 Found 853 hits with Last Name = 'song' and Initial = 'c'
Found 853 hits with Last Name = 'song' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50606766
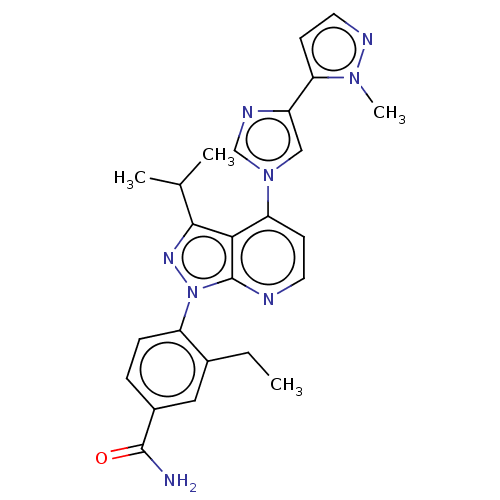
(CHEMBL5218680)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1ccnn1C)C(N)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114516
BindingDB Entry DOI: 10.7270/Q2TQ65N5 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50606766

(CHEMBL5218680)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1ccnn1C)C(N)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114516
BindingDB Entry DOI: 10.7270/Q2TQ65N5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50420244
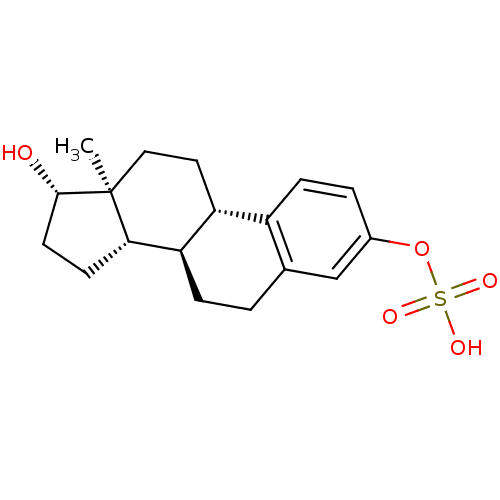
(CHEMBL1628111)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(O)(=O)=O)ccc34)[C@@H]1CC[C@@H]2O |r| Show InChI InChI=1S/C18H24O5S/c1-18-9-8-14-13-5-3-12(23-24(20,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)19/h3,5,10,14-17,19H,2,4,6-9H2,1H3,(H,20,21,22)/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's University
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of LTC4 uptake in membrane vesicle from MRP1-expressing HeLa cells |
J Biol Chem 276: 6404-11 (2001)
Article DOI: 10.1074/jbc.m008251200
BindingDB Entry DOI: 10.7270/Q29S1TW9 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50366524
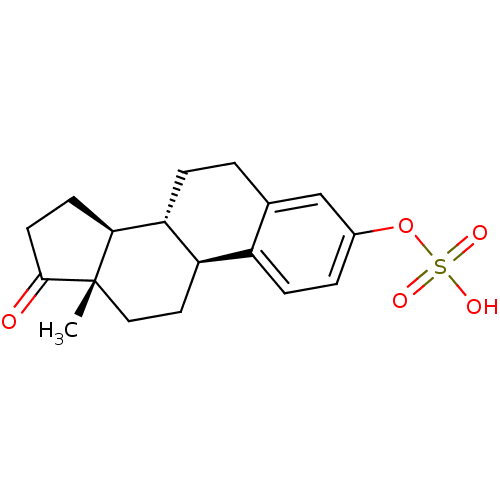
(ESTRONE | ESTROPIPATE | Estrone 3-sulfate | Estron...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(O)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H22O5S/c1-18-9-8-14-13-5-3-12(23-24(20,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)19/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,20,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's University
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of LTC4 uptake in membrane vesicle from MRP1-expressing HeLa cells |
J Biol Chem 276: 6404-11 (2001)
Article DOI: 10.1074/jbc.m008251200
BindingDB Entry DOI: 10.7270/Q29S1TW9 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50308110
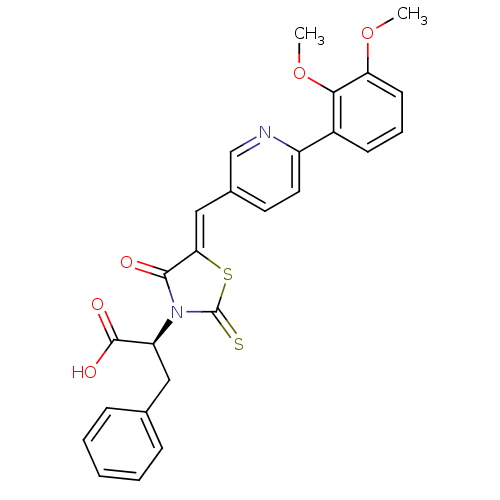
((S,Z)-2-(5-((6-(2,3-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1cccc(c1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-10-6-9-18(23(21)33-2)19-12-11-17(15-27-19)14-22-24(29)28(26(34)35-22)20(25(30)31)13-16-7-4-3-5-8-16/h3-12,14-15,20H,13H2,1-2H3,(H,30,31)/b22-14-/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human Bcl-XL by isothermal titration calorimetry |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50308110

((S,Z)-2-(5-((6-(2,3-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1cccc(c1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-10-6-9-18(23(21)33-2)19-12-11-17(15-27-19)14-22-24(29)28(26(34)35-22)20(25(30)31)13-16-7-4-3-5-8-16/h3-12,14-15,20H,13H2,1-2H3,(H,30,31)/b22-14-/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50107130
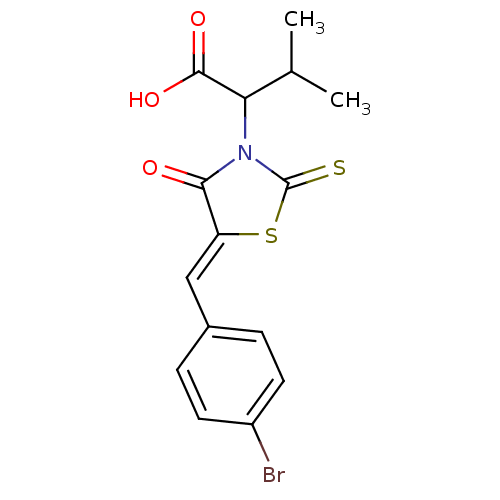
((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...)Show SMILES CC(C)C(N1C(=S)S\C(=C/c2ccc(Br)cc2)C1=O)C(O)=O Show InChI InChI=1S/C15H14BrNO3S2/c1-8(2)12(14(19)20)17-13(18)11(22-15(17)21)7-9-3-5-10(16)6-4-9/h3-8,12H,1-2H3,(H,19,20)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human Bcl-XL by isothermal titration calorimetry |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50107130

