44.24,;11.41,-39.61,;11.42,-38.07,;10.07,-40.37,;8.74,-39.59,;8.84,-38.05,;7.56,-37.19,;6.12,-37.73,;5.16,-36.53,;6.01,-35.24,;5.61,-33.74,;6.71,-32.66,;8.21,-33.06,;8.6,-34.57,;7.5,-35.65,;7.4,-40.35,;7.39,-41.89,;6.08,-39.57,;4.74,-40.33,;4.73,-41.87,;6.05,-42.65,;6.04,-44.18,;7.37,-44.97,;8.71,-44.21,;8.72,-42.66,;10.04,-44.99,;3.41,-39.55,;3.42,-38.01,;2.07,-40.31,;.75,-39.53,;-.59,-40.29,;-1.92,-39.51,;-3.26,-40.27,;.76,-37.99,;-.57,-37.21,;2.1,-37.23,;2.11,-35.69,;3.45,-34.93,;3.46,-33.39,;4.8,-32.63,;4.81,-31.09,;6.15,-30.33,;7.48,-31.11,;6.16,-28.8,;.78,-34.91,;-.55,-35.67,;.8,-33.37,;-.53,-32.59,;-1.87,-33.35,;-3.2,-32.58,;-3.18,-31.03,;-4.52,-30.26,;-5.85,-31.02,;-7.17,-30.24,;-5.86,-32.56,;-4.53,-33.33,;-.52,-31.05,;-1.85,-30.27,;.82,-30.3,)| Show InChI InChI=1S/C111H159N37O18/c1-4-19-77(136-96(157)80(28-13-46-126-106(114)115)135-92(153)73(112)55-64-58-132-74-25-10-7-22-70(64)74)94(155)140-84(32-17-50-130-110(122)123)100(161)145-87(53-62-36-42-68(150)43-37-62)102(163)148-90(57-66-60-134-76-27-12-9-24-72(66)76)105(166)143-82(30-15-48-128-108(118)119)98(159)138-79(21-6-3)95(156)141-85(33-18-51-131-111(124)125)101(162)146-88(54-63-38-44-69(151)45-39-63)103(164)147-89(56-65-59-133-75-26-11-8-23-71(65)75)104(165)142-81(29-14-47-127-107(116)117)97(158)137-78(20-5-2)93(154)139-83(31-16-49-129-109(120)121)99(160)144-86(91(113)152)52-61-34-40-67(149)41-35-61/h7-12,22-27,34-45,58-60,73,77-90,132-134,149-151H,4-6,13-21,28-33,46-57,112H2,1-3H3,(H2,113,152)(H,135,153)(H,136,157)(H,137,158)(H,138,159)(H,139,154)(H,140,155)(H,141,156)(H,142,165)(H,143,166)(H,144,160)(H,145,161)(H,146,162)(H,147,164)(H,148,163)(H4,114,115,126)(H4,116,117,127)(H4,118,119,128)(H4,120,121,129)(H4,122,123,130)(H4,124,125,131)/t73-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 expressed in HEK-293 cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185365

(Adp[-Trp-Arg-Nva-Arg-Tyr-NH2]2 | CHEMBL2371909 | C...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:99.104,3.3,18.18,73.77,66.69,41.43,wD:110.115,7.7,84.88,55.59,32.35,36.39,(.45,-20.37,;.5,-18.83,;-.81,-18.01,;-.76,-16.47,;.6,-15.75,;1.91,-16.56,;1.86,-18.1,;3.26,-15.83,;3.31,-14.29,;2.01,-13.48,;2.06,-11.94,;.74,-11.13,;.79,-9.59,;2.16,-8.86,;-.51,-8.78,;4.57,-16.65,;5.93,-15.92,;5.98,-14.38,;7.24,-16.73,;7.19,-18.27,;5.83,-19,;4.44,-18.35,;3.38,-19.47,;4.11,-20.83,;3.6,-22.29,;4.61,-23.46,;6.13,-23.19,;6.64,-21.73,;5.64,-20.55,;8.6,-16,;8.56,-14.47,;7.22,-13.75,;9.86,-13.67,;11.2,-14.39,;9.82,-12.14,;11.11,-11.35,;11.07,-9.82,;9.73,-9.1,;12.37,-9.03,;12.33,-7.5,;13.71,-9.75,;15.05,-8.98,;15.05,-7.44,;16.38,-6.67,;17.79,-7.28,;18.82,-6.12,;18.04,-4.78,;18.5,-3.31,;17.46,-2.17,;15.95,-2.49,;15.48,-3.97,;16.52,-5.12,;16.38,-9.75,;16.38,-11.29,;17.72,-8.98,;19.05,-9.75,;19.05,-11.29,;20.38,-12.06,;20.38,-13.6,;21.72,-14.37,;21.72,-15.91,;20.38,-16.68,;23.05,-16.68,;20.38,-8.98,;20.38,-7.44,;21.72,-9.75,;23.05,-8.98,;23.05,-7.44,;21.72,-6.67,;21.72,-5.13,;24.39,-9.75,;24.39,-11.29,;25.72,-8.98,;25.72,-7.44,;24.39,-6.67,;24.39,-5.13,;23.05,-4.36,;23.05,-2.82,;21.72,-2.05,;20.38,-2.82,;21.72,-.51,;27.05,-6.67,;28.39,-7.44,;27.05,-5.13,;28.39,-4.36,;29.72,-5.13,;31.05,-4.36,;31.05,-2.96,;32.27,-2.26,;33.47,-2.96,;34.81,-2.19,;33.47,-4.36,;32.26,-5.06,;28.39,-2.82,;29.72,-2.05,;27.05,-2.05,;-2.07,-15.66,;-2.02,-14.12,;-3.43,-16.39,;-3.48,-17.93,;-2.17,-18.74,;-2.22,-20.28,;-.91,-21.09,;-.96,-22.63,;.35,-23.44,;1.71,-22.72,;.3,-24.98,;-4.83,-18.66,;-6.14,-17.84,;-4.88,-20.19,;-6.24,-20.92,;-7.55,-20.11,;-8.91,-20.84,;-8.95,-22.24,;-10.19,-22.9,;-11.37,-22.16,;-12.74,-22.89,;-11.34,-20.76,;-10.1,-20.1,;-6.29,-22.46,;-7.65,-23.19,;-4.98,-23.27,)| Show InChI InChI=1S/C80H118N28O14/c1-3-13-55(69(115)101-59(21-11-35-95-79(89)90)73(119)105-61(65(83)111)37-43-23-27-47(109)28-24-43)99-71(117)57(19-9-33-93-77(85)86)103-75(121)63(39-45-41-97-53-17-7-5-15-49(45)53)107-67(113)51(81)31-32-52(82)68(114)108-64(40-46-42-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-34-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-36-96-80(91)92)74(120)106-62(66(84)112)38-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,41-42,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-40,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51?,52?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185369

