 Found 347 hits with Last Name = 'smith' and Initial = 'mt'
Found 347 hits with Last Name = 'smith' and Initial = 'mt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594821
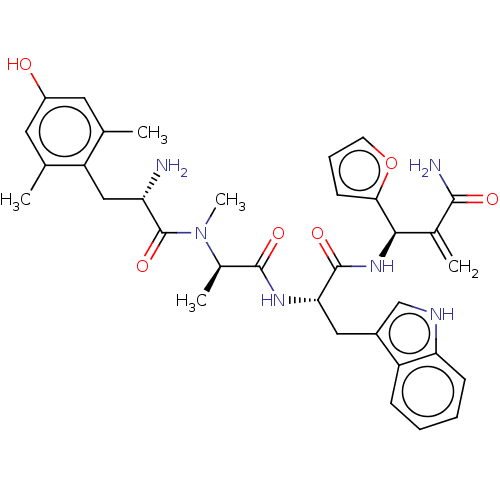
(CHEMBL5175179)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594822
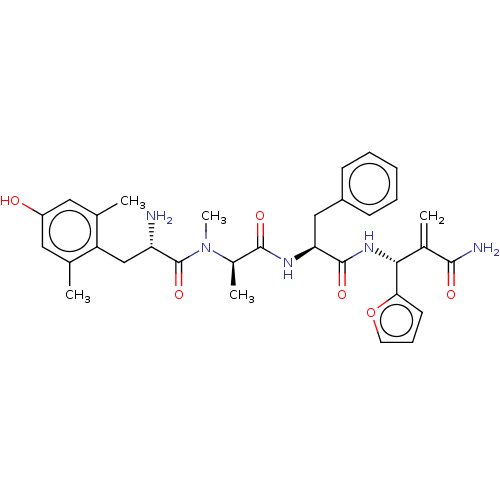
(CHEMBL5182849)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594820
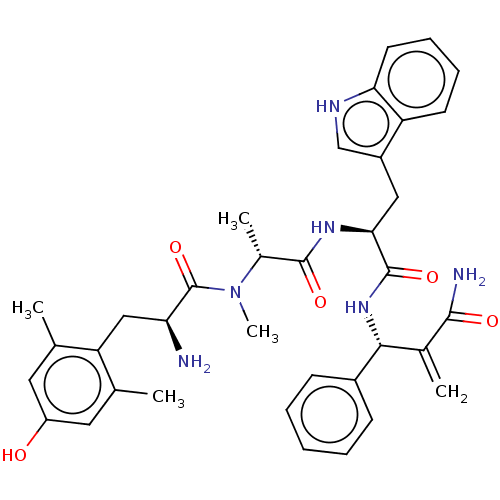
(CHEMBL5176887)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594825
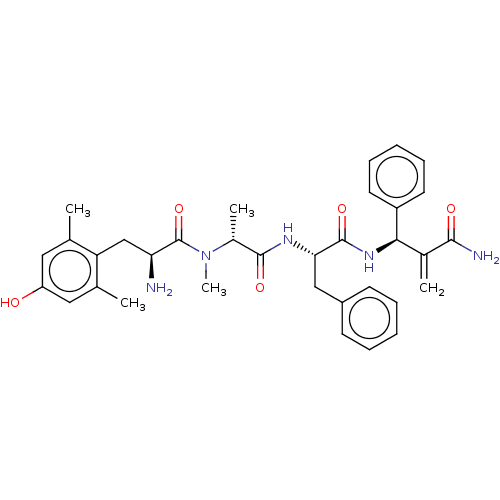
(CHEMBL5198856)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50594846

(CHEMBL5209004)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50149381
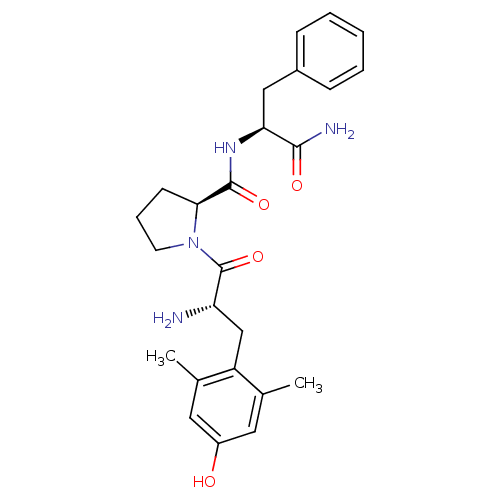
(1-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-p...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C25H32N4O4/c1-15-11-18(30)12-16(2)19(15)14-20(26)25(33)29-10-6-9-22(29)24(32)28-21(23(27)31)13-17-7-4-3-5-8-17/h3-5,7-8,11-12,20-22,30H,6,9-10,13-14,26H2,1-2H3,(H2,27,31)(H,28,32)/t20-,21-,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009271

