 Found 317 hits with Last Name = 'stassen' and Initial = 'f'
Found 317 hits with Last Name = 'stassen' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226415
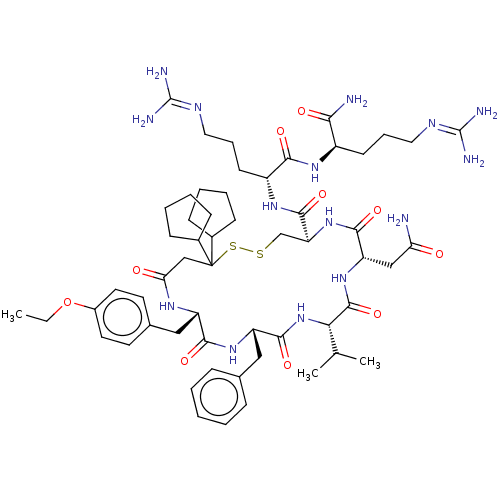
(CHEMBL3142312)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C57H87N15O10S2/c1-4-82-38-24-22-35(23-25-38)29-41-50(77)69-42(28-34-14-6-5-7-15-34)52(79)72-47(33(2)3)54(81)70-43(30-45(58)73)51(78)71-44(32-83-84-57(31-46(74)66-41,36-16-8-9-17-36)37-18-10-11-19-37)53(80)68-40(21-13-27-65-56(62)63)49(76)67-39(48(59)75)20-12-26-64-55(60)61/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,73)(H2,59,75)(H,66,74)(H,67,76)(H,68,80)(H,69,77)(H,70,81)(H,71,78)(H,72,79)(H4,60,61,64)(H4,62,63,65)/t39-,40-,41+,42+,43+,44+,47+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226412

(CHEMBL3142332)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C57H87N15O10S2/c1-4-82-38-24-22-35(23-25-38)29-41-50(77)69-42(28-34-14-6-5-7-15-34)52(79)72-47(33(2)3)54(81)70-43(30-45(58)73)51(78)71-44(32-83-84-57(31-46(74)66-41,36-16-8-9-17-36)37-18-10-11-19-37)53(80)68-40(21-13-27-65-56(62)63)49(76)67-39(48(59)75)20-12-26-64-55(60)61/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,73)(H2,59,75)(H,66,74)(H,67,76)(H,68,80)(H,69,77)(H,70,81)(H,71,78)(H,72,79)(H4,60,61,64)(H4,62,63,65)/t39-,40+,41+,42+,43+,44+,47+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226410
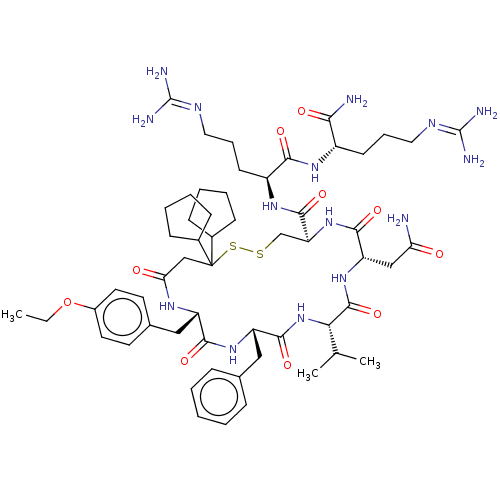
(CHEMBL3142318)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C57H87N15O10S2/c1-4-82-38-24-22-35(23-25-38)29-41-50(77)69-42(28-34-14-6-5-7-15-34)52(79)72-47(33(2)3)54(81)70-43(30-45(58)73)51(78)71-44(32-83-84-57(31-46(74)66-41,36-16-8-9-17-36)37-18-10-11-19-37)53(80)68-40(21-13-27-65-56(62)63)49