

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM16596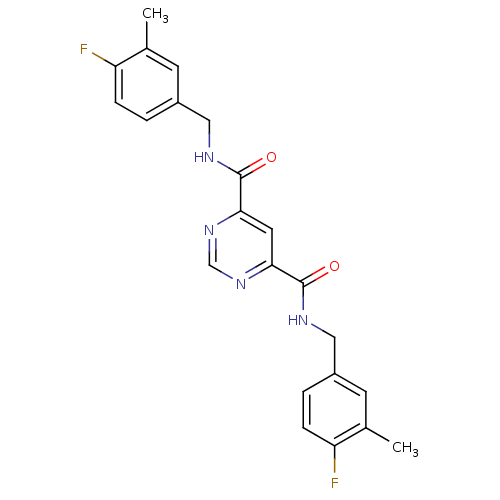 (4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human MMP-13 assessed as bovine type-2 collagen hydrolysis after 18 hrs by ELISA | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM16596 (4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361639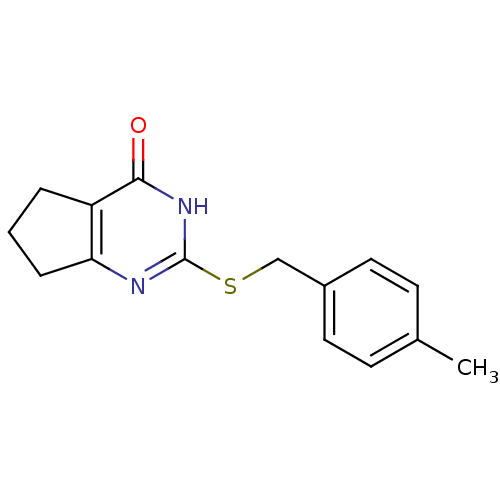 (CHEMBL1371684) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human MMP-13 assessed as bovine type-2 collagen hydrolysis after 18 hrs by ELISA | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314727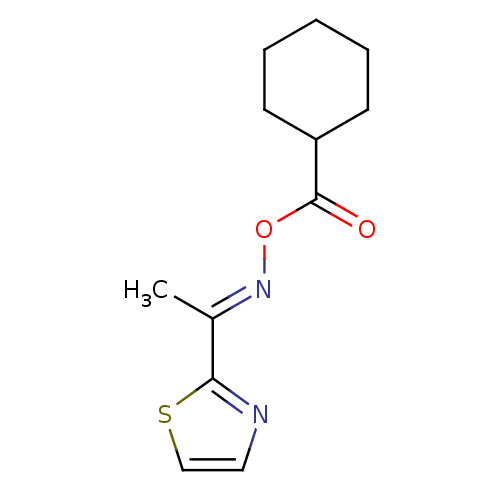 (1-(thiazol-2-yl)ethanone O-cyclohexanecarbonyl oxi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50031314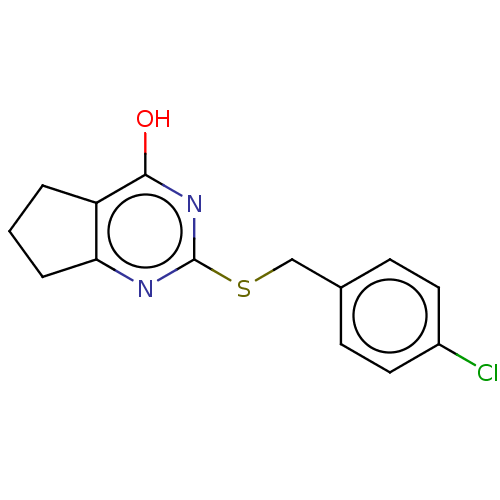 (CHEMBL473326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human MMP-13 assessed as bovine type-2 collagen hydrolysis after 18 hrs by ELISA | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154536 (2-(4-tert-butylphenyl)-1-(4-methylbenzenesulfonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154531 (2-(4-bromophenyl)-6-(4-chlorophenyl)-1-(4-methylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154529 (2-(4-fluorophenyl)-1-(4-methylbenzenesulfonyl)-6-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314725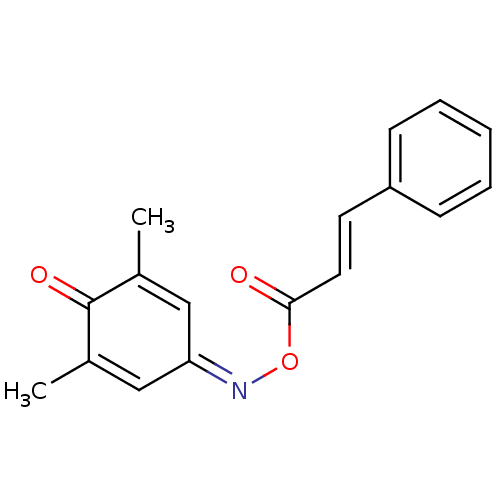 (4-(cinnamoyloxyimino)-2,6-dimethylcyclohexa-2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154534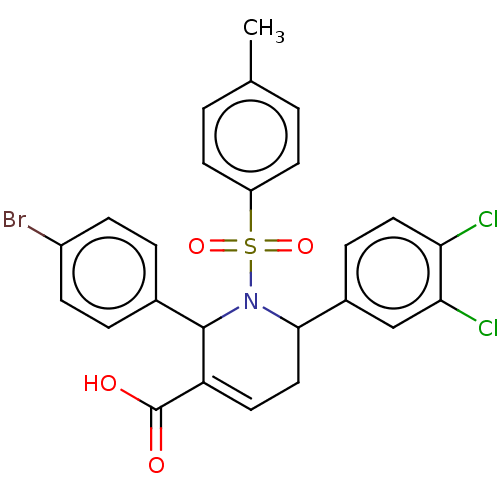 (2-(4-bromophenyl)-6-(3,4-dichlorophenyl)-1-(4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314728 (4-(4-methoxybenzoyloxyimino)-2,6-dimethylcyclohexa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from recombinant RBBP9 transfected in mouse brain membrane proteomes after 30 mins by SDS-PAGE gel fluoresc... | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314728 (4-(4-methoxybenzoyloxyimino)-2,6-dimethylcyclohexa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154540 (2-(4-(tert-butyl)phenyl)-N-(2-methoxyethyl)-6-(p-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314729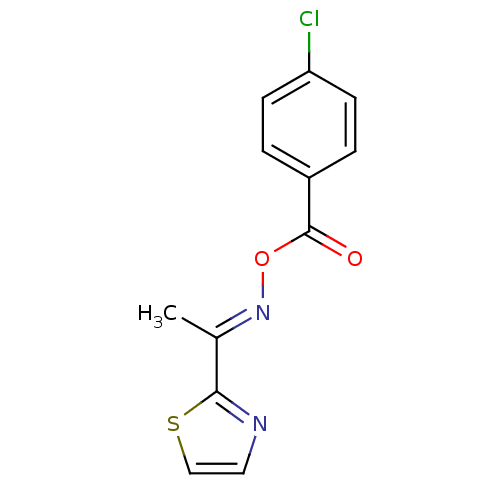 (1-(thiazol-2-yl)ethanone O-4-chlorobenzoyl oxime |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154535 (2-(4-fluorophenyl)-1-(4-methylbenzenesulfonyl)-6-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361640 (CHEMBL1515648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human MMP-13 assessed as bovine type-2 collagen hydrolysis after 18 hrs by ELISA | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361639 (CHEMBL1371684) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) using (S)-mephentoin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) using dextromethophan substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) using amodiaquine substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2B6 (unknown origin) using bupropion substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) using tacrin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50265079 ((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using testosterone substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361640 (CHEMBL1515648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50361639 (CHEMBL1371684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) using tacrin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154530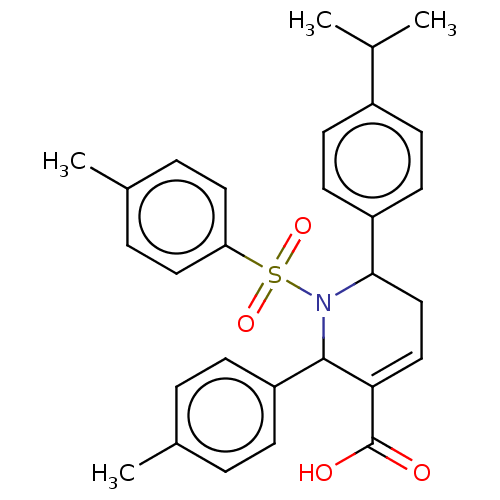 (1-(4-methylbenzenesulfonyl)-2-(4-methylphenyl)-6-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154542 (5-(bromomethyl)-6-(4-(tert-butyl)phenyl)-2-(p-toly...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314726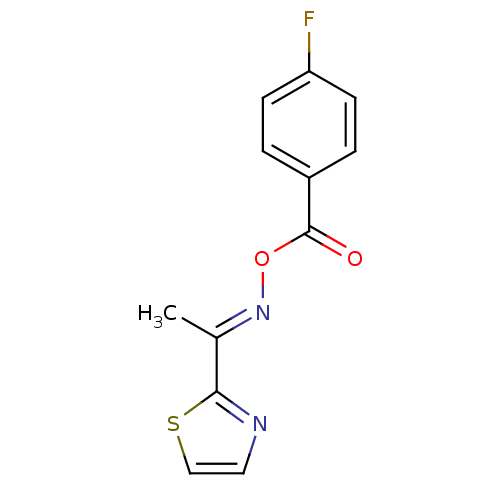 (1-(thiazol-2-yl)ethanone O-4-fluorobenzoyl oxime |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) using (S)-mephentoin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154541 (N-allyl-2-(4-(tert-butyl)phenyl)-6-(p-tolyl)-1-tos...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154543 (N-((2-(4-(tert-butyl)phenyl)-6-(p-tolyl)-1-tosyl-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50216297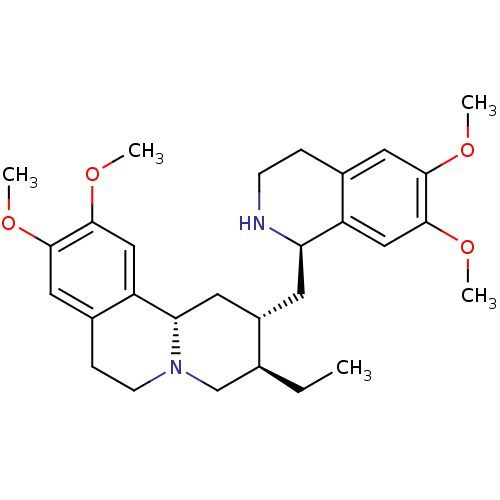 (6',7',10,11-tetramethoxyemetan | CHEMBL50588 | Eme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50361639 (CHEMBL1371684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) using (S)-mephentoin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154533 (2-(3-chlorophenyl)-1-(4-methylbenzenesulfonyl)-6-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) using tacrin substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50361639 (CHEMBL1371684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydrolase RBBP9 (Homo sapiens (Human)) | BDBM50314724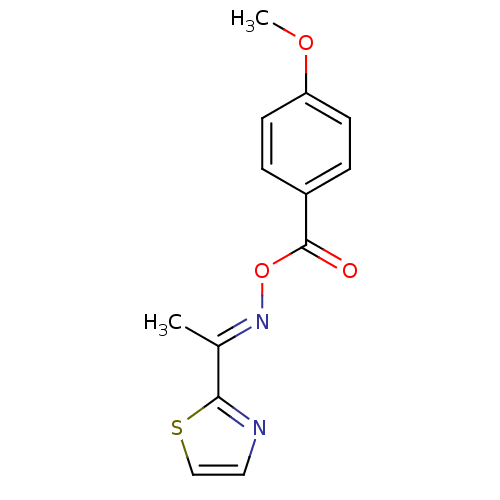 (1-(thiazol-2-yl)ethanone O-4-methoxybenzoyl oxime ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluorophosphate-rhodamine from RBBP9 transfected in human HEK293T cells proteomes after 30 mins by SDS-PAGE gel fluorescence assay | Bioorg Med Chem Lett 20: 2254-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.011 BindingDB Entry DOI: 10.7270/Q27D2V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154538 (2-(4-(tert-butyl)phenyl)-N-cyclopropyl-6-(p-tolyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154532 (2-(3-chlorophenyl)-6-(4-ethylphenyl)-1-(4-methylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154537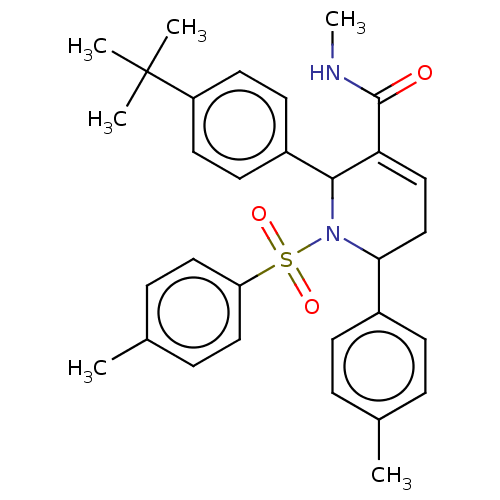 (2-(4-(tert-butyl)phenyl)-N-methyl-6-(p-tolyl)-1-to...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154544 (Methyl-2-(4-(tert-butyl)phenyl)-6-(p-tolyl)-1-tosy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase IB subunit alpha2 (Mus musculus (Mouse)) | BDBM154539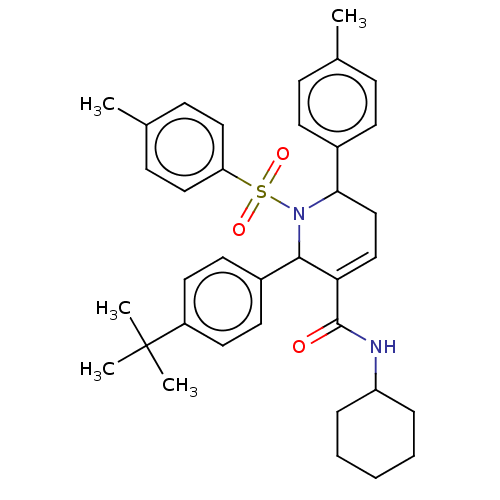 (2-(4-(tert-butyl)phenyl)-N-cyclohexyl-6-(p-tolyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, Berkeley | Assay Description For in vitro experiments, proteomes were diluted to 1 mg/mL in PBS (pH 7.5, 50 μL total reaction volume), doped with 1 μM recombinant PAFAH... | ACS Chem Biol 10: 925-32 (2015) Article DOI: 10.1021/cb500893q BindingDB Entry DOI: 10.7270/Q2CJ8C6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using testosterone substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) using amodiaquine substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50031314 (CHEMBL473326) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2B6 (unknown origin) using bupropion substrate | J Med Chem 57: 9598-611 (2014) Article DOI: 10.1021/jm501284e BindingDB Entry DOI: 10.7270/Q2P55Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 146 total ) | Next | Last >> |