(CHEMBL408891 | H-[Trp-Arg-Nva-Arg-Tyr]2-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:88.92,56.58,wD:99.103,81.85,70.74,33.34,44.45,3.3,18.18,7.14,(1.08,-23.2,;1.08,-21.66,;2.42,-20.89,;2.42,-19.35,;1.08,-18.58,;1.08,-17.04,;2.42,-16.27,;-.25,-16.27,;-1.58,-17.04,;-2.92,-16.27,;-4.25,-17.04,;-5.59,-16.27,;-6.92,-17.04,;-6.92,-18.58,;-8.25,-16.27,;-.25,-14.73,;1.08,-13.96,;2.42,-14.73,;1.08,-12.42,;2.42,-11.65,;-.25,-11.65,;-1.58,-12.42,;-1.74,-13.96,;-3.25,-14.29,;-4.03,-12.96,;-5.54,-12.63,;-6.02,-11.17,;-4.99,-10.02,;-3.48,-10.33,;-2.99,-11.79,;3.75,-18.58,;3.75,-17.04,;5.08,-19.35,;5.08,-20.89,;3.75,-21.66,;3.75,-23.2,;2.42,-23.97,;2.42,-25.51,;1.08,-26.28,;-.25,-25.51,;1.08,-27.82,;6.42,-21.66,;7.75,-20.89,;6.42,-23.2,;7.75,-23.97,;7.75,-25.51,;6.42,-26.28,;5.21,-25.59,;4,-26.3,;4.01,-27.69,;2.67,-28.46,;5.22,-28.39,;6.42,-27.68,;9.09,-23.2,;9.09,-21.66,;10.42,-23.97,;11.75,-23.2,;11.75,-21.66,;13.09,-20.89,;14.49,-21.5,;15.53,-20.35,;14.76,-19.01,;15.23,-17.54,;14.19,-16.39,;12.67,-16.71,;12.2,-18.19,;13.24,-19.34,;13.09,-23.97,;13.09,-25.51,;14.42,-23.2,;15.76,-23.97,;15.76,-25.51,;14.42,-26.28,;14.42,-27.82,;13.09,-28.59,;11.75,-27.82,;11.75,-26.28,;10.42,-28.59,;17.09,-23.2,;17.09,-21.66,;18.42,-23.97,;19.76,-23.2,;21.09,-23.97,;22.43,-23.2,;23.76,-23.97,;19.76,-21.66,;21.09,-20.89,;18.42,-20.89,;18.42,-19.35,;17.09,-18.58,;17.09,-17.04,;15.76,-16.27,;15.76,-14.73,;14.42,-13.96,;13.09,-14.73,;14.42,-12.42,;19.76,-18.58,;21.09,-19.35,;19.76,-17.04,;21.09,-16.27,;22.43,-17.04,;23.76,-16.27,;23.75,-14.86,;24.96,-14.16,;26.17,-14.86,;27.51,-14.09,;26.18,-16.26,;24.97,-16.96,;21.09,-14.73,;22.43,-13.96,;19.76,-13.96,)| Show InChI InChI=1S/C74H107N25O12/c1-3-13-52(92-65(106)54(19-9-31-85-71(77)78)91-62(103)49(75)37-43-39-89-50-17-7-5-15-47(43)50)64(105)95-57(22-12-34-88-74(83)84)68(109)98-59(36-42-25-29-46(101)30-26-42)69(110)99-60(38-44-40-90-51-18-8-6-16-48(44)51)70(111)96-55(20-10-32-86-72(79)80)66(107)93-53(14-4-2)63(104)94-56(21-11-33-87-73(81)82)67(108)97-58(61(76)102)35-41-23-27-45(100)28-24-41/h5-8,15-18,23-30,39-40,49,52-60,89-90,100-101H,3-4,9-14,19-22,31-38,75H2,1-2H3,(H2,76,102)(H,91,103)(H,92,106)(H,93,107)(H,94,104)(H,95,105)(H,96,111)(H,97,108)(H,98,109)(H,99,110)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t49-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50049744

(CHEMBL438548 | NPY-analogs | [Cys-Trp-Arg-Nva-Arg-...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CSSC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C80H118N28O14S2/c1-3-13-55(69(115)101-59(21-11-33-95-79(89)90)73(119)105-61(65(83)111)35-43-23-27-47(109)28-24-43)99-71(117)57(19-9-31-93-77(85)86)103-75(121)63(37-45-39-97-53-17-7-5-15-49(45)53)107-67(113)51(81)41-123-124-42-52(82)68(114)108-64(38-46-40-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-32-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-34-96-80(91)92)74(120)106-62(66(84)112)36-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,39-40,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-38,41-42,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51-,52-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY1 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185365

(Adp[-Trp-Arg-Nva-Arg-Tyr-NH2]2 | CHEMBL2371909 | C...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:99.104,3.3,18.18,73.77,66.69,41.43,wD:110.115,7.7,84.88,55.59,32.35,36.39,(.45,-20.37,;.5,-18.83,;-.81,-18.01,;-.76,-16.47,;.6,-15.75,;1.91,-16.56,;1.86,-18.1,;3.26,-15.83,;3.31,-14.29,;2.01,-13.48,;2.06,-11.94,;.74,-11.13,;.79,-9.59,;2.16,-8.86,;-.51,-8.78,;4.57,-16.65,;5.93,-15.92,;5.98,-14.38,;7.24,-16.73,;7.19,-18.27,;5.83,-19,;4.44,-18.35,;3.38,-19.47,;4.11,-20.83,;3.6,-22.29,;4.61,-23.46,;6.13,-23.19,;6.64,-21.73,;5.64,-20.55,;8.6,-16,;8.56,-14.47,;7.22,-13.75,;9.86,-13.67,;11.2,-14.39,;9.82,-12.14,;11.11,-11.35,;11.07,-9.82,;9.73,-9.1,;12.37,-9.03,;12.33,-7.5,;13.71,-9.75,;15.05,-8.98,;15.05,-7.44,;16.38,-6.67,;17.79,-7.28,;18.82,-6.12,;18.04,-4.78,;18.5,-3.31,;17.46,-2.17,;15.95,-2.49,;15.48,-3.97,;16.52,-5.12,;16.38,-9.75,;16.38,-11.29,;17.72,-8.98,;19.05,-9.75,;19.05,-11.29,;20.38,-12.06,;20.38,-13.6,;21.72,-14.37,;21.72,-15.91,;20.38,-16.68,;23.05,-16.68,;20.38,-8.98,;20.38,-7.44,;21.72,-9.75,;23.05,-8.98,;23.05,-7.44,;21.72,-6.67,;21.72,-5.13,;24.39,-9.75,;24.39,-11.29,;25.72,-8.98,;25.72,-7.44,;24.39,-6.67,;24.39,-5.13,;23.05,-4.36,;23.05,-2.82,;21.72,-2.05,;20.38,-2.82,;21.72,-.51,;27.05,-6.67,;28.39,-7.44,;27.05,-5.13,;28.39,-4.36,;29.72,-5.13,;31.05,-4.36,;31.05,-2.96,;32.27,-2.26,;33.47,-2.96,;34.81,-2.19,;33.47,-4.36,;32.26,-5.06,;28.39,-2.82,;29.72,-2.05,;27.05,-2.05,;-2.07,-15.66,;-2.02,-14.12,;-3.43,-16.39,;-3.48,-17.93,;-2.17,-18.74,;-2.22,-20.28,;-.91,-21.09,;-.96,-22.63,;.35,-23.44,;1.71,-22.72,;.3,-24.98,;-4.83,-18.66,;-6.14,-17.84,;-4.88,-20.19,;-6.24,-20.92,;-7.55,-20.11,;-8.91,-20.84,;-8.95,-22.24,;-10.19,-22.9,;-11.37,-22.16,;-12.74,-22.89,;-11.34,-20.76,;-10.1,-20.1,;-6.29,-22.46,;-7.65,-23.19,;-4.98,-23.27,)| Show InChI InChI=1S/C80H118N28O14/c1-3-13-55(69(115)101-59(21-11-35-95-79(89)90)73(119)105-61(65(83)111)37-43-23-27-47(109)28-24-43)99-71(117)57(19-9-33-93-77(85)86)103-75(121)63(39-45-41-97-53-17-7-5-15-49(45)53)107-67(113)51(81)31-32-52(82)68(114)108-64(40-46-42-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-34-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-36-96-80(91)92)74(120)106-62(66(84)112)38-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,41-42,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-40,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51?,52?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185364

(CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C80H122N24O18/c1-43(2)37-59(71(115)95-57(15-9-35-93-79(87)88)69(113)103-63(75(119)120)41-47-21-29-51(107)30-22-47)101-67(111)55(13-7-33-91-77(83)84)97-73(117)61(39-45-17-25-49(105)26-18-45)99-65(109)53(81)11-5-6-12-54(82)66(110)100-62(40-46-19-27-50(106)28-20-46)74(118)98-56(14-8-34-92-78(85)86)68(112)102-60(38-44(3)4)72(116)96-58(16-10-36-94-80(89)90)70(114)104-64(76(121)122)42-48-23-31-52(108)32-24-48/h17-32,43-44,53-64,105-108H,5-16,33-42,81-82H2,1-4H3,(H,95,115)(H,96,116)(H,97,117)(H,98,118)(H,99,109)(H,100,110)(H,101,111)(H,102,112)(H,103,113)(H,104,114)(H,119,120)(H,121,122)(H4,83,84,91)(H4,85,86,92)(H4,87,88,93)(H4,89,90,94)/t53?,54?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185369

(CHEMBL408891 | H-[Trp-Arg-Nva-Arg-Tyr]2-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:88.92,56.58,wD:99.103,81.85,70.74,33.34,44.45,3.3,18.18,7.14,(1.08,-23.2,;1.08,-21.66,;2.42,-20.89,;2.42,-19.35,;1.08,-18.58,;1.08,-17.04,;2.42,-16.27,;-.25,-16.27,;-1.58,-17.04,;-2.92,-16.27,;-4.25,-17.04,;-5.59,-16.27,;-6.92,-17.04,;-6.92,-18.58,;-8.25,-16.27,;-.25,-14.73,;1.08,-13.96,;2.42,-14.73,;1.08,-12.42,;2.42,-11.65,;-.25,-11.65,;-1.58,-12.42,;-1.74,-13.96,;-3.25,-14.29,;-4.03,-12.96,;-5.54,-12.63,;-6.02,-11.17,;-4.99,-10.02,;-3.48,-10.33,;-2.99,-11.79,;3.75,-18.58,;3.75,-17.04,;5.08,-19.35,;5.08,-20.89,;3.75,-21.66,;3.75,-23.2,;2.42,-23.97,;2.42,-25.51,;1.08,-26.28,;-.25,-25.51,;1.08,-27.82,;6.42,-21.66,;7.75,-20.89,;6.42,-23.2,;7.75,-23.97,;7.75,-25.51,;6.42,-26.28,;5.21,-25.59,;4,-26.3,;4.01,-27.69,;2.67,-28.46,;5.22,-28.39,;6.42,-27.68,;9.09,-23.2,;9.09,-21.66,;10.42,-23.97,;11.75,-23.2,;11.75,-21.66,;13.09,-20.89,;14.49,-21.5,;15.53,-20.35,;14.76,-19.01,;15.23,-17.54,;14.19,-16.39,;12.67,-16.71,;12.2,-18.19,;13.24,-19.34,;13.09,-23.97,;13.09,-25.51,;14.42,-23.2,;15.76,-23.97,;15.76,-25.51,;14.42,-26.28,;14.42,-27.82,;13.09,-28.59,;11.75,-27.82,;11.75,-26.28,;10.42,-28.59,;17.09,-23.2,;17.09,-21.66,;18.42,-23.97,;19.76,-23.2,;21.09,-23.97,;22.43,-23.2,;23.76,-23.97,;19.76,-21.66,;21.09,-20.89,;18.42,-20.89,;18.42,-19.35,;17.09,-18.58,;17.09,-17.04,;15.76,-16.27,;15.76,-14.73,;14.42,-13.96,;13.09,-14.73,;14.42,-12.42,;19.76,-18.58,;21.09,-19.35,;19.76,-17.04,;21.09,-16.27,;22.43,-17.04,;23.76,-16.27,;23.75,-14.86,;24.96,-14.16,;26.17,-14.86,;27.51,-14.09,;26.18,-16.26,;24.97,-16.96,;21.09,-14.73,;22.43,-13.96,;19.76,-13.96,)| Show InChI InChI=1S/C74H107N25O12/c1-3-13-52(92-65(106)54(19-9-31-85-71(77)78)91-62(103)49(75)37-43-39-89-50-17-7-5-15-47(43)50)64(105)95-57(22-12-34-88-74(83)84)68(109)98-59(36-42-25-29-46(101)30-26-42)69(110)99-60(38-44-40-90-51-18-8-6-16-48(44)51)70(111)96-55(20-10-32-86-72(79)80)66(107)93-53(14-4-2)63(104)94-56(21-11-33-87-73(81)82)67(108)97-58(61(76)102)35-41-23-27-45(100)28-24-41/h5-8,15-18,23-30,39-40,49,52-60,89-90,100-101H,3-4,9-14,19-22,31-38,75H2,1-2H3,(H2,76,102)(H,91,103)(H,92,106)(H,93,107)(H,94,104)(H,95,105)(H,96,111)(H,97,108)(H,98,109)(H,99,110)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t49-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50049744