((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...)Show SMILES CC(C)C(N1C(=S)S\C(=C/c2ccc(Br)cc2)C1=O)C(O)=O Show InChI InChI=1S/C15H14BrNO3S2/c1-8(2)12(14(19)20)17-13(18)11(22-15(17)21)7-9-3-5-10(16)6-4-9/h3-8,12H,1-2H3,(H,19,20)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Endoplasmin
(Canis familiaris) | BDBM50606766

(CHEMBL5218680)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1ccnn1C)C(N)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114516
BindingDB Entry DOI: 10.7270/Q2TQ65N5 |
More data for this
Ligand-Target Pair | |
Heat shock protein 75 kDa, mitochondrial
(Homo sapiens (Human)) | BDBM50606766

(CHEMBL5218680)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1ccnn1C)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114516
BindingDB Entry DOI: 10.7270/Q2TQ65N5 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50308111
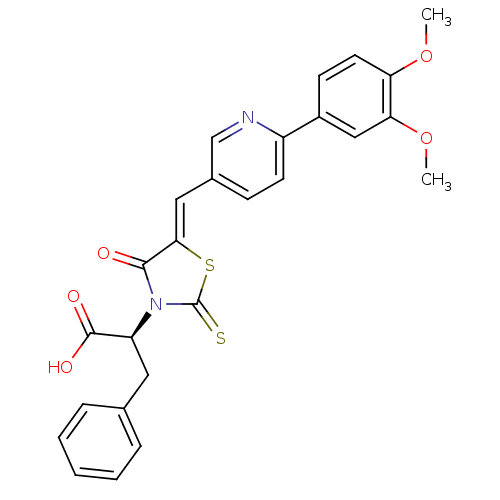
((S,Z)-2-(5-((6-(3,4-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1ccc(cc1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-11-9-18(14-22(21)33-2)19-10-8-17(15-27-19)13-23-24(29)28(26(34)35-23)20(25(30)31)12-16-6-4-3-5-7-16/h3-11,13-15,20H,12H2,1-2H3,(H,30,31)/b23-13-/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50308111

((S,Z)-2-(5-((6-(3,4-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1ccc(cc1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-11-9-18(14-22(21)33-2)19-10-8-17(15-27-19)13-23-24(29)28(26(34)35-23)20(25(30)31)12-16-6-4-3-5-7-16/h3-11,13-15,20H,12H2,1-2H3,(H,30,31)/b23-13-/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human Bcl-XL by isothermal titration calorimetry |
J Med Chem 53: 2314-8 (2010)
Article DOI: 10.1021/jm901469p
BindingDB Entry DOI: 10.7270/Q2K937NJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636214

(US20230365541, Compound 60, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N1CC2COCC2C1 |r,wU:29.35,wD:26.28,(-6.03,-23.5,;-6.03,-21.96,;-7.36,-21.19,;-8.7,-21.96,;-10.03,-21.19,;-11.36,-21.96,;-10.03,-19.65,;-8.7,-18.88,;-8.7,-17.34,;-7.36,-19.65,;-6.03,-18.88,;-4.7,-19.65,;-3.36,-18.88,;-3.36,-17.34,;-2.03,-19.65,;-2.03,-21.19,;-.69,-21.96,;-.69,-23.5,;.64,-21.19,;2.1,-21.67,;3.01,-20.42,;4.34,-21.19,;2.1,-19.18,;.64,-19.65,;-.69,-18.88,;-.69,-17.34,;4.34,-19.65,;5.68,-20.42,;7.01,-19.65,;7.01,-18.11,;5.68,-17.34,;4.34,-18.11,;8.34,-17.34,;9.81,-17.82,;10.71,-16.57,;12.18,-16.09,;12.18,-14.55,;10.71,-14.08,;9.81,-15.32,;8.34,-15.8,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636198

(US20230365541, Compound 42, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N1CC2CN(C)CC2C1 |r,wU:26.28,29.35,(-9.14,-19.26,;-9.14,-17.72,;-10.48,-16.95,;-11.81,-17.72,;-13.15,-16.95,;-14.48,-17.72,;-13.15,-15.41,;-11.81,-14.64,;-11.81,-13.1,;-10.48,-15.41,;-9.14,-14.64,;-7.81,-15.41,;-6.48,-14.64,;-6.48,-13.1,;-5.14,-15.41,;-5.14,-16.95,;-3.81,-17.72,;-3.81,-19.26,;-2.48,-16.95,;-1.01,-17.43,;-.11,-16.18,;.98,-17.27,;-1.01,-14.94,;-2.48,-15.41,;-3.81,-14.64,;-3.81,-13.1,;1.23,-15.41,;2.56,-16.18,;3.89,-15.41,;3.89,-13.87,;2.56,-13.1,;1.23,-13.87,;5.23,-13.1,;6.64,-13.73,;7.67,-12.59,;9.17,-12.27,;9.33,-10.73,;10.67,-9.96,;7.93,-10.11,;6.9,-11.25,;5.39,-11.57,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636217
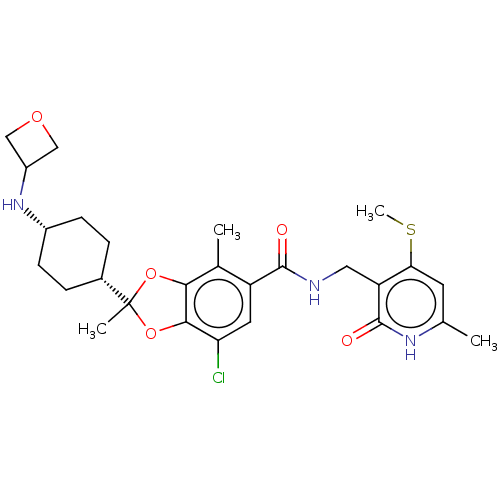
(US20230365541, Compound 61, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)NC1COC1 |r,wU:26.28,29.35,(-8.52,-6.52,;-8.52,-4.98,;-9.85,-4.21,;-11.19,-4.98,;-12.52,-4.21,;-13.85,-4.98,;-12.52,-2.67,;-11.19,-1.9,;-11.19,-.36,;-9.85,-2.67,;-8.52,-1.9,;-7.19,-2.67,;-5.85,-1.9,;-5.85,-.36,;-4.52,-2.67,;-4.52,-4.21,;-3.19,-4.98,;-3.19,-6.52,;-1.85,-4.21,;-.39,-4.68,;.52,-3.44,;1.85,-4.21,;-.39,-2.19,;-1.85,-2.67,;-3.19,-1.9,;-3.19,-.36,;1.85,-2.67,;3.19,-3.44,;4.52,-2.67,;4.52,-1.13,;3.19,-.36,;1.85,-1.13,;5.85,-.36,;7.19,-1.13,;7.59,-2.62,;9.07,-2.22,;8.67,-.73,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636203