(76)67-39(48(59)75)20-12-26-64-55(60)61/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,73)(H2,59,75)(H,66,74)(H,67,76)(H,68,80)(H,69,77)(H,70,81)(H,71,78)(H,72,79)(H4,60,61,64)(H4,62,63,65)/t39-,40-,41-,42-,43-,44-,47-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226411
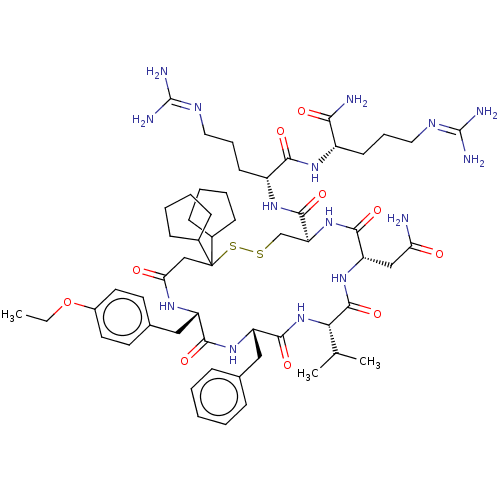
(CHEMBL3142329)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C57H87N15O10S2/c1-4-82-38-24-22-35(23-25-38)29-41-50(77)69-42(28-34-14-6-5-7-15-34)52(79)72-47(33(2)3)54(81)70-43(30-45(58)73)51(78)71-44(32-83-84-57(31-46(74)66-41,36-16-8-9-17-36)37-18-10-11-19-37)53(80)68-40(21-13-27-65-56(62)63)49(76)67-39(48(59)75)20-12-26-64-55(60)61/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,73)(H2,59,75)(H,66,74)(H,67,76)(H,68,80)(H,69,77)(H,70,81)(H,71,78)(H,72,79)(H4,60,61,64)(H4,62,63,65)/t39-,40+,41-,42-,43-,44-,47-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226417
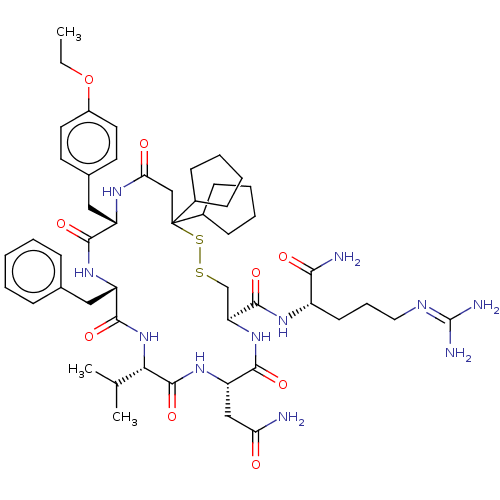
(CHEMBL3142331)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C51H75N11O9S2/c1-4-71-35-22-20-32(21-23-35)26-37-45(66)59-38(25-31-13-6-5-7-14-31)47(68)62-43(30(2)3)49(70)60-39(27-41(52)63)46(67)61-40(48(69)58-36(44(53)65)19-12-24-56-50(54)55)29-72-73-51(28-42(64)57-37,33-15-8-9-16-33)34-17-10-11-18-34/h5-7,13-14,20-23,30,33-34,36-40,43H,4,8-12,15-19,24-29H2,1-3H3,(H2,52,63)(H2,53,65)(H,57,64)(H,58,69)(H,59,66)(H,60,70)(H,61,67)(H,62,68)(H4,54,55,56)/t36-,37-,38-,39-,40-,43-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226413