(CHEMBL438548 | NPY-analogs | [Cys-Trp-Arg-Nva-Arg-...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CSSC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C80H118N28O14S2/c1-3-13-55(69(115)101-59(21-11-33-95-79(89)90)73(119)105-61(65(83)111)35-43-23-27-47(109)28-24-43)99-71(117)57(19-9-31-93-77(85)86)103-75(121)63(37-45-39-97-53-17-7-5-15-49(45)53)107-67(113)51(81)41-123-124-42-52(82)68(114)108-64(38-46-40-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-32-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-34-96-80(91)92)74(120)106-62(66(84)112)36-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,39-40,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-38,41-42,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51-,52-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human NPY2 receptor expressed in CHO cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK Tyr397 phosphorylation in human A431 cells by ELISA |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK Tyr397 phosphorylation in human FG cells after 60 mins by western blot analysis |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK Tyr397 phosphorylation in human SKOV3 cells after 60 mins by western blot analysis |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK Tyr397 phosphorylation in human PC3 cells after 60 mins by western blot analysis |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of human CDK7/cyclinH/MAT1 |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of FAK Tyr397 phosphorylation in human L3.6pl cells after 60 mins by western blot analysis |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 486 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of human CDK1/cyclinB |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50269948

(6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)CCc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C22H20F3N5O3S/c1-34(32,33)16-4-2-3-13(9-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(10-15)5-8-19(31)29-18/h2-4,6-7,9-10,12H,5,8,11H2,1H3,(H,29,31)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Pyk2 (unknown origin) |
J Biol Chem 282: 14845-52 (2007)
Article DOI: 10.1074/jbc.M606695200
BindingDB Entry DOI: 10.7270/Q2J67GQH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVLL) by bovine geranygeranyltransferase |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185368

(CHEMBL2371910 | CHEMBL439884 | Pim[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:111.116,18.18,85.89,42.44,32.35,wD:3.2,74.77,67.71,56.60,7.7,37.38,100.104,(-6.32,-13.79,;-5,-13.01,;-3.66,-13.78,;-2.33,-13,;-2.33,-11.46,;-1,-10.69,;.33,-11.45,;-1,-9.14,;-2.34,-8.38,;-2.35,-6.84,;-3.69,-6.08,;-3.69,-4.53,;-2.36,-3.76,;-2.37,-2.22,;-1.02,-4.52,;.32,-8.36,;.31,-6.82,;-1.03,-6.06,;1.65,-6.05,;2.98,-6.81,;2.98,-8.36,;1.74,-9.26,;2.23,-10.72,;3.77,-10.72,;4.8,-11.86,;6.3,-11.54,;6.78,-10.07,;5.74,-8.93,;4.24,-9.25,;1.64,-4.51,;2.97,-3.73,;4.31,-4.48,;2.96,-2.18,;1.61,-1.43,;4.28,-1.41,;4.27,.14,;5.59,.92,;5.58,2.46,;4.24,3.21,;6.91,3.24,;6.89,4.78,;8.25,2.49,;9.59,3.25,;9.59,4.79,;10.94,5.55,;12.34,4.91,;13.37,6.05,;12.61,7.39,;13.1,8.86,;12.08,10,;10.57,9.7,;10.09,8.23,;11.11,7.08,;10.91,2.47,;10.9,.93,;12.25,3.23,;13.58,2.45,;13.57,.91,;12.23,.15,;12.22,-1.39,;10.88,-2.15,;9.55,-1.37,;8.22,-2.13,;9.56,.17,;14.92,3.21,;14.93,4.75,;16.25,2.43,;17.59,3.19,;18.92,2.41,;18.91,.87,;20.24,.09,;17.6,4.73,;16.27,5.51,;18.94,5.49,;20.26,4.71,;20.25,3.17,;21.58,2.39,;21.57,.85,;22.9,.07,;22.89,-1.47,;21.55,-2.23,;24.22,-2.25,;21.61,5.47,;21.62,7.01,;22.94,4.69,;24.27,5.45,;24.29,6.99,;25.63,7.75,;25.63,9.29,;26.97,10.05,;28.3,9.28,;29.64,10.04,;28.29,7.74,;26.95,6.97,;25.6,4.67,;26.94,5.43,;25.59,3.13,;-.99,-13.76,;.34,-12.99,;-.99,-15.31,;-2.31,-16.08,;-3.65,-15.31,;-4.98,-16.09,;-6.32,-15.32,;-7.65,-16.1,;-8.99,-15.34,;-8.99,-13.79,;-10.31,-16.11,;-2.31,-17.62,;-.97,-18.38,;-3.64,-18.4,;-3.63,-19.94,;-2.3,-20.71,;-2.29,-22.24,;-.95,-23,;-.95,-24.54,;-2.28,-25.32,;-2.27,-26.86,;-3.62,-24.55,;-3.62,-23.02,;-4.96,-20.71,;-4.95,-22.25,;-6.3,-19.95,)| Show InChI InChI=1S/C81H120N28O14/c1-3-14-56(70(116)102-60(24-12-36-96-80(90)91)74(120)106-62(66(84)112)38-44-26-30-48(110)31-27-44)100-72(118)58(22-10-34-94-78(86)87)104-76(122)64(40-46-42-98-54-20-7-5-16-50(46)54)108-68(114)52(82)18-9-19-53(83)69(115)109-65(41-47-43-99-55-21-8-6-17-51(47)55)77(123)105-59(23-11-35-95-79(88)89)73(119)101-57(15-4-2)71(117)103-61(25-13-37-97-81(92)93)75(121)107-63(67(85)113)39-45-28-32-49(111)33-29-45/h5-8,16-17,20-21,26-33,42-43,52-53,56-65,98-99,110-111H,3-4,9-15,18-19,22-25,34-41,82-83H2,1-2H3,(H2,84,112)(H2,85,113)(H,100,118)(H,101,119)(H,102,116)(H,103,117)(H,104,122)(H,105,123)(H,106,120)(H,107,121)(H,108,114)(H,109,115)(H4,86,87,94)(H4,88,89,95)(H4,90,91,96)(H4,92,93,97)/t52?,53?,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 expressed in HEK-293 cells |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185367