(US20230365541, Compound 45, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N[C@H]1C[C@H](O)C1 |r,wU:26.28,33.36,wD:29.35,35.39,(-6.8,22.49,;-6.8,24.03,;-8.13,24.8,;-9.46,24.03,;-10.8,24.8,;-12.13,24.03,;-10.8,26.34,;-9.46,27.11,;-9.46,28.65,;-8.13,26.34,;-6.8,27.11,;-5.46,26.34,;-4.13,27.11,;-4.13,28.65,;-2.79,26.34,;-2.79,24.8,;-1.46,24.03,;-1.46,22.49,;-.13,24.8,;1.34,24.32,;2.24,25.57,;3.58,24.8,;1.34,26.81,;-.13,26.34,;-1.46,27.11,;-1.46,28.65,;3.58,26.34,;3.58,27.88,;4.91,28.65,;6.24,27.88,;6.24,26.34,;4.91,25.57,;7.58,28.65,;8.91,27.88,;10.4,28.27,;10.8,26.79,;12.13,26.02,;9.31,26.39,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636208
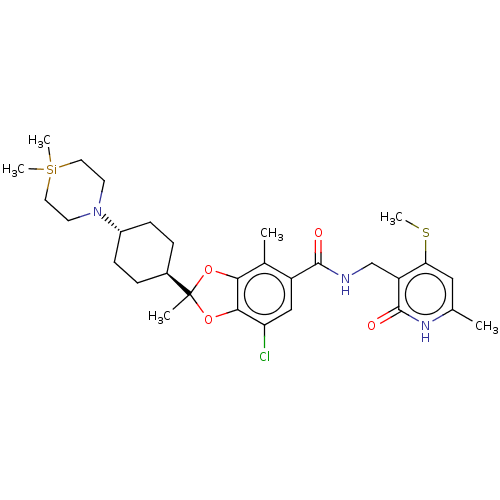
(US20230365541, Compound 49, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N1CC[Si](C)(C)CC1 |r,wU:29.35,wD:26.28,(5.74,-4.62,;5.74,-3.08,;4.41,-2.31,;3.07,-3.08,;1.74,-2.31,;.41,-3.08,;1.74,-.77,;3.07,,;3.07,1.54,;4.41,-.77,;5.74,,;7.07,-.77,;8.41,,;8.41,1.54,;9.74,-.77,;9.74,-2.31,;11.07,-3.08,;11.07,-4.62,;12.41,-2.31,;13.87,-2.79,;14.78,-1.54,;15.73,-2.58,;13.87,-.29,;12.41,-.77,;11.07,,;11.07,1.54,;16.11,-.77,;17.45,-1.54,;18.78,-.77,;18.78,.77,;17.45,1.54,;16.11,.77,;20.11,1.54,;20.11,3.08,;21.45,3.85,;22.78,3.08,;24.11,3.85,;22.78,4.62,;22.78,1.54,;21.45,.77,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636209

(US20230365541, Compound 53, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N[C@H]1C[C@H](F)C1 |r,wU:29.35,33.36,wD:26.28,35.39,(1.04,-17.42,;1.04,-15.88,;-.3,-15.11,;-1.63,-15.88,;-2.96,-15.11,;-4.3,-15.88,;-2.96,-13.57,;-1.63,-12.8,;-1.63,-11.26,;-.3,-13.57,;1.04,-12.8,;2.37,-13.57,;3.7,-12.8,;3.7,-11.26,;5.04,-13.57,;5.04,-15.11,;6.37,-15.88,;6.37,-17.42,;7.7,-15.11,;9.17,-15.59,;10.07,-14.34,;11.41,-15.11,;9.17,-13.1,;7.7,-13.57,;6.37,-12.8,;6.37,-11.26,;11.41,-13.57,;12.74,-14.34,;14.08,-13.57,;14.08,-12.03,;12.74,-11.26,;11.41,-12.03,;15.41,-11.26,;16.74,-12.03,;18.23,-11.63,;18.63,-13.12,;19.96,-13.89,;17.14,-13.52,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636215

(US20230365541, Compound 60, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N1CC2COCC2C1 |r,wU:26.28,29.35,(-6.03,-23.5,;-6.03,-21.96,;-7.36,-21.19,;-8.7,-21.96,;-10.03,-21.19,;-11.36,-21.96,;-10.03,-19.66,;-8.7,-18.89,;-8.7,-17.35,;-7.36,-19.66,;-6.03,-18.89,;-4.7,-19.66,;-3.36,-18.89,;-3.36,-17.35,;-2.03,-19.66,;-2.03,-21.2,;-.69,-21.97,;-.69,-23.51,;.64,-21.2,;2.1,-21.67,;3.01,-20.43,;4.34,-21.2,;2.1,-19.18,;.64,-19.66,;-.69,-18.89,;-.69,-17.35,;4.34,-19.66,;5.68,-20.42,;7.01,-19.66,;7.01,-18.12,;5.68,-17.35,;4.34,-18.12,;8.34,-17.35,;9.81,-17.82,;10.71,-16.58,;12.18,-16.1,;12.18,-14.56,;10.71,-14.08,;9.81,-15.33,;8.34,-15.81,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636205