(CHEMBL3233014)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C28H38N4O4/c1-15-9-16(2)22(17(3)10-15)14-24(26(30)34)31-27(35)25-7-6-8-32(25)28(36)23(29)13-21-18(4)11-20(33)12-19(21)5/h9-12,23-25,33H,6-8,13-14,29H2,1-5H3,(H2,30,34)(H,31,35)/t23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50016867
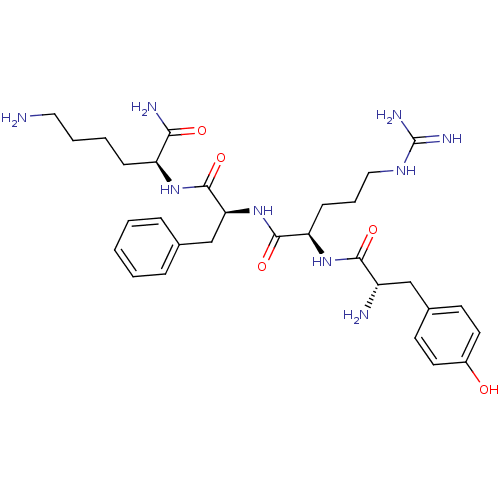
((S)-6-Amino-2-((S)-2-{(R)-2-[(S)-2-amino-3-(4-hydr...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C30H45N9O5/c31-15-5-4-9-23(26(33)41)37-29(44)25(18-19-7-2-1-3-8-19)39-28(43)24(10-6-16-36-30(34)35)38-27(42)22(32)17-20-11-13-21(40)14-12-20/h1-3,7-8,11-14,22-25,40H,4-6,9-10,15-18,31-32H2,(H2,33,41)(H,37,44)(H,38,42)(H,39,43)(H4,34,35,36)/t22-,23-,24+,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(CALF) | BDBM50010483
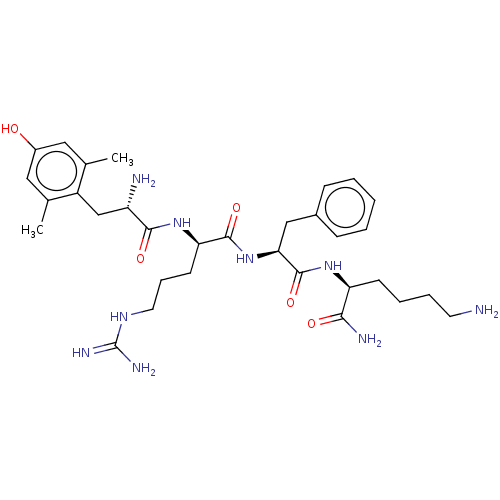
(CHEMBL2181202)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009272
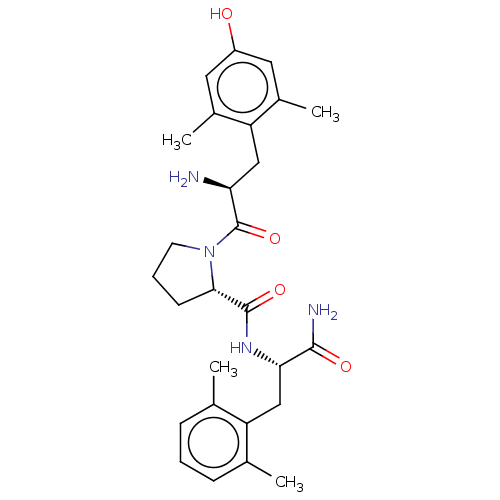
(CHEMBL3233200)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C27H36N4O4/c1-15-7-5-8-16(2)21(15)14-23(25(29)33)30-26(34)24-9-6-10-31(24)27(35)22(28)13-20-17(3)11-19(32)12-18(20)4/h5,7-8,11-12,22-24,32H,6,9-10,13-14,28H2,1-4H3,(H2,29,33)(H,30,34)/t22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009268