(CHEMBL413871 | H-[Trp-Arg-Nva-Arg-Tyr]3-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:154.161,136.143,125.132,88.92,99.103,81.85,56.58,70.74,33.34,3.3,18.18,7.14,wD:143.150,111.116,44.45,(19.56,-19.66,;18.24,-18.88,;16.9,-19.64,;15.57,-18.87,;15.58,-17.32,;14.25,-16.54,;12.91,-17.3,;14.26,-15.01,;15.6,-14.25,;15.61,-12.71,;16.95,-11.95,;16.96,-10.41,;18.3,-9.65,;19.63,-10.43,;18.31,-8.11,;12.93,-14.23,;11.59,-14.99,;11.58,-16.53,;10.27,-14.2,;8.93,-14.96,;10.28,-12.67,;11.62,-11.91,;13.02,-12.54,;14.06,-11.39,;13.29,-10.05,;13.78,-8.59,;12.76,-7.43,;11.24,-7.73,;10.76,-9.2,;11.79,-10.37,;14.23,-19.63,;12.9,-18.84,;14.22,-21.16,;15.55,-21.94,;16.88,-21.18,;18.21,-21.96,;19.55,-21.2,;20.88,-21.98,;22.21,-21.22,;22.23,-19.69,;23.54,-22,;15.53,-23.48,;14.19,-24.24,;16.86,-24.26,;16.85,-25.8,;15.51,-26.56,;15.5,-28.1,;16.71,-28.8,;16.7,-30.21,;15.49,-30.9,;15.47,-32.44,;14.26,-30.19,;14.27,-28.79,;18.17,-26.58,;19.51,-25.82,;18.16,-28.11,;19.49,-28.9,;20.83,-28.14,;22.15,-28.92,;22.3,-30.46,;23.82,-30.79,;24.6,-29.45,;26.12,-29.14,;26.6,-27.67,;25.58,-26.51,;24.06,-26.82,;23.57,-28.29,;19.47,-30.44,;18.14,-31.2,;20.8,-31.21,;20.79,-32.75,;22.12,-33.54,;23.45,-32.78,;24.78,-33.55,;26.12,-32.79,;27.45,-33.57,;27.44,-35.11,;28.79,-32.81,;19.45,-33.51,;18.12,-32.73,;19.44,-35.06,;18.1,-35.81,;18.09,-37.35,;19.42,-38.13,;19.41,-39.67,;16.77,-35.03,;16.78,-33.49,;15.43,-35.79,;15.42,-37.33,;16.75,-38.11,;16.74,-39.65,;18.07,-40.43,;18.06,-41.97,;19.39,-42.75,;20.72,-41.99,;19.37,-44.29,;14.08,-38.09,;12.76,-37.31,;14.08,-39.63,;12.74,-40.39,;12.73,-41.93,;14.05,-42.71,;15.39,-41.95,;16.72,-42.73,;16.71,-44.26,;18.04,-45.04,;15.37,-45.02,;14.05,-44.24,;11.41,-39.61,;11.42,-38.07,;10.07,-40.37,;8.74,-39.59,;8.84,-38.05,;7.56,-37.19,;6.12,-37.73,;5.16,-36.53,;6.01,-35.24,;5.61,-33.74,;6.71,-32.66,;8.21,-33.06,;8.6,-34.57,;7.5,-35.65,;7.4,-40.35,;7.39,-41.89,;6.08,-39.57,;4.74,-40.33,;4.73,-41.87,;6.05,-42.65,;6.04,-44.18,;7.37,-44.97,;8.71,-44.21,;8.72,-42.66,;10.04,-44.99,;3.41,-39.55,;3.42,-38.01,;2.07,-40.31,;.75,-39.53,;-.59,-40.29,;-1.92,-39.51,;-3.26,-40.27,;.76,-37.99,;-.57,-37.21,;2.1,-37.23,;2.11,-35.69,;3.45,-34.93,;3.46,-33.39,;4.8,-32.63,;4.81,-31.09,;6.15,-30.33,;7.48,-31.11,;6.16,-28.8,;.78,-34.91,;-.55,-35.67,;.8,-33.37,;-.53,-32.59,;-1.87,-33.35,;-3.2,-32.58,;-3.18,-31.03,;-4.52,-30.26,;-5.85,-31.02,;-7.17,-30.24,;-5.86,-32.56,;-4.53,-33.33,;-.52,-31.05,;-1.85,-30.27,;.82,-30.3,)| Show InChI InChI=1S/C111H159N37O18/c1-4-19-77(136-96(157)80(28-13-46-126-106(114)115)135-92(153)73(112)55-64-58-132-74-25-10-7-22-70(64)74)94(155)140-84(32-17-50-130-110(122)123)100(161)145-87(53-62-36-42-68(150)43-37-62)102(163)148-90(57-66-60-134-76-27-12-9-24-72(66)76)105(166)143-82(30-15-48-128-108(118)119)98(159)138-79(21-6-3)95(156)141-85(33-18-51-131-111(124)125)101(162)146-88(54-63-38-44-69(151)45-39-63)103(164)147-89(56-65-59-133-75-26-11-8-23-71(65)75)104(165)142-81(29-14-47-127-107(116)117)97(158)137-78(20-5-2)93(154)139-83(31-16-49-129-109(120)121)99(160)144-86(91(113)152)52-61-34-40-67(149)41-35-61/h7-12,22-27,34-45,58-60,73,77-90,132-134,149-151H,4-6,13-21,28-33,46-57,112H2,1-3H3,(H2,113,152)(H,135,153)(H,136,157)(H,137,158)(H,138,159)(H,139,154)(H,140,155)(H,141,156)(H,142,165)(H,143,166)(H,144,160)(H,145,161)(H,146,162)(H,147,164)(H,148,163)(H4,114,115,126)(H4,116,117,127)(H4,118,119,128)(H4,120,121,129)(H4,122,123,130)(H4,124,125,131)/t73-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185369