(US20230365541, Compound 47, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)C(=O)N1CC(F)(F)C1 |r,wU:29.35,wD:26.28,(-6.86,-17.49,;-6.86,-15.95,;-8.2,-15.18,;-9.53,-15.95,;-10.86,-15.18,;-12.2,-15.95,;-10.86,-13.64,;-9.53,-12.87,;-9.53,-11.33,;-8.2,-13.64,;-6.86,-12.87,;-5.53,-13.64,;-4.2,-12.87,;-4.2,-11.33,;-2.86,-13.64,;-2.86,-15.18,;-1.53,-15.95,;-1.53,-17.49,;-.2,-15.18,;1.27,-15.66,;2.17,-14.41,;3.51,-15.18,;1.27,-13.16,;-.2,-13.64,;-1.53,-12.87,;-1.53,-11.33,;3.51,-13.64,;4.84,-14.41,;6.18,-13.64,;6.18,-12.1,;4.84,-11.33,;3.51,-12.1,;7.51,-11.33,;7.51,-9.79,;8.84,-12.1,;9.24,-13.59,;10.73,-13.19,;12.27,-13.19,;11.5,-14.52,;10.33,-11.7,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636197
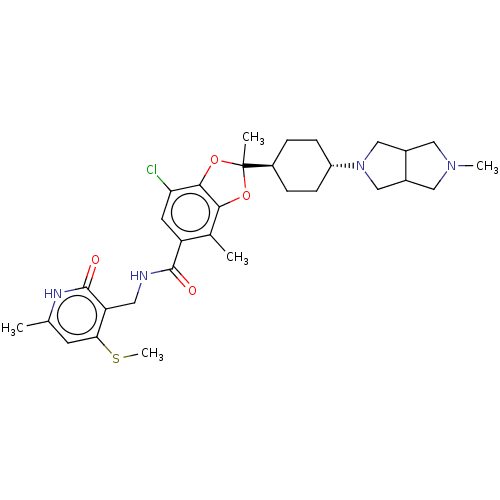
(US20230365541, Compound 42, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N1CC2CN(C)CC2C1 |r,wU:26.28,wD:29.35,(-9.14,-19.26,;-9.14,-17.72,;-10.48,-16.95,;-11.81,-17.72,;-13.15,-16.95,;-14.48,-17.72,;-13.15,-15.41,;-11.81,-14.64,;-11.81,-13.1,;-10.48,-15.41,;-9.14,-14.64,;-7.81,-15.41,;-6.48,-14.64,;-6.48,-13.1,;-5.14,-15.41,;-5.14,-16.95,;-3.81,-17.72,;-3.81,-19.26,;-2.48,-16.95,;-1.01,-17.43,;-.11,-16.18,;.98,-17.27,;-1.01,-14.94,;-2.48,-15.41,;-3.81,-14.64,;-3.81,-13.1,;1.23,-15.41,;1.23,-13.87,;2.56,-13.1,;3.89,-13.87,;3.89,-15.41,;2.56,-16.18,;5.23,-13.1,;6.64,-13.73,;7.67,-12.59,;9.17,-12.27,;9.33,-10.73,;10.67,-9.96,;7.93,-10.11,;6.9,-11.25,;5.39,-11.57,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636204

(US20230365541, Compound 45, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N[C@H]1C[C@H](O)C1 |r,wU:26.28,29.35,33.36,wD:35.39,(-6.8,22.49,;-6.8,24.03,;-8.13,24.8,;-9.46,24.03,;-10.8,24.8,;-12.13,24.03,;-10.8,26.34,;-9.46,27.11,;-9.46,28.65,;-8.13,26.34,;-6.8,27.11,;-5.46,26.34,;-4.13,27.11,;-4.13,28.65,;-2.79,26.34,;-2.79,24.8,;-1.46,24.03,;-1.46,22.49,;-.13,24.8,;1.34,24.32,;2.24,25.57,;3.58,24.8,;1.34,26.81,;-.13,26.34,;-1.46,27.11,;-1.46,28.65,;3.58,26.34,;4.91,25.57,;6.24,26.34,;6.24,27.88,;4.91,28.65,;3.58,27.88,;7.58,28.65,;8.91,27.88,;10.4,28.27,;10.8,26.79,;12.13,26.02,;9.31,26.39,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50042559

(5-Butyl-2-(2-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1ccccc1Cl Show InChI InChI=1S/C28H25ClN6O2/c1-2-3-11-24-22(26(28(36)37)35(32-24)25-12-7-6-10-23(25)29)17-18-13-15-19(16-14-18)20-8-4-5-9-21(20)27-30-33-34-31-27/h4-10,12-16H,2-3,11,17H2,1H3,(H,36,37)(H,30,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00206j
BindingDB Entry DOI: 10.7270/Q2PC36F2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636200
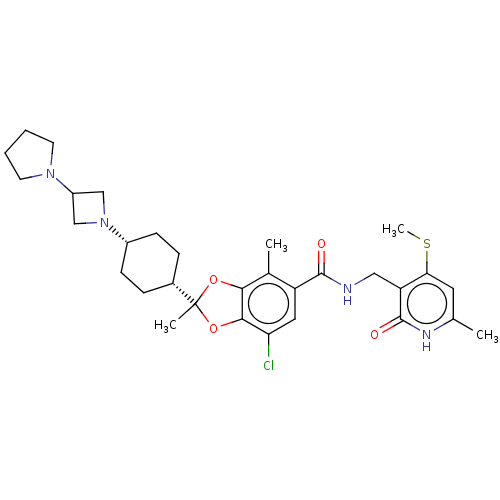
(US20230365541, Compound 43, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N1CC(C1)N1CCCC1 |r,wU:26.28,29.35,(-5.2,-36.99,;-5.2,-35.45,;-6.53,-34.68,;-7.87,-35.45,;-9.2,-34.68,;-10.53,-35.45,;-9.2,-33.14,;-7.87,-32.37,;-7.87,-30.83,;-6.53,-33.14,;-5.2,-32.37,;-3.87,-33.14,;-2.53,-32.37,;-2.53,-30.83,;-1.2,-33.14,;-1.2,-34.68,;.13,-35.45,;.13,-36.99,;1.47,-34.68,;2.93,-35.15,;3.84,-33.91,;4.93,-34.99,;2.93,-32.66,;1.47,-33.14,;.13,-32.37,;.13,-30.83,;5.17,-33.14,;6.51,-33.91,;7.84,-33.14,;7.84,-31.6,;6.51,-30.83,;5.17,-31.6,;9.17,-30.83,;10.66,-31.22,;11.06,-29.74,;9.57,-29.34,;12.39,-28.97,;13.8,-29.59,;14.83,-28.45,;14.06,-27.12,;12.55,-27.44,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636191
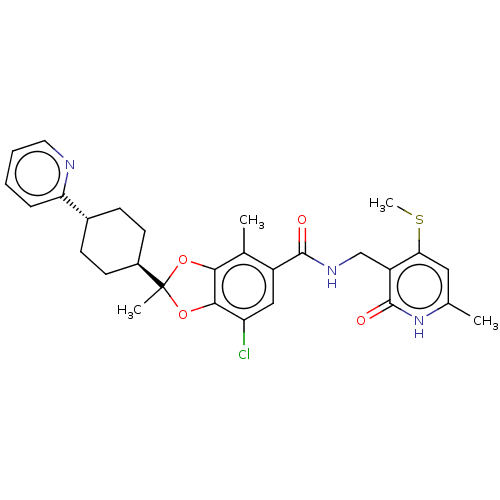
(US20230365541, Compound 39, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)c1ccccn1 |r,wU:26.28,wD:29.35,(-6.18,-37.37,;-6.18,-35.83,;-7.51,-35.06,;-8.85,-35.83,;-10.18,-35.06,;-11.51,-35.83,;-10.18,-33.52,;-8.85,-32.75,;-8.85,-31.21,;-7.51,-33.52,;-6.18,-32.75,;-4.84,-33.52,;-3.51,-32.75,;-3.51,-31.21,;-2.18,-33.52,;-2.18,-35.06,;-.84,-35.83,;-.84,-37.37,;.49,-35.06,;1.96,-35.54,;2.86,-34.29,;3.95,-35.38,;1.96,-33.05,;.49,-33.52,;-.84,-32.75,;-.84,-31.21,;3.95,-33.21,;3.55,-31.72,;4.64,-30.63,;6.13,-31.03,;6.53,-32.52,;5.44,-33.6,;7.22,-29.94,;6.82,-28.45,;7.91,-27.36,;9.39,-27.76,;9.79,-29.25,;8.7,-30.34,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636172
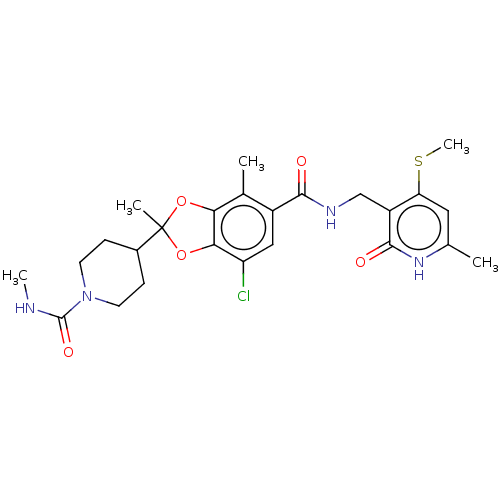
(4-(7-chloro-2,4-dimethyl-5-(((6-methyl-4-(methylth...)Show SMILES CNC(=O)N1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(SC)cc(C)[nH]c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636196