(CHEMBL3233197)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C27H33N5O4/c1-15-10-18(33)11-16(2)20(15)13-21(28)27(36)32-9-5-8-24(32)26(35)31-23(25(29)34)12-17-14-30-22-7-4-3-6-19(17)22/h3-4,6-7,10-11,14,21,23-24,30,33H,5,8-9,12-13,28H2,1-2H3,(H2,29,34)(H,31,35)/t21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143182
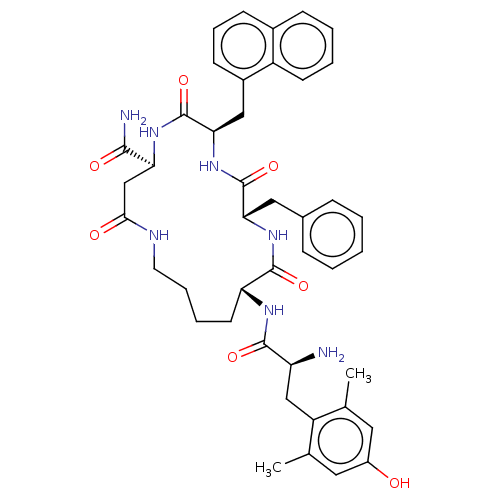
(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594830
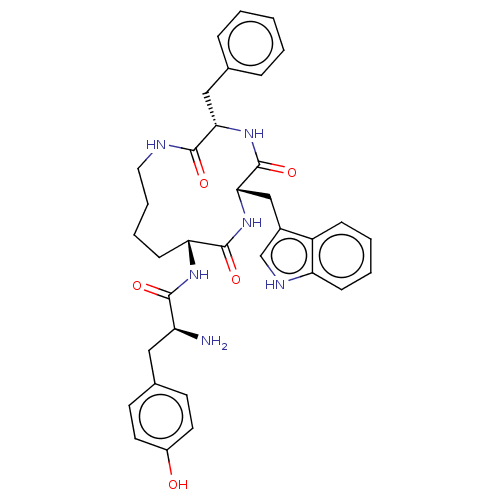
(CHEMBL5186493)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009266
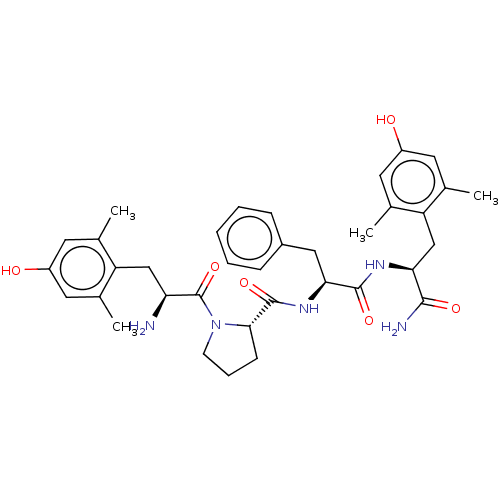
(CHEMBL3233195)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C36H45N5O6/c1-20-13-25(42)14-21(2)27(20)18-29(37)36(47)41-12-8-11-32(41)35(46)40-31(17-24-9-6-5-7-10-24)34(45)39-30(33(38)44)19-28-22(3)15-26(43)16-23(28)4/h5-7,9-10,13-16,29-32,42-43H,8,11-12,17-19,37H2,1-4H3,(H2,38,44)(H,39,45)(H,40,46)/t29-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009251

(CHEMBL3233191)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-18-28(44)19-24(2)30(23)22-31(39)38(48)43-17-9-16-34(43)37(47)42-33(20-25-10-4-3-5-11-25)36(46)41-32(35(40)45)21-27-14-8-13-26-12-6-7-15-29(26)27/h3-8,10-15,18-19,31-34,44H,9,16-17,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor expressed in CHOK1 cells after overnight incubation by scintillation proximity assay |
J Med Chem 55: 5859-67 (2012)
Article DOI: 10.1021/jm300418d
BindingDB Entry DOI: 10.7270/Q2PR7X3K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594819
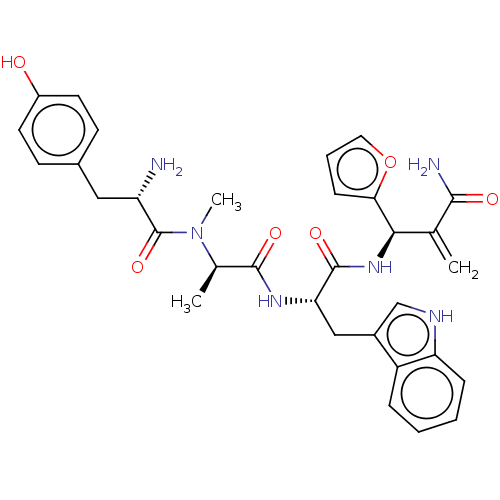
(CHEMBL5208747)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009265