(CHEMBL2369777)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C57H87N13O10S2/c1-4-80-39-25-23-36(24-26-39)30-42-51(75)67-43(29-35-15-6-5-7-16-35)53(77)70-48(34(2)3)55(79)68-44(31-46(59)71)52(76)69-45(33-81-82-57(32-47(72)64-42,37-17-8-9-18-37)38-19-10-11-20-38)54(78)66-41(22-14-28-63-56(61)62)50(74)65-40(49(60)73)21-12-13-27-58/h5-7,15-16,23-26,34,37-38,40-45,48H,4,8-14,17-22,27-33,58H2,1-3H3,(H2,59,71)(H2,60,73)(H,64,72)(H,65,74)(H,66,78)(H,67,75)(H,68,79)(H,69,76)(H,70,77)(H4,61,62,63)/t40-,41-,42+,43-,44-,45-,48-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226416
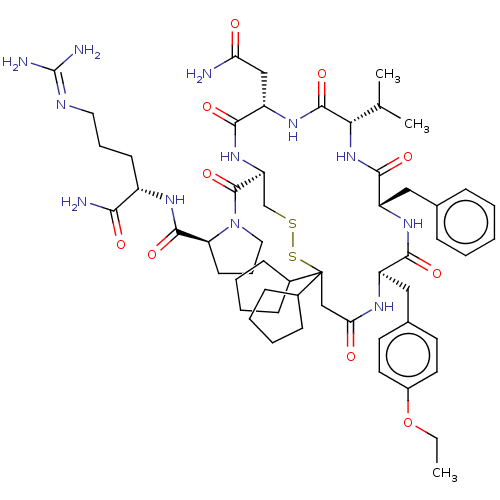
(CHEMBL2369525)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C56H82N12O10S2/c1-4-78-38-24-22-35(23-25-38)29-40-49(72)64-41(28-34-14-6-5-7-15-34)51(74)67-47(33(2)3)53(76)65-42(30-45(57)69)50(73)66-43(32-79-80-56(31-46(70)62-40,36-16-8-9-17-36)37-18-10-11-19-37)54(77)68-27-13-21-44(68)52(75)63-39(48(58)71)20-12-26-61-55(59)60/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,57,69)(H2,58,71)(H,62,70)(H,63,75)(H,64,72)(H,65,76)(H,66,73)(H,67,74)(H4,59,60,61)/t39-,40+,41-,42-,43-,44-,47-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020654
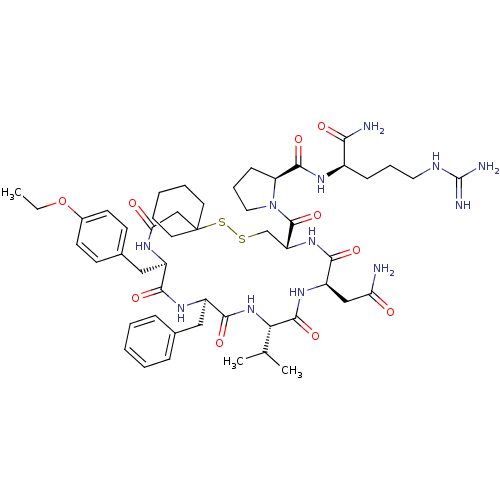
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35-,36-,37-,38-,39+,42+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226414
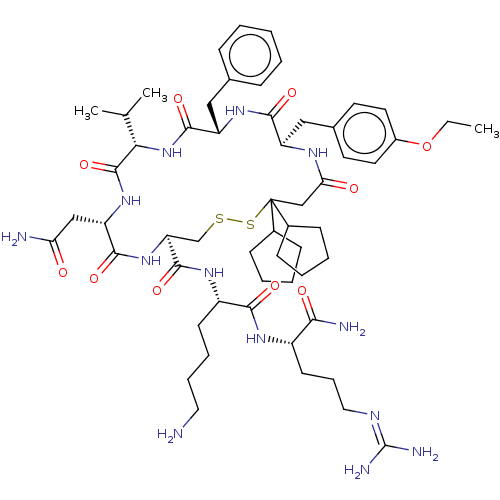
(CHEMBL2369778)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C57H87N13O10S2/c1-4-80-39-25-23-36(24-26-39)30-42-51(75)67-43(29-35-15-6-5-7-16-35)53(77)70-48(34(2)3)55(79)68-44(31-46(59)71)52(76)69-45(33-81-82-57(32-47(72)64-42,37-17-8-9-18-37)38-19-10-11-20-38)54(78)66-41(21-12-13-27-58)50(74)65-40(49(60)73)22-14-28-63-56(61)62/h5-7,15-16,23-26,34,37-38,40-45,48H,4,8-14,17-22,27-33,58H2,1-3H3,(H2,59,71)(H2,60,73)(H,64,72)(H,65,74)(H,66,78)(H,67,75)(H,68,79)(H,69,76)(H,70,77)(H4,61,62,63)/t40-,41-,42+,43-,44-,45-,48-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020675