(US20230365541, Compound 41, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N[C@@H]1C[C@H](F)C1 |r,wU:26.28,29.35,wD:33.36,35.39,(-16.15,-22.58,;-16.15,-21.04,;-17.49,-20.27,;-18.82,-21.04,;-20.16,-20.27,;-21.49,-21.04,;-20.16,-18.73,;-18.82,-17.96,;-18.82,-16.42,;-17.49,-18.73,;-16.15,-17.96,;-14.82,-18.73,;-13.49,-17.96,;-13.49,-16.42,;-12.15,-18.73,;-12.15,-20.27,;-10.82,-21.04,;-10.82,-22.58,;-9.49,-20.27,;-8.02,-20.74,;-7.12,-19.5,;-5.78,-20.27,;-8.02,-18.25,;-9.49,-18.73,;-10.82,-17.96,;-10.82,-16.42,;-5.78,-18.73,;-4.45,-19.5,;-3.12,-18.73,;-3.12,-17.19,;-4.45,-16.42,;-5.78,-17.19,;-1.78,-16.42,;-.45,-17.19,;1.04,-16.79,;1.44,-18.28,;2.77,-19.05,;-.05,-18.68,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636189

(7-chloro-2-(1-(cyclobutylmethyl)piperidin-4-yl)-2,...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCN(CC2CCC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636180

(US20230365541, Compound 29 | methyl-4-(7-chloro-2,...)Show SMILES COC(=O)N1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(SC)cc(C)[nH]c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636213

(US20230365541, Compound 59, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)NC1COC1 |r,wU:29.35,wD:26.28,(14.26,6.69,;12.93,5.92,;12.93,4.38,;14.26,3.61,;14.26,2.07,;15.59,1.3,;12.93,1.3,;11.59,2.07,;10.26,1.3,;11.59,3.61,;10.26,4.38,;8.93,3.61,;7.59,4.38,;7.59,5.92,;6.26,3.61,;6.26,2.07,;4.92,1.3,;4.92,-.24,;3.59,2.07,;2.13,1.6,;1.22,2.84,;.08,3.87,;2.13,4.09,;3.59,3.61,;4.92,4.38,;4.92,5.92,;.08,1.81,;-1.39,2.29,;-2.53,1.26,;-2.21,-.25,;-.75,-.72,;.4,.31,;-3.36,-1.28,;-4.82,-.8,;-6.19,-1.5,;-6.89,-.13,;-5.52,.57,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636210