(CHEMBL3233194)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C36H45N5O5/c1-21-10-8-11-22(2)28(21)20-30(33(38)43)39-34(44)31(18-25-12-6-5-7-13-25)40-35(45)32-14-9-15-41(32)36(46)29(37)19-27-23(3)16-26(42)17-24(27)4/h5-8,10-13,16-17,29-32,42H,9,14-15,18-20,37H2,1-4H3,(H2,38,43)(H,39,44)(H,40,45)/t29-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166065
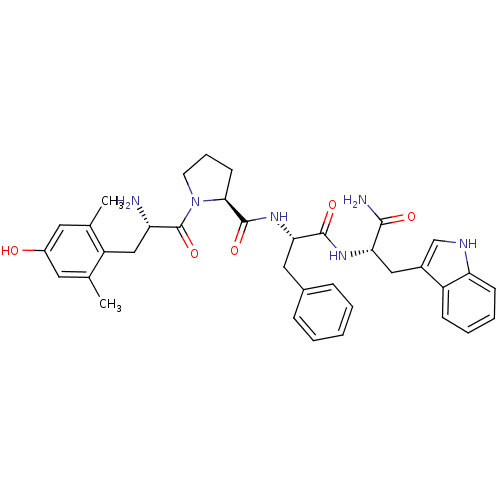
((S)-1-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(17-23-9-4-3-5-10-23)34(45)40-30(33(38)44)18-24-20-39-29-12-7-6-11-26(24)29/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009264
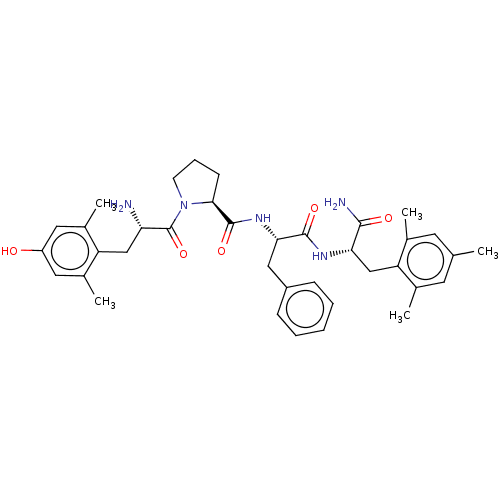
(CHEMBL3233193)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C37H47N5O5/c1-21-14-22(2)29(23(3)15-21)20-31(34(39)44)40-35(45)32(18-26-10-7-6-8-11-26)41-36(46)33-12-9-13-42(33)37(47)30(38)19-28-24(4)16-27(43)17-25(28)5/h6-8,10-11,14-17,30-33,43H,9,12-13,18-20,38H2,1-5H3,(H2,39,44)(H,40,45)(H,41,46)/t30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594824

(CHEMBL5190232)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009267
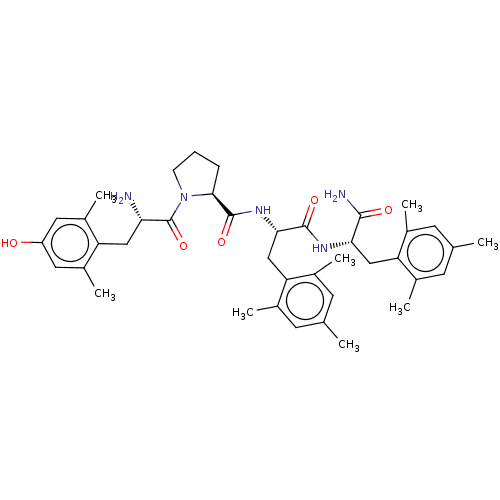
(CHEMBL3233196)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2c(C)cc(C)cc2C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C40H53N5O5/c1-21-12-23(3)31(24(4)13-21)19-34(37(42)47)43-38(48)35(20-32-25(5)14-22(2)15-26(32)6)44-39(49)36-10-9-11-45(36)40(50)33(41)18-30-27(7)16-29(46)17-28(30)8/h12-17,33-36,46H,9-11,18-20,41H2,1-8H3,(H2,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594836