(CHEMBL408891 | H-[Trp-Arg-Nva-Arg-Tyr]2-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:88.92,56.58,wD:99.103,81.85,70.74,33.34,44.45,3.3,18.18,7.14,(1.08,-23.2,;1.08,-21.66,;2.42,-20.89,;2.42,-19.35,;1.08,-18.58,;1.08,-17.04,;2.42,-16.27,;-.25,-16.27,;-1.58,-17.04,;-2.92,-16.27,;-4.25,-17.04,;-5.59,-16.27,;-6.92,-17.04,;-6.92,-18.58,;-8.25,-16.27,;-.25,-14.73,;1.08,-13.96,;2.42,-14.73,;1.08,-12.42,;2.42,-11.65,;-.25,-11.65,;-1.58,-12.42,;-1.74,-13.96,;-3.25,-14.29,;-4.03,-12.96,;-5.54,-12.63,;-6.02,-11.17,;-4.99,-10.02,;-3.48,-10.33,;-2.99,-11.79,;3.75,-18.58,;3.75,-17.04,;5.08,-19.35,;5.08,-20.89,;3.75,-21.66,;3.75,-23.2,;2.42,-23.97,;2.42,-25.51,;1.08,-26.28,;-.25,-25.51,;1.08,-27.82,;6.42,-21.66,;7.75,-20.89,;6.42,-23.2,;7.75,-23.97,;7.75,-25.51,;6.42,-26.28,;5.21,-25.59,;4,-26.3,;4.01,-27.69,;2.67,-28.46,;5.22,-28.39,;6.42,-27.68,;9.09,-23.2,;9.09,-21.66,;10.42,-23.97,;11.75,-23.2,;11.75,-21.66,;13.09,-20.89,;14.49,-21.5,;15.53,-20.35,;14.76,-19.01,;15.23,-17.54,;14.19,-16.39,;12.67,-16.71,;12.2,-18.19,;13.24,-19.34,;13.09,-23.97,;13.09,-25.51,;14.42,-23.2,;15.76,-23.97,;15.76,-25.51,;14.42,-26.28,;14.42,-27.82,;13.09,-28.59,;11.75,-27.82,;11.75,-26.28,;10.42,-28.59,;17.09,-23.2,;17.09,-21.66,;18.42,-23.97,;19.76,-23.2,;21.09,-23.97,;22.43,-23.2,;23.76,-23.97,;19.76,-21.66,;21.09,-20.89,;18.42,-20.89,;18.42,-19.35,;17.09,-18.58,;17.09,-17.04,;15.76,-16.27,;15.76,-14.73,;14.42,-13.96,;13.09,-14.73,;14.42,-12.42,;19.76,-18.58,;21.09,-19.35,;19.76,-17.04,;21.09,-16.27,;22.43,-17.04,;23.76,-16.27,;23.75,-14.86,;24.96,-14.16,;26.17,-14.86,;27.51,-14.09,;26.18,-16.26,;24.97,-16.96,;21.09,-14.73,;22.43,-13.96,;19.76,-13.96,)| Show InChI InChI=1S/C74H107N25O12/c1-3-13-52(92-65(106)54(19-9-31-85-71(77)78)91-62(103)49(75)37-43-39-89-50-17-7-5-15-47(43)50)64(105)95-57(22-12-34-88-74(83)84)68(109)98-59(36-42-25-29-46(101)30-26-42)69(110)99-60(38-44-40-90-51-18-8-6-16-48(44)51)70(111)96-55(20-10-32-86-72(79)80)66(107)93-53(14-4-2)63(104)94-56(21-11-33-87-73(81)82)67(108)97-58(61(76)102)35-41-23-27-45(100)28-24-41/h5-8,15-18,23-30,39-40,49,52-60,89-90,100-101H,3-4,9-14,19-22,31-38,75H2,1-2H3,(H2,76,102)(H,91,103)(H,92,106)(H,93,107)(H,94,104)(H,95,105)(H,96,111)(H,97,108)(H,98,109)(H,99,110)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t49-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50185367

(CHEMBL413871 | H-[Trp-Arg-Nva-Arg-Tyr]3-NH2)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:154.161,136.143,125.132,88.92,99.103,81.85,56.58,70.74,33.34,3.3,18.18,7.14,wD:143.150,111.116,44.45,(19.56,-19.66,;18.24,-18.88,;16.9,-19.64,;15.57,-18.87,;15.58,-17.32,;14.25,-16.54,;12.91,-17.3,;14.26,-15.01,;15.6,-14.25,;15.61,-12.71,;16.95,-11.95,;16.96,-10.41,;18.3,-9.65,;19.63,-10.43,;18.31,-8.11,;12.93,-14.23,;11.59,-14.99,;11.58,-16.53,;10.27,-14.2,;8.93,-14.96,;10.28,-12.67,;11.62,-11.91,;13.02,-12.54,;14.06,-11.39,;13.29,-10.05,;13.78,-8.59,;12.76,-7.43,;11.24,-7.73,;10.76,-9.2,;11.79,-10.37,;14.23,-19.63,;12.9,-18.84,;14.22,-21.16,;15.55,-21.94,;16.88,-21.18,;18.21,-21.96,;19.55,-21.2,;20.88,-21.98,;22.21,-21.22,;22.23,-19.69,;23.54,-22,;15.53,-23.48,;14.19,-24.24,;16.86,-24.26,;16.85,-25.8,;15.51,-26.56,;15.5,-28.1,;16.71,-28.8,;16.7,-30.21,;15.49,-30.9,;15.47,-32.44,;14.26,-30.19,;14.27,-28.79,;18.17,-26.58,;19.51,-25.82,;18.16,-28.11,;19.49,-28.9,;20.83,-28.14,;22.15,-28.92,;22.3,-30.46,;23.82,-30.79,;24.6,-29.45,;26.12,-29.14,;26.6,-27.67,;25.58,-26.51,;24.06,-26.82,;23.57,-28.29,;19.47,-30.44,;18.14,-31.2,;20.8,-31.21,;20.79,-32.75,;22.12,-33.54,;23.45,-32.78,;24.78,-33.55,;26.12,-32.79,;27.45,-33.57,;27.44,-35.11,;28.79,-32.81,;19.45,-33.51,;18.12,-32.73,;19.44,-35.06,;18.1,-35.81,;18.09,-37.35,;19.42,-38.13,;19.41,-39.67,;16.77,-35.03,;16.78,-33.49,;15.43,-35.79,;15.42,-37.33,;16.75,-38.11,;16.74,-39.65,;18.07,-40.43,;18.06,-41.97,;19.39,-42.75,;20.72,-41.99,;19.37,-44.29,;14.08,-38.09,;12.76,-37.31,;14.08,-39.63,;12.74,-40.39,;12.73,-41.93,;14.05,-42.71,;15.39,-41.95,;16.72,-42.73,;16.71,-44.26,;18.04,-45.04,;15.37,-45.02,;14.05,-44.24,;11.41,-39.61,;11.42,-38.07,;10.07,-40.37,;8.74,-39.59,;8.84,-38.05,;7.56,-37.19,;6.12,-37.73,;5.16,-36.53,;6.01,-35.24,;5.61,-33.74,;6.71,-32.66,;8.21,-33.06,;8.6,-34.57,;7.5,-35.65,;7.4,-40.35,;7.39,-41.89,;6.08,-39.57,;4.74,-40.33,;4.73,-41.87,;6.05,-42.65,;6.04,-44.18,;7.37,-44.97,;8.71,-44.21,;8.72,-42.66,;10.04,-44.99,;3.41,-39.55,;3.42,-38.01,;2.07,-40.31,;.75,-39.53,;-.59,-40.29,;-1.92,-39.51,;-3.26,-40.27,;.76,-37.99,;-.57,-37.21,;2.1,-37.23,;2.11,-35.69,;3.45,-34.93,;3.46,-33.39,;4.8,-32.63,;4.81,-31.09,;6.15,-30.33,;7.48,-31.11,;6.16,-28.8,;.78,-34.91,;-.55,-35.67,;.8,-33.37,;-.53,-32.59,;-1.87,-33.35,;-3.2,-32.58,;-3.18,-31.03,;-4.52,-30.26,;-5.85,-31.02,;-7.17,-30.24,;-5.86,-32.56,;-4.53,-33.33,;-.52,-31.05,;-1.85,-30.27,;.82,-30.3,)| Show InChI InChI=1S/C111H159N37O18/c1-4-19-77(136-96(157)80(28-13-46-126-106(114)115)135-92(153)73(112)55-64-58-132-74-25-10-7-22-70(64)74)94(155)140-84(32-17-50-130-110(122)123)100(161)145-87(53-62-36-42-68(150)43-37-62)102(163)148-90(57-66-60-134-76-27-12-9-24-72(66)76)105(166)143-82(30-15-48-128-108(118)119)98(159)138-79(21-6-3)95(156)141-85(33-18-51-131-111(124)125)101(162)146-88(54-63-38-44-69(151)45-39-63)103(164)147-89(56-65-59-133-75-26-11-8-23-71(65)75)104(165)142-81(29-14-47-127-107(116)117)97(158)137-78(20-5-2)93(154)139-83(31-16-49-129-109(120)121)99(160)144-86(91(113)152)52-61-34-40-67(149)41-35-61/h7-12,22-27,34-45,58-60,73,77-90,132-134,149-151H,4-6,13-21,28-33,46-57,112H2,1-3H3,(H2,113,152)(H,135,153)(H,136,157)(H,137,158)(H,138,159)(H,139,154)(H,140,155)(H,141,156)(H,142,165)(H,143,166)(H,144,160)(H,145,161)(H,146,162)(H,147,164)(H,148,163)(H4,114,115,126)(H4,116,117,127)(H4,118,119,128)(H4,120,121,129)(H4,122,123,130)(H4,124,125,131)/t73-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185364

(CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C80H122N24O18/c1-43(2)37-59(71(115)95-57(15-9-35-93-79(87)88)69(113)103-63(75(119)120)41-47-21-29-51(107)30-22-47)101-67(111)55(13-7-33-91-77(83)84)97-73(117)61(39-45-17-25-49(105)26-18-45)99-65(109)53(81)11-5-6-12-54(82)66(110)100-62(40-46-19-27-50(106)28-20-46)74(118)98-56(14-8-34-92-78(85)86)68(112)102-60(38-44(3)4)72(116)96-58(16-10-36-94-80(89)90)70(114)104-64(76(121)122)42-48-23-31-52(108)32-24-48/h17-32,43-44,53-64,105-108H,5-16,33-42,81-82H2,1-4H3,(H,95,115)(H,96,116)(H,97,117)(H,98,118)(H,99,109)(H,100,110)(H,101,111)(H,102,112)(H,103,113)(H,104,114)(H,119,120)(H,121,122)(H4,83,84,91)(H4,85,86,92)(H4,87,88,93)(H4,89,90,94)/t53?,54?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185364

(CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C80H122N24O18/c1-43(2)37-59(71(115)95-57(15-9-35-93-79(87)88)69(113)103-63(75(119)120)41-47-21-29-51(107)30-22-47)101-67(111)55(13-7-33-91-77(83)84)97-73(117)61(39-45-17-25-49(105)26-18-45)99-65(109)53(81)11-5-6-12-54(82)66(110)100-62(40-46-19-27-50(106)28-20-46)74(118)98-56(14-8-34-92-78(85)86)68(112)102-60(38-44(3)4)72(116)96-58(16-10-36-94-80(89)90)70(114)104-64(76(121)122)42-48-23-31-52(108)32-24-48/h17-32,43-44,53-64,105-108H,5-16,33-42,81-82H2,1-4H3,(H,95,115)(H,96,116)(H,97,117)(H,98,118)(H,99,109)(H,100,110)(H,101,111)(H,102,112)(H,103,113)(H,104,114)(H,119,120)(H,121,122)(H4,83,84,91)(H4,85,86,92)(H4,87,88,93)(H4,89,90,94)/t53?,54?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185365

(Adp[-Trp-Arg-Nva-Arg-Tyr-NH2]2 | CHEMBL2371909 | C...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:99.104,3.3,18.18,73.77,66.69,41.43,wD:110.115,7.7,84.88,55.59,32.35,36.39,(.45,-20.37,;.5,-18.83,;-.81,-18.01,;-.76,-16.47,;.6,-15.75,;1.91,-16.56,;1.86,-18.1,;3.26,-15.83,;3.31,-14.29,;2.01,-13.48,;2.06,-11.94,;.74,-11.13,;.79,-9.59,;2.16,-8.86,;-.51,-8.78,;4.57,-16.65,;5.93,-15.92,;5.98,-14.38,;7.24,-16.73,;7.19,-18.27,;5.83,-19,;4.44,-18.35,;3.38,-19.47,;4.11,-20.83,;3.6,-22.29,;4.61,-23.46,;6.13,-23.19,;6.64,-21.73,;5.64,-20.55,;8.6,-16,;8.56,-14.47,;7.22,-13.75,;9.86,-13.67,;11.2,-14.39,;9.82,-12.14,;11.11,-11.35,;11.07,-9.82,;9.73,-9.1,;12.37,-9.03,;12.33,-7.5,;13.71,-9.75,;15.05,-8.98,;15.05,-7.44,;16.38,-6.67,;17.79,-7.28,;18.82,-6.12,;18.04,-4.78,;18.5,-3.31,;17.46,-2.17,;15.95,-2.49,;15.48,-3.97,;16.52,-5.12,;16.38,-9.75,;16.38,-11.29,;17.72,-8.98,;19.05,-9.75,;19.05,-11.29,;20.38,-12.06,;20.38,-13.6,;21.72,-14.37,;21.72,-15.91,;20.38,-16.68,;23.05,-16.68,;20.38,-8.98,;20.38,-7.44,;21.72,-9.75,;23.05,-8.98,;23.05,-7.44,;21.72,-6.67,;21.72,-5.13,;24.39,-9.75,;24.39,-11.29,;25.72,-8.98,;25.72,-7.44,;24.39,-6.67,;24.39,-5.13,;23.05,-4.36,;23.05,-2.82,;21.72,-2.05,;20.38,-2.82,;21.72,-.51,;27.05,-6.67,;28.39,-7.44,;27.05,-5.13,;28.39,-4.36,;29.72,-5.13,;31.05,-4.36,;31.05,-2.96,;32.27,-2.26,;33.47,-2.96,;34.81,-2.19,;33.47,-4.36,;32.26,-5.06,;28.39,-2.82,;29.72,-2.05,;27.05,-2.05,;-2.07,-15.66,;-2.02,-14.12,;-3.43,-16.39,;-3.48,-17.93,;-2.17,-18.74,;-2.22,-20.28,;-.91,-21.09,;-.96,-22.63,;.35,-23.44,;1.71,-22.72,;.3,-24.98,;-4.83,-18.66,;-6.14,-17.84,;-4.88,-20.19,;-6.24,-20.92,;-7.55,-20.11,;-8.91,-20.84,;-8.95,-22.24,;-10.19,-22.9,;-11.37,-22.16,;-12.74,-22.89,;-11.34,-20.76,;-10.1,-20.1,;-6.29,-22.46,;-7.65,-23.19,;-4.98,-23.27,)| Show InChI InChI=1S/C80H118N28O14/c1-3-13-55(69(115)101-59(21-11-35-95-79(89)90)73(119)105-61(65(83)111)37-43-23-27-47(109)28-24-43)99-71(117)57(19-9-33-93-77(85)86)103-75(121)63(39-45-41-97-53-17-7-5-15-49(45)53)107-67(113)51(81)31-32-52(82)68(114)108-64(40-46-42-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-34-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-36-96-80(91)92)74(120)106-62(66(84)112)38-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,41-42,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-40,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51?,52?,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Agonist activity at human NPY4 receptor in HEK293 cells by inhibition of forskolin-stimulated cAMP synthesis |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50049744