(US20230365541, Compound 57, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@H](CN2CCOCC2)CC1 |r,wU:29.32,wD:26.28,(-5.9,-26.5,;-5.9,-24.96,;-7.23,-24.19,;-8.57,-24.96,;-9.9,-24.19,;-11.24,-24.96,;-9.9,-22.65,;-8.57,-21.88,;-8.57,-20.34,;-7.23,-22.65,;-5.9,-21.88,;-4.57,-22.65,;-3.23,-21.88,;-3.23,-20.34,;-1.9,-22.65,;-1.9,-24.19,;-.57,-24.96,;-.57,-26.5,;.77,-24.19,;2.23,-24.66,;3.14,-23.42,;4.47,-24.19,;2.23,-22.17,;.77,-22.65,;-.57,-21.88,;-.57,-20.34,;4.47,-22.65,;5.8,-23.42,;7.14,-22.65,;7.14,-21.11,;8.47,-20.34,;9.81,-21.11,;9.81,-22.65,;11.14,-23.42,;12.47,-22.65,;12.47,-21.11,;11.14,-20.34,;5.8,-20.34,;4.47,-21.11,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636158
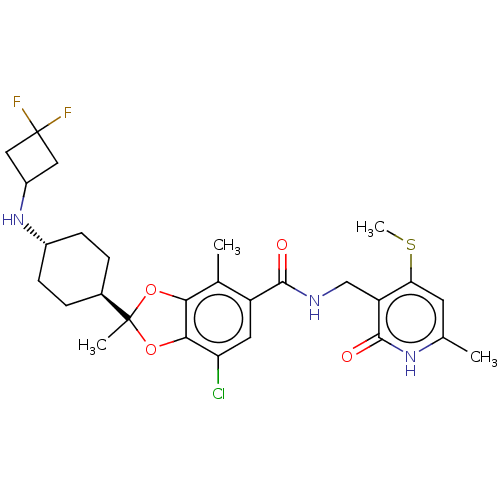
(US20230365541, Compound 4, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)NC1CC(F)(F)C1 |r,wU:26.28,wD:29.35,(6.57,-23.57,;6.57,-22.03,;5.23,-21.26,;3.9,-22.03,;2.56,-21.26,;1.23,-22.03,;2.56,-19.72,;3.9,-18.95,;3.9,-17.41,;5.23,-19.72,;6.57,-18.95,;7.9,-19.72,;9.23,-18.95,;9.23,-17.41,;10.57,-19.72,;10.57,-21.26,;11.9,-22.03,;11.9,-23.57,;13.23,-21.26,;14.7,-21.73,;15.6,-20.49,;16.69,-21.58,;14.7,-19.24,;13.23,-19.72,;11.9,-18.95,;11.9,-17.41,;16.94,-19.72,;16.94,-18.18,;18.27,-17.41,;19.6,-18.18,;19.6,-19.72,;18.27,-20.49,;20.94,-17.41,;22.27,-18.18,;22.67,-19.67,;24.16,-19.27,;25.7,-19.27,;24.93,-20.6,;23.76,-17.78,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636173
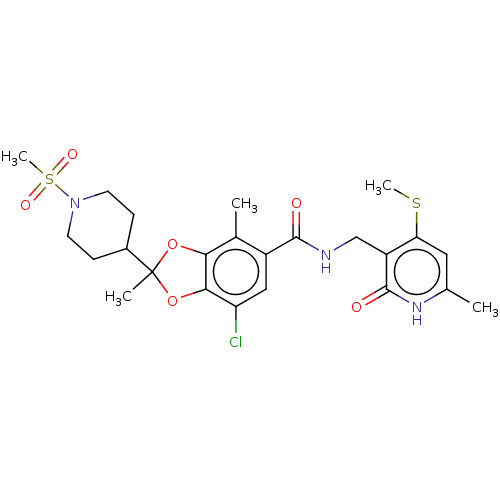
(7-chloro-2,4-dimethyl-N-((6-methyl-4-(methylthio)-...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCN(CC1)S(C)(=O)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636201
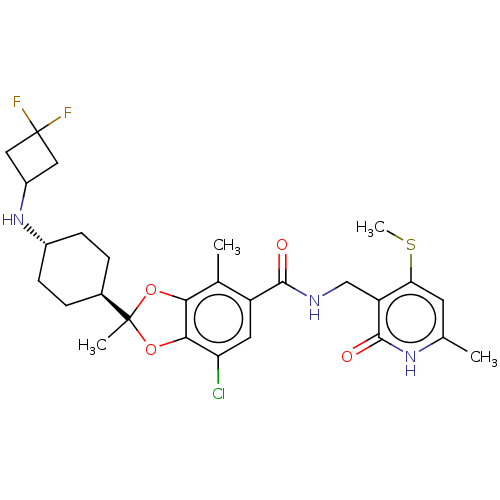
(US20230365541, Compound 44, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)NC1CC(F)(F)C1 |r,wU:29.35,wD:26.28,(-6.57,-12.84,;-6.57,-11.3,;-7.91,-10.53,;-9.24,-11.3,;-10.58,-10.53,;-11.91,-11.3,;-10.58,-8.99,;-9.24,-8.22,;-9.24,-6.68,;-7.91,-8.99,;-6.57,-8.22,;-5.24,-8.99,;-3.91,-8.22,;-3.91,-6.68,;-2.57,-8.99,;-2.57,-10.53,;-1.24,-11.3,;-1.24,-12.84,;.09,-10.53,;1.56,-11.01,;2.46,-9.76,;3.55,-10.85,;1.56,-8.51,;.09,-8.99,;-1.24,-8.22,;-1.24,-6.68,;3.8,-8.99,;5.13,-9.76,;6.46,-8.99,;6.46,-7.45,;5.13,-6.68,;3.8,-7.45,;7.8,-6.68,;9.13,-7.45,;9.53,-8.94,;11.02,-8.54,;12.56,-8.54,;11.79,-9.87,;10.62,-7.05,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636167

(US20230365541, Compound 9, isomer 2)Show SMILES CO[C@H]1C[C@@H](C1)N[C@H]1CC[C@H](CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(SC)cc(C)[nH]c1=O |r,wU:10.14,7.7,4.6,wD:2.1,(-.82,-22.62,;-2.15,-23.39,;-3.49,-22.62,;-3.88,-21.14,;-5.37,-21.54,;-4.97,-23.02,;-6.71,-20.77,;-8.04,-21.54,;-8.04,-23.08,;-9.37,-23.85,;-10.71,-23.08,;-10.71,-21.54,;-9.37,-20.77,;-12.04,-23.85,;-10.95,-24.93,;-12.95,-25.09,;-14.41,-24.62,;-14.41,-23.08,;-12.95,-22.6,;-15.74,-22.31,;-15.74,-20.77,;-17.08,-23.08,;-17.08,-24.62,;-15.74,-25.39,;-15.74,-26.93,;-18.41,-22.31,;-18.41,-20.77,;-19.74,-23.08,;-21.08,-22.31,;-22.41,-23.08,;-22.41,-24.62,;-21.08,-25.39,;-21.08,-26.93,;-23.75,-25.39,;-25.08,-24.62,;-26.41,-25.39,;-25.08,-23.08,;-23.75,-22.31,;-23.75,-20.77,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636179
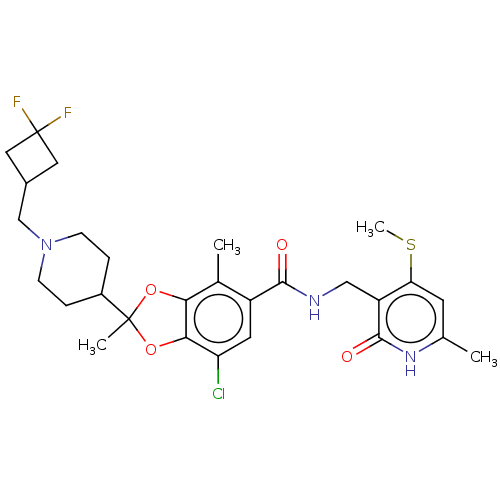
(7-chloro-2-(1-((3,3-difluorocyclobutyl)methyl)pipe...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCN(CC2CC(F)(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636199
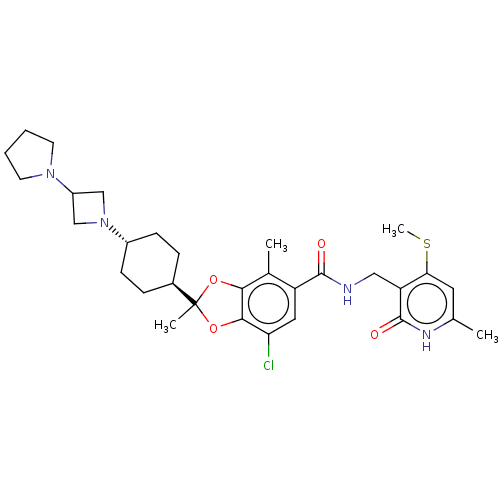
(US20230365541, Compound 43, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N1CC(C1)N1CCCC1 |r,wU:29.35,wD:26.28,(-5.2,-36.99,;-5.2,-35.45,;-6.53,-34.68,;-7.87,-35.45,;-9.2,-34.68,;-10.53,-35.45,;-9.2,-33.14,;-7.87,-32.37,;-7.87,-30.83,;-6.53,-33.14,;-5.2,-32.37,;-3.87,-33.14,;-2.53,-32.37,;-2.53,-30.83,;-1.2,-33.14,;-1.2,-34.68,;.14,-35.45,;.14,-36.99,;1.47,-34.68,;2.93,-35.16,;3.84,-33.91,;4.93,-35,;2.93,-32.66,;1.47,-33.14,;.14,-32.37,;.14,-30.83,;5.17,-33.14,;6.51,-33.91,;7.84,-33.14,;7.84,-31.6,;6.51,-30.83,;5.17,-31.6,;9.17,-30.83,;10.66,-31.23,;11.06,-29.74,;9.57,-29.34,;12.39,-28.97,;13.8,-29.6,;14.83,-28.45,;14.06,-27.12,;12.55,-27.44,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636211