(CHEMBL5199625)Show SMILES CCCCCCCCC(N)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594827

(CHEMBL5171745)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM50594853

(CHEMBL1355638)Show SMILES [#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594818

(CHEMBL5173837)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50491417
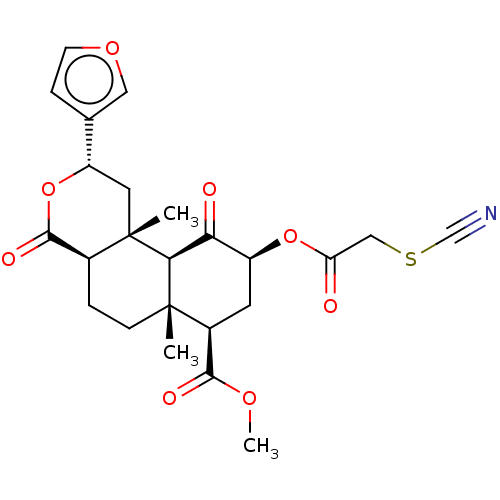
(22-THIOCYANATOSALVINORIN A)Show SMILES [H][C@@]12CC[C@@]3(C)[C@@H](C[C@H](OC(=O)CSC#N)C(=O)[C@]3([H])[C@@]1(C)C[C@H](OC2=O)c1ccoc1)C(=O)OC |r| Show InChI InChI=1S/C24H27NO8S/c1-23-6-4-14-22(29)33-17(13-5-7-31-10-13)9-24(14,2)20(23)19(27)16(8-15(23)21(28)30-3)32-18(26)11-34-12-25/h5,7,10,14-17,20H,4,6,8-9,11H2,1-3H3/t14-,15-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594823

(CHEMBL5188741)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594826
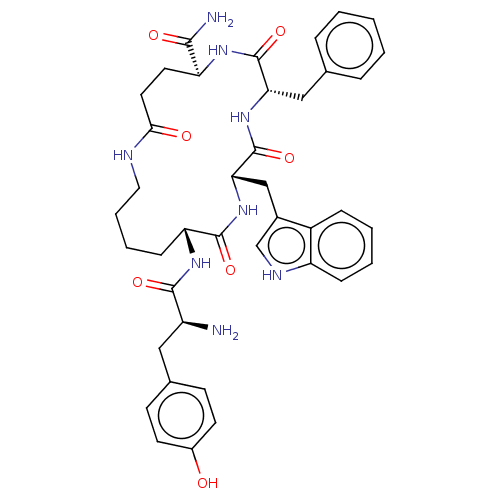
(CHEMBL5197271)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50594854

(CHEMBL5169797)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594828
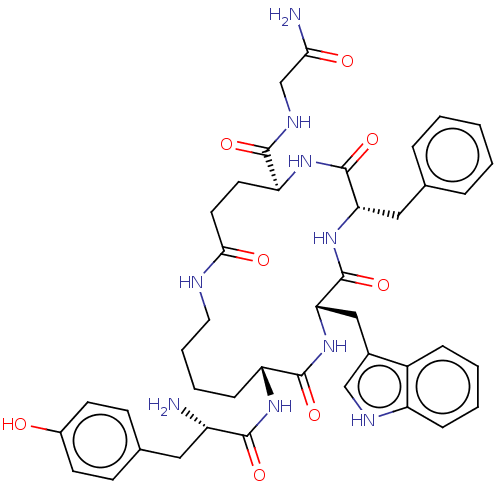
(CHEMBL5198915)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009269
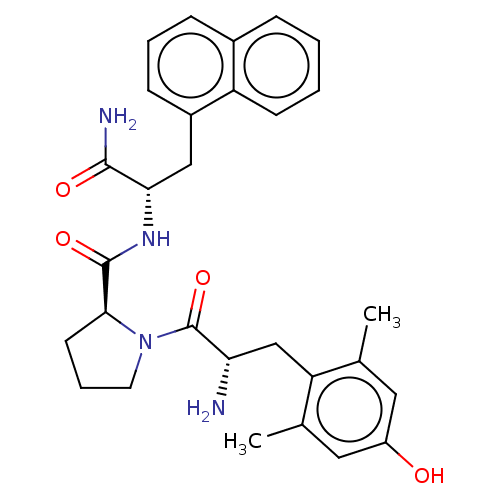
(CHEMBL3233198)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C29H34N4O4/c1-17-13-21(34)14-18(2)23(17)16-24(30)29(37)33-12-6-11-26(33)28(36)32-25(27(31)35)15-20-9-5-8-19-7-3-4-10-22(19)20/h3-5,7-10,13-14,24-26,34H,6,11-12,15-16,30H2,1-2H3,(H2,31,35)(H,32,36)/t24-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009254