(CHEMBL438548 | NPY-analogs | [Cys-Trp-Arg-Nva-Arg-...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CSSC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C80H118N28O14S2/c1-3-13-55(69(115)101-59(21-11-33-95-79(89)90)73(119)105-61(65(83)111)35-43-23-27-47(109)28-24-43)99-71(117)57(19-9-31-93-77(85)86)103-75(121)63(37-45-39-97-53-17-7-5-15-49(45)53)107-67(113)51(81)41-123-124-42-52(82)68(114)108-64(38-46-40-98-54-18-8-6-16-50(46)54)76(122)104-58(20-10-32-94-78(87)88)72(118)100-56(14-4-2)70(116)102-60(22-12-34-96-80(91)92)74(120)106-62(66(84)112)36-44-25-29-48(110)30-26-44/h5-8,15-18,23-30,39-40,51-52,55-64,97-98,109-110H,3-4,9-14,19-22,31-38,41-42,81-82H2,1-2H3,(H2,83,111)(H2,84,112)(H,99,117)(H,100,118)(H,101,115)(H,102,116)(H,103,121)(H,104,122)(H,105,119)(H,106,120)(H,107,113)(H,108,114)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t51-,52-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Agonist activity at human NPY4 receptor in HEK293 cells by inhibition of forskolin-stimulated cAMP synthesis |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Agonist activity at human NPY1 receptor in CHO cells by inhibition of forskolin-stimulated cAMP synthesis |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50185366

(CHEMBL2371911 | CHEMBL436499 | Sub[-Trp-Arg-Nva-Ar...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:3.3,18.18,75.79,68.71,43.45,101.106,32.35,wD:112.117,7.7,86.90,57.61,38.39,(13.8,-37.49,;13.73,-39.03,;15.03,-39.86,;14.95,-41.4,;13.59,-42.11,;12.29,-41.28,;12.35,-39.74,;10.92,-41.99,;10.85,-43.53,;12.15,-44.36,;12.08,-45.89,;13.39,-46.72,;13.31,-48.26,;11.94,-48.97,;14.61,-49.09,;9.62,-41.16,;8.26,-41.87,;8.19,-43.41,;6.96,-41.04,;7.03,-39.5,;8.39,-38.79,;9.78,-39.46,;10.85,-38.35,;10.14,-36.98,;10.66,-35.53,;9.67,-34.34,;8.15,-34.59,;7.62,-36.04,;8.6,-37.24,;5.58,-41.75,;4.22,-41.06,;4.14,-39.53,;2.94,-41.89,;3.02,-43.41,;1.58,-41.19,;1.51,-39.66,;.15,-38.97,;.07,-37.45,;-1.29,-36.75,;-2.57,-37.58,;-1.37,-35.23,;-.09,-34.4,;-2.73,-34.54,;-2.73,-32.99,;-4.07,-32.23,;-4.07,-30.69,;-2.85,-29.76,;-3.35,-28.3,;-4.89,-28.31,;-5.94,-27.17,;-7.45,-27.52,;-7.91,-28.99,;-6.87,-30.12,;-5.36,-29.79,;-1.4,-32.22,;-.06,-32.98,;-1.41,-30.68,;-.08,-29.9,;1.26,-30.66,;2.59,-29.89,;3.93,-30.65,;5.26,-29.87,;6.6,-30.64,;6.61,-32.18,;7.93,-29.87,;-.08,-28.36,;-1.42,-27.6,;1.25,-27.58,;1.24,-26.05,;-.1,-25.27,;-1.43,-26.05,;-2.76,-25.29,;2.57,-25.26,;3.91,-26.03,;2.57,-23.72,;1.23,-22.96,;-.1,-23.73,;-1.44,-22.97,;-2.77,-23.75,;-4.11,-22.98,;-5.43,-23.77,;-5.43,-25.3,;-6.78,-22.99,;1.22,-21.42,;2.55,-20.64,;-.11,-20.67,;-.12,-19.11,;1.22,-18.34,;1.21,-16.79,;-.01,-16.09,;-.02,-14.7,;1.19,-14,;1.19,-12.45,;2.41,-14.68,;2.41,-16.09,;-1.46,-18.34,;-1.46,-16.79,;-2.79,-19.13,;16.26,-42.22,;16.19,-43.76,;17.62,-41.51,;17.69,-39.97,;16.39,-39.15,;16.46,-37.61,;15.16,-36.78,;15.23,-35.24,;13.93,-34.41,;12.56,-35.12,;14,-32.87,;19.06,-39.26,;20.36,-40.09,;19.12,-37.72,;20.5,-37.01,;21.79,-37.84,;23.16,-37.14,;23.22,-35.74,;24.47,-35.09,;25.64,-35.85,;27.01,-35.13,;25.59,-37.25,;24.34,-37.89,;20.57,-35.48,;21.93,-34.76,;19.26,-34.65,)| Show InChI InChI=1S/C82H122N28O14/c1-3-15-57(71(117)103-61(25-13-37-97-81(91)92)75(121)107-63(67(85)113)39-45-27-31-49(111)32-28-45)101-73(119)59(23-11-35-95-79(87)88)105-77(123)65(41-47-43-99-55-21-9-5-17-51(47)55)109-69(115)53(83)19-7-8-20-54(84)70(116)110-66(42-48-44-100-56-22-10-6-18-52(48)56)78(124)106-60(24-12-36-96-80(89)90)74(120)102-58(16-4-2)72(118)104-62(26-14-38-98-82(93)94)76(122)108-64(68(86)114)40-46-29-33-50(112)34-30-46/h5-6,9-10,17-18,21-22,27-34,43-44,53-54,57-66,99-100,111-112H,3-4,7-8,11-16,19-20,23-26,35-42,83-84H2,1-2H3,(H2,85,113)(H2,86,114)(H,101,119)(H,102,120)(H,103,117)(H,104,118)(H,105,123)(H,106,124)(H,107,121)(H,108,122)(H,109,115)(H,110,116)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t53?,54?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Agonist activity at human NPY4 receptor in HEK293 cells by inhibition of forskolin-stimulated cAMP synthesis |
J Med Chem 49: 2661-5 (2006)
Article DOI: 10.1021/jm050907d
BindingDB Entry DOI: 10.7270/Q2XS5TZ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data