(US20230365541, Compound 57, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@H](CN2CCOCC2)CC1 |r,wU:26.28,29.32,(-5.9,-26.5,;-5.9,-24.96,;-7.23,-24.19,;-8.57,-24.96,;-9.9,-24.19,;-11.24,-24.96,;-9.9,-22.65,;-8.57,-21.88,;-8.57,-20.34,;-7.23,-22.65,;-5.9,-21.88,;-4.57,-22.65,;-3.23,-21.88,;-3.23,-20.34,;-1.9,-22.65,;-1.9,-24.19,;-.57,-24.96,;-.57,-26.5,;.77,-24.19,;2.23,-24.66,;3.14,-23.42,;4.47,-24.19,;2.23,-22.17,;.77,-22.65,;-.57,-21.88,;-.57,-20.34,;4.47,-22.65,;5.8,-23.42,;7.14,-22.65,;7.14,-21.11,;8.47,-20.34,;9.81,-21.11,;9.81,-22.65,;11.14,-23.42,;12.47,-22.65,;12.47,-21.11,;11.14,-20.34,;5.8,-20.34,;4.47,-21.11,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00206j
BindingDB Entry DOI: 10.7270/Q2PC36F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM418817

(N-methyl-4-({4-[({3- [methyl(methylsulfonyl)amino]...)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3nccnc3N(C)S(C)(=O)=O)n2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H21F3N8O3S/c1-24-18(32)12-4-6-13(7-5-12)29-19-28-10-14(20(21,22)23)16(30-19)27-11-15-17(26-9-8-25-15)31(2)35(3,33)34/h4-10H,11H2,1-3H3,(H,24,32)(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant FAK (unknown origin) by radiometric kinase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00459
BindingDB Entry DOI: 10.7270/Q2TT4VSH |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636207
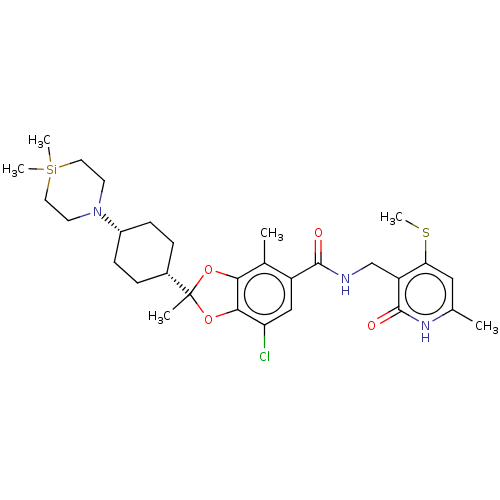
(US20230365541, Compound 49, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)N1CC[Si](C)(C)CC1 |r,wD:26.28,29.35,(-19.84,-4.8,;-19.84,-3.26,;-21.17,-2.49,;-22.51,-3.26,;-23.84,-2.49,;-25.18,-3.26,;-23.84,-.95,;-22.51,-.18,;-22.51,1.36,;-21.17,-.95,;-19.84,-.18,;-18.51,-.95,;-17.17,-.18,;-17.17,1.36,;-15.84,-.95,;-15.84,-2.49,;-14.51,-3.26,;-14.51,-4.8,;-13.17,-2.49,;-11.71,-2.97,;-10.8,-1.72,;-9.87,-2.78,;-11.71,-.48,;-13.17,-.95,;-14.51,-.18,;-14.51,1.36,;-9.47,-.95,;-9.47,.59,;-8.14,1.36,;-6.8,.59,;-6.8,-.95,;-8.14,-1.72,;-5.47,1.36,;-5.47,2.9,;-4.13,3.67,;-2.8,2.9,;-1.47,3.67,;-2.8,4.44,;-2.8,1.36,;-4.13,.59,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636202
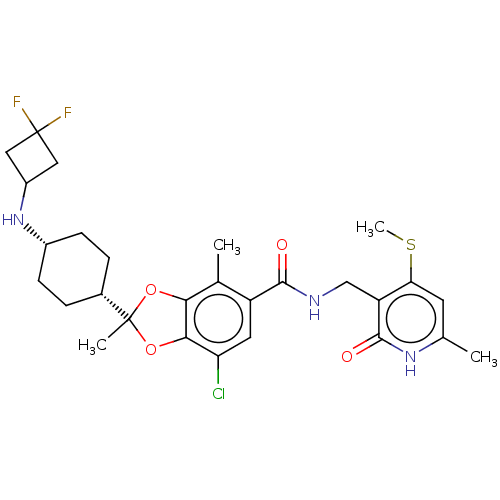
(US20230365541, Compound 44, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@@H](CC1)NC1CC(F)(F)C1 |r,wU:26.28,29.35,(-6.57,-12.84,;-6.57,-11.3,;-7.91,-10.53,;-9.24,-11.3,;-10.58,-10.53,;-11.91,-11.3,;-10.58,-8.99,;-9.24,-8.22,;-9.24,-6.68,;-7.91,-8.99,;-6.57,-8.22,;-5.24,-8.99,;-3.91,-8.22,;-3.91,-6.68,;-2.57,-8.99,;-2.57,-10.53,;-1.24,-11.3,;-1.24,-12.84,;.09,-10.53,;1.56,-11.01,;2.46,-9.76,;3.55,-10.85,;1.56,-8.51,;.09,-8.99,;-1.24,-8.22,;-1.24,-6.68,;3.8,-8.99,;5.13,-9.76,;6.46,-8.99,;6.46,-7.45,;5.13,-6.68,;3.8,-7.45,;7.8,-6.68,;9.13,-7.45,;9.53,-8.94,;11.02,-8.54,;12.56,-8.54,;11.79,-9.87,;10.62,-7.05,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636164