(1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC(CC)(CC)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C52H77N13O11S2/c1-6-52(7-2)27-42(68)59-35(25-32-18-20-33(21-19-32)76-8-3)45(70)61-36(24-31-14-10-9-11-15-31)47(72)64-43(30(4)5)49(74)62-37(26-40(53)66)46(71)63-38(29-77-78-52)50(75)65-23-13-17-39(65)48(73)60-34(16-12-22-57-51(55)56)44(69)58-28-41(54)67/h9-11,14-15,18-21,30,34-39,43H,6-8,12-13,16-17,22-29H2,1-5H3,(H2,53,66)(H2,54,67)(H,58,69)(H,59,68)(H,60,73)(H,61,70)(H,62,74)(H,63,71)(H,64,72)(H4,55,56,57)/t34-,35-,36-,37-,38-,39+,43+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020653
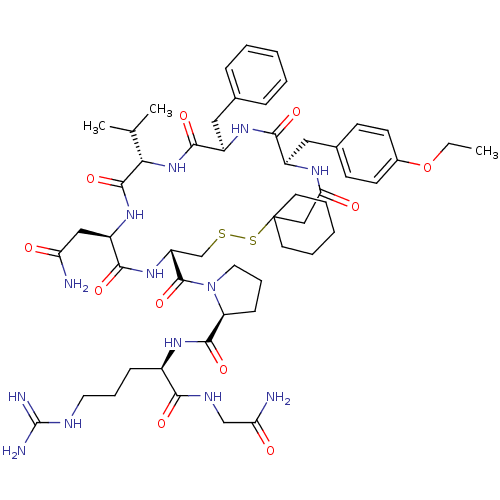
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C53H77N13O11S2/c1-4-77-34-19-17-33(18-20-34)26-36-46(71)62-37(25-32-13-7-5-8-14-32)48(73)65-44(31(2)3)50(75)63-38(27-41(54)67)47(72)64-39(30-78-79-53(28-43(69)60-36)21-9-6-10-22-53)51(76)66-24-12-16-40(66)49(74)61-35(15-11-23-58-52(56)57)45(70)59-29-42(55)68/h5,7-8,13-14,17-20,31,35-40,44H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,67)(H2,55,68)(H,59,70)(H,60,69)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,73)(H4,56,57,58)/t35-,36-,37-,38-,39-,40+,44+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226418

(CHEMBL3142313)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C57H86N14O11S2/c1-4-82-38-24-22-35(23-25-38)29-41-49(75)68-42(28-34-14-6-5-7-15-34)51(77)71-47(33(2)3)53(79)69-43(30-45(58)72)50(76)70-44(32-83-84-57(31-46(73)65-41,36-16-8-9-17-36)37-18-10-11-19-37)52(78)66-39(20-12-26-63-55(59)60)48(74)67-40(54(80)81)21-13-27-64-56(61)62/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,72)(H,65,73)(H,66,78)(H,67,74)(H,68,75)(H,69,79)(H,70,76)(H,71,77)(H,80,81)(H4,59,60,63)(H4,61,62,64)/t39-,40-,41-,42-,43-,44-,47-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020674