(CHEMBL3233192)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-17-29(44)18-24(2)30(23)22-31(39)38(48)43-16-8-13-34(43)37(47)42-33(20-25-9-4-3-5-10-25)36(46)41-32(35(40)45)21-26-14-15-27-11-6-7-12-28(27)19-26/h3-7,9-12,14-15,17-19,31-34,44H,8,13,16,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594829
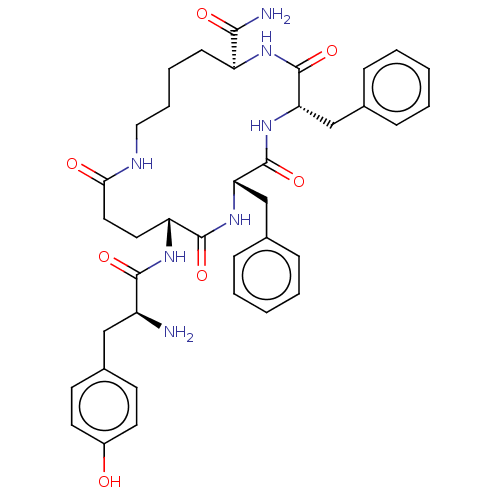
(CHEMBL5196331)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594832

(CHEMBL5185581)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594831

(CHEMBL5185861)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009267

(CHEMBL3233196)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2c(C)cc(C)cc2C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C40H53N5O5/c1-21-12-23(3)31(24(4)13-21)19-34(37(42)47)43-38(48)35(20-32-25(5)14-22(2)15-26(32)6)44-39(49)36-10-9-11-45(36)40(50)33(41)18-30-27(7)16-29(46)17-28(30)8/h12-17,33-36,46H,9-11,18-20,41H2,1-8H3,(H2,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin-2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143182

(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50594868
(CHEMBL5178271)Show SMILES [H][C@]12Nc3ccc(F)c(OC)c3[C@@]1(O)CCN1C[C@@H](CC)[C@]([H])(C[C@@]21[H])C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50268531

(CHEMBL499595 | c[L-Ala-D-pro-L-Phe-D-trp])Show SMILES C[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C1=O |r| Show InChI InChI=1S/C28H31N5O4/c1-17-28(37)33-13-7-12-24(33)27(36)32-22(14-18-8-3-2-4-9-18)26(35)31-23(25(34)30-17)15-19-16-29-21-11-6-5-10-20(19)21/h2-6,8-11,16-17,22-24,29H,7,12-15H2,1H3,(H,30,34)(H,31,35)(H,32,36)/t17-,22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM21025

((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C29H39N5O7/c1-17(2)13-24(29(40)41)34-28(39)23(15-19-7-5-4-6-8-19)33-25(36)16-31-26(37)18(3)32-27(38)22(30)14-20-9-11-21(35)12-10-20/h4-12,17-18,22-24,35H,13-16,30H2,1-3H3,(H,31,37)(H,32,38)(H,33,36)(H,34,39)(H,40,41)/t18-,22+,23+,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in guinea pig brain membranes after 1 hr |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50594867

(CHEMBL5187788)Show SMILES [H][C@]12Nc3cccc(OC)c3[C@@]1(O)CCN1C[C@@H](CC)[C@]([H])(C[C@@]21[H])C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50492099
(CHEMBL2396991)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]13OCCO[C@@]21Nc1ccc(F)c(OC)c31)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C25H33FN2O6/c1-5-15-13-28-9-8-24-21-19(7-6-18(26)22(21)31-3)27-25(24,34-11-10-33-24)20(28)12-16(15)17(14-30-2)23(29)32-4/h6-7,14-16,20,27H,5,8-13H2,1-4H3/b17-14+/t15-,16+,20+,24+,25+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data