(US20230365541, Compound 8, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CCC(CC1)c1cnn(C)c1 |r,wD:26.28,(7.22,-24.92,;7.22,-23.38,;5.89,-22.61,;4.55,-23.38,;3.22,-22.61,;1.89,-23.38,;3.22,-21.07,;4.55,-20.3,;4.55,-18.76,;5.89,-21.07,;7.22,-20.3,;8.56,-21.07,;9.89,-20.3,;9.89,-18.76,;11.22,-21.07,;11.22,-22.61,;12.56,-23.38,;12.56,-24.92,;13.89,-22.61,;15.35,-23.09,;16.26,-21.84,;17.35,-22.93,;15.35,-20.6,;13.89,-21.07,;12.56,-20.3,;12.56,-18.76,;17.59,-21.07,;18.93,-21.84,;20.26,-21.07,;20.26,-19.53,;18.93,-18.76,;17.59,-19.53,;21.59,-18.76,;23,-19.39,;24.03,-18.24,;23.26,-16.91,;23.89,-15.5,;21.76,-17.23,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636157

(US20230365541, Compound 3, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)NCC1(C)COC1 |r,wU:29.35,wD:26.28,(6.67,-27.07,;6.67,-25.53,;5.34,-24.76,;4,-25.53,;2.67,-24.76,;1.33,-25.53,;2.67,-23.22,;4,-22.45,;4,-20.91,;5.34,-23.22,;6.67,-22.45,;8,-23.22,;9.34,-22.45,;9.34,-20.91,;10.67,-23.22,;10.67,-24.76,;12,-25.53,;12,-27.07,;13.34,-24.76,;14.8,-25.24,;15.71,-23.99,;16.8,-25.08,;14.8,-22.74,;13.34,-23.22,;12,-22.45,;12,-20.91,;17.04,-23.22,;18.37,-23.99,;19.71,-23.22,;19.71,-21.68,;18.37,-20.91,;17.04,-21.68,;21.04,-20.91,;22.38,-21.68,;23.71,-20.91,;22.38,-20.14,;24.8,-22,;25.89,-20.91,;24.8,-19.82,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636194

(US20230365541, Compound 40, isomer 2)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@@H]1CC[C@H](CNC2CC(F)(F)C2)CC1 |r,wU:26.28,29.32,(-7.47,-18.93,;-7.47,-17.39,;-8.8,-16.62,;-10.13,-17.39,;-11.47,-16.62,;-12.8,-17.39,;-11.47,-15.08,;-10.13,-14.31,;-10.13,-12.77,;-8.8,-15.08,;-7.47,-14.31,;-6.13,-15.08,;-4.8,-14.31,;-4.8,-12.77,;-3.47,-15.08,;-3.47,-16.62,;-2.13,-17.39,;-2.13,-18.93,;-.8,-16.62,;.67,-17.1,;1.57,-15.85,;2.91,-16.62,;.67,-14.61,;-.8,-15.08,;-2.13,-14.31,;-2.13,-12.77,;2.91,-15.08,;4.24,-15.85,;5.57,-15.08,;5.57,-13.54,;6.91,-12.77,;8.24,-13.54,;9.57,-12.77,;11.06,-13.17,;11.46,-11.69,;13,-11.69,;12.23,-10.35,;9.97,-11.29,;4.24,-12.77,;2.91,-13.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636163

(US20230365541, Compound 11, isomer 1 | US202303655...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2O[C@@](C)(Oc2c1C)C1CCN(CC1)C(=O)C1CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636177

(7-chloro-2,4-dimethyl-N-((6-methyl-4-(methylthio)-...)Show SMILES CNS(=O)(=O)N1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(SC)cc(C)[nH]c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636178

(7-chloro-2-(1-(cyclopropylmethyl)piperidin-4-yl)-2...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCN(CC2CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636216

(US20230365541, Compound 61, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)NC1COC1 |r,wU:26.28,wD:29.35,(-8.52,-6.52,;-8.52,-4.98,;-9.85,-4.21,;-11.19,-4.98,;-12.52,-4.21,;-13.85,-4.98,;-12.52,-2.67,;-11.19,-1.9,;-11.19,-.36,;-9.85,-2.67,;-8.52,-1.9,;-7.19,-2.67,;-5.85,-1.9,;-5.85,-.36,;-4.52,-2.67,;-4.52,-4.21,;-3.19,-4.98,;-3.19,-6.52,;-1.85,-4.21,;-.39,-4.68,;.52,-3.44,;1.85,-4.21,;-.39,-2.19,;-1.85,-2.67,;-3.19,-1.9,;-3.19,-.36,;1.85,-2.67,;1.85,-1.13,;3.19,-.36,;4.52,-1.13,;4.52,-2.67,;3.19,-3.44,;5.85,-.36,;7.19,-1.13,;7.59,-2.62,;9.07,-2.22,;8.67,-.73,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM636195

(US20230365541, Compound 41, isomer 1)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N[C@@H]1C[C@H](F)C1 |r,wU:26.28,wD:29.35,33.36,35.39,(-16.15,-22.58,;-16.15,-21.04,;-17.49,-20.27,;-18.82,-21.04,;-20.16,-20.27,;-21.49,-21.04,;-20.16,-18.73,;-18.82,-17.96,;-18.82,-16.42,;-17.49,-18.73,;-16.15,-17.96,;-14.82,-18.73,;-13.49,-17.96,;-13.49,-16.42,;-12.15,-18.73,;-12.15,-20.27,;-10.82,-21.04,;-10.82,-22.58,;-9.49,-20.27,;-8.02,-20.74,;-7.12,-19.5,;-5.78,-20.27,;-8.02,-18.25,;-9.49,-18.73,;-10.82,-17.96,;-10.82,-16.42,;-5.78,-18.73,;-5.78,-17.19,;-4.45,-16.42,;-3.12,-17.19,;-3.12,-18.73,;-4.45,-19.5,;-1.78,-16.42,;-.45,-17.19,;1.04,-16.79,;1.44,-18.28,;2.77,-19.05,;-.05,-18.68,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data