

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase TEM (Escherichia coli) | BDBM50021963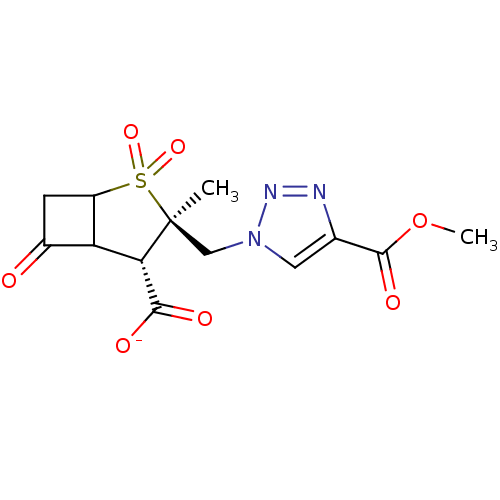 (CHEMBL33711 | Sodium; 3-(4-methoxycarbonyl-[1,2,3]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021958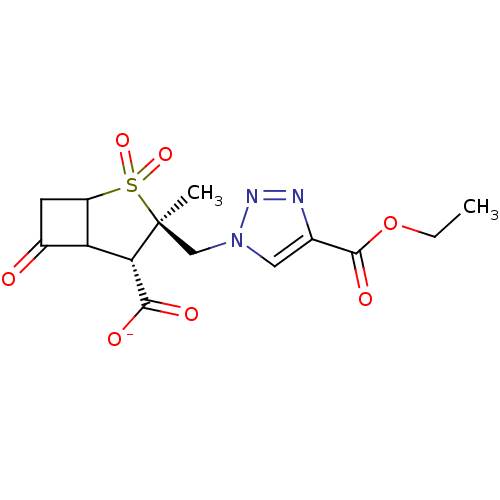 (CHEMBL33950 | Sodium; 3-(4-ethoxycarbonyl-[1,2,3]t...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289135 ((S)-4-(Bis-methylsulfanyl-methylene)-7-methoxy-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human sputum elastase (HSE) | Bioorg Med Chem Lett 6: 823-826 (1996) Article DOI: 10.1016/0960-894X(96)00114-X BindingDB Entry DOI: 10.7270/Q2CV4HR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Penicillinase from Staphylococcus aureus TH-14 using piperacillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021956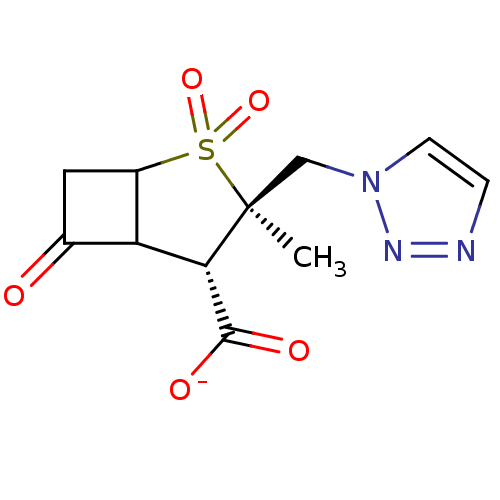 (CHEMBL285387 | Sodium; 3-methyl-2,2,6-trioxo-3-[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Penicillinase from Staphylococcus aureus TH-14 using piperacillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021960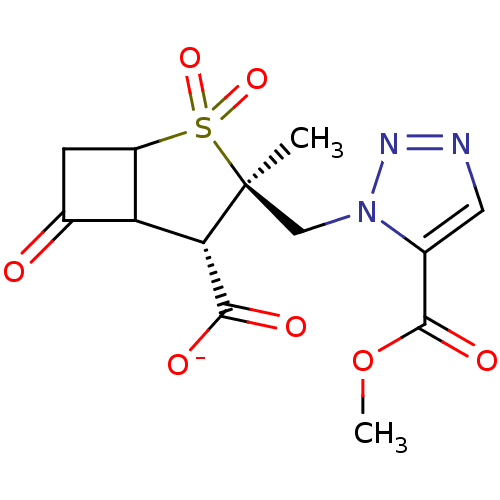 (CHEMBL286323 | Sodium; 3-(5-methoxycarbonyl-[1,2,3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021957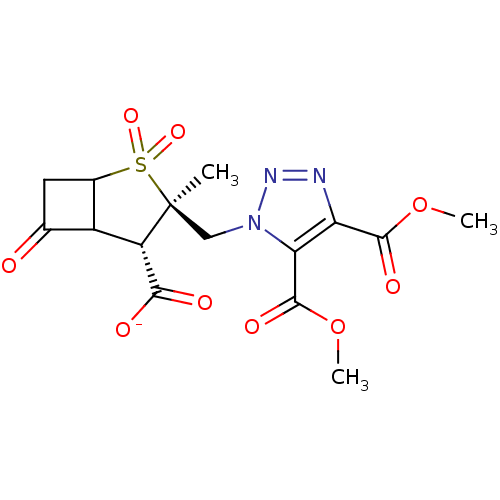 (CHEMBL284273 | Sodium; 3-(4,5-bis-methoxycarbonyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289134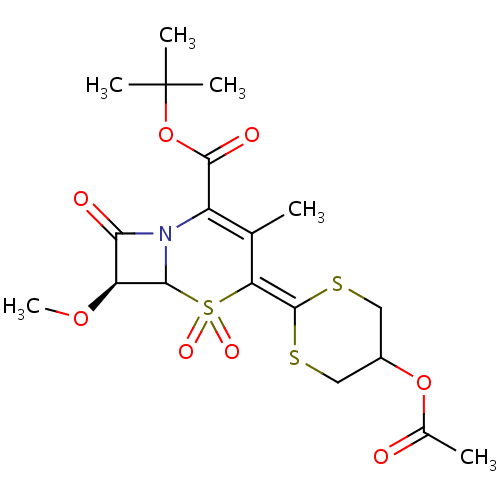 ((S)-4-(5-Acetoxy-[1,3]dithian-2-ylidene)-7-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human sputum elastase (HSE) | Bioorg Med Chem Lett 6: 823-826 (1996) Article DOI: 10.1016/0960-894X(96)00114-X BindingDB Entry DOI: 10.7270/Q2CV4HR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289132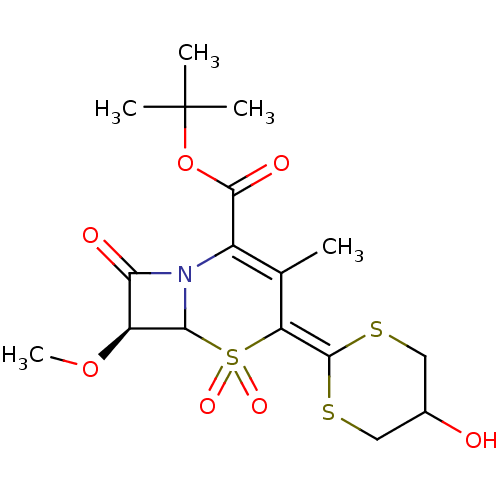 ((S)-4-(5-Hydroxy-[1,3]dithian-2-ylidene)-7-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human sputum elastase (HSE) | Bioorg Med Chem Lett 6: 823-826 (1996) Article DOI: 10.1016/0960-894X(96)00114-X BindingDB Entry DOI: 10.7270/Q2CV4HR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021965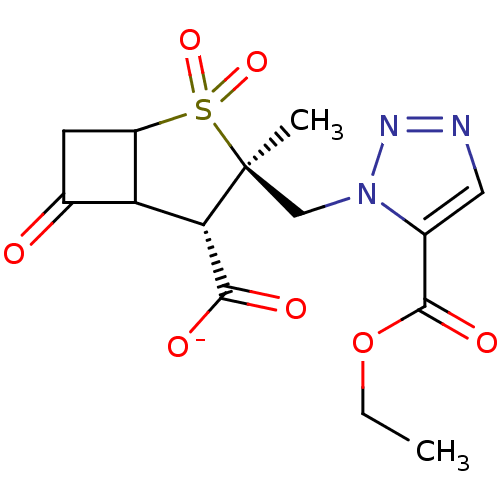 (CHEMBL35418 | Sodium; 3-(5-ethoxycarbonyl-[1,2,3]t...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50289133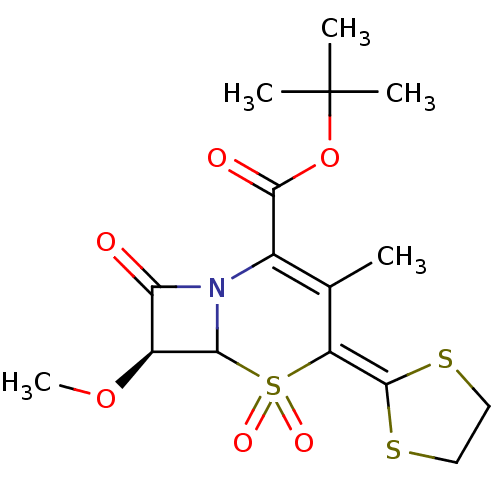 ((S)-4-[1,3]Dithiolan-2-ylidene-7-methoxy-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human sputum elastase (HSE) | Bioorg Med Chem Lett 6: 823-826 (1996) Article DOI: 10.1016/0960-894X(96)00114-X BindingDB Entry DOI: 10.7270/Q2CV4HR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021964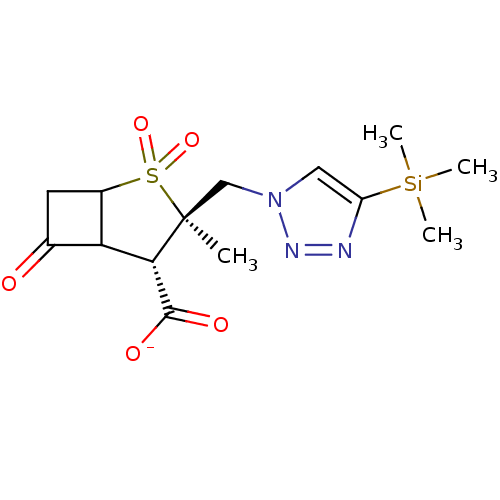 (CHEMBL284828 | Sodium; 3-methyl-2,2,6-trioxo-3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021961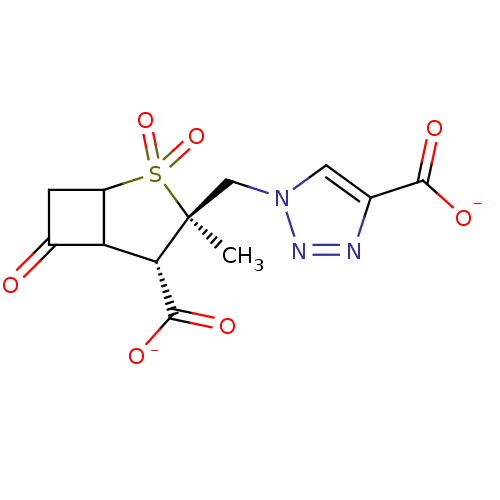 (CHEMBL286829 | dipotassium 1-{[(3R,4S)-4-carboxyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021956 (CHEMBL285387 | Sodium; 3-methyl-2,2,6-trioxo-3-[1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021956 (CHEMBL285387 | Sodium; 3-methyl-2,2,6-trioxo-3-[1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021955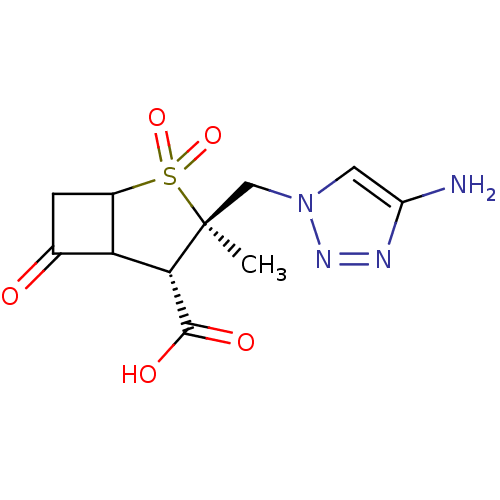 (3-(4-Amino-[1,2,3]triazol-1-ylmethyl)-3-methyl-2,2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021962 (CHEMBL288566 | dipotassium 1-{[(3R,4S)-4-carboxyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from Bacillus sp. using penicillin G as substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015927 ((S)-7-Methoxy-3-methyl-5,5,8-trioxo-5lambda*6*-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human sputum elastase (HSE) | Bioorg Med Chem Lett 6: 823-826 (1996) Article DOI: 10.1016/0960-894X(96)00114-X BindingDB Entry DOI: 10.7270/Q2CV4HR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type OXA1 (penicillinase) from E. coli OXA1 using ampicillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50021956 (CHEMBL285387 | Sodium; 3-methyl-2,2,6-trioxo-3-[1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type OXA1 (penicillinase) from E. coli OXA1 using ampicillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021954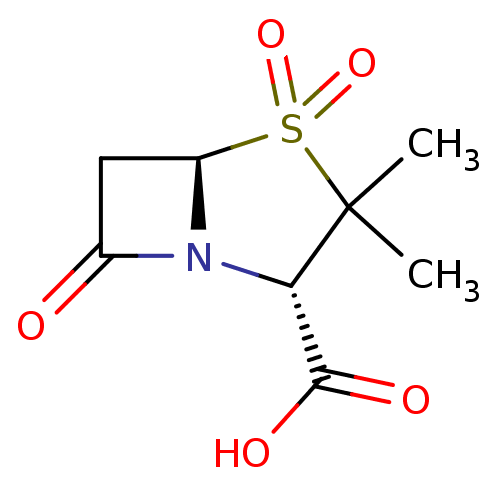 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Penicillinase from Staphylococcus aureus TH-14 using piperacillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type TEM2 (penicillinase) from E. coli TEM2 using penicillin G (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type TEM2 (penicillinase) from E. coli TEM2 using penicillin G (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type OXA1 (penicillinase) from E. coli OXA1 using ampicillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type TEM2 (penicillinase) from E. coli TEM2 using penicillin G (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||