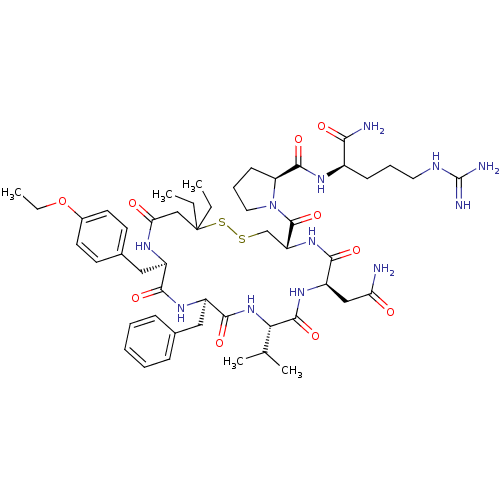
(1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC(CC)(CC)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C50H74N12O10S2/c1-6-50(7-2)27-40(64)56-34(25-31-18-20-32(21-19-31)72-8-3)43(66)58-35(24-30-14-10-9-11-15-30)45(68)61-41(29(4)5)47(70)59-36(26-39(51)63)44(67)60-37(28-73-74-50)48(71)62-23-13-17-38(62)46(69)57-33(42(52)65)16-12-22-55-49(53)54/h9-11,14-15,18-21,29,33-38,41H,6-8,12-13,16-17,22-28H2,1-5H3,(H2,51,63)(H2,52,65)(H,56,64)(H,57,69)(H,58,66)(H,59,70)(H,60,67)(H,61,68)(H4,53,54,55)/t33-,34-,35-,36-,37-,38+,41+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020701
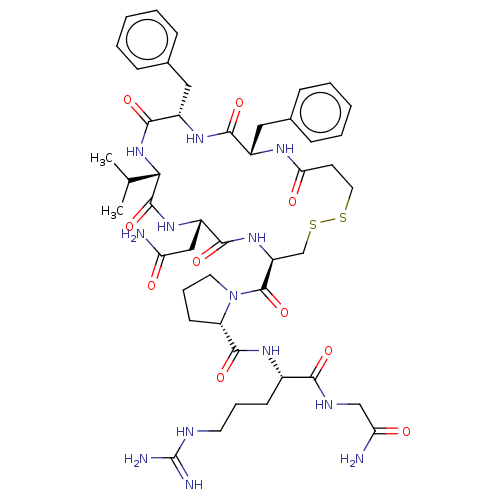
(1-(13,16-Dibenzyl-7-carbamoylmethyl-10-isopropyl-6...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N13O10S2/c1-26(2)38-44(68)56-32(23-35(47)60)41(65)57-33(45(69)59-19-10-16-34(59)43(67)54-29(15-9-18-51-46(49)50)39(63)52-24-36(48)61)25-71-70-20-17-37(62)53-30(21-27-11-5-3-6-12-27)40(64)55-31(42(66)58-38)22-28-13-7-4-8-14-28/h3-8,11-14,26,29-34,38H,9-10,15-25H2,1-2H3,(H2,47,60)(H2,48,61)(H,52,63)(H,53,62)(H,54,67)(H,55,64)(H,56,68)(H,57,65)(H,58,66)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation |
J Med Chem 31: 742-4 (1988)
BindingDB Entry DOI: 10.7270/Q2VD6XFS |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020698
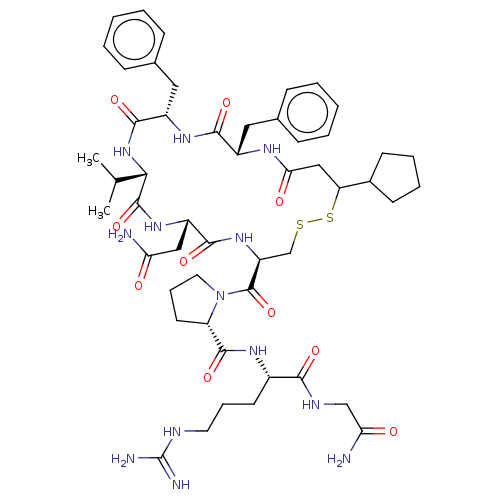
(1-(13,16-Dibenzyl-7-carbamoylmethyl-20-cyclopentyl...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)CC(SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O)C1CCCC1 Show InChI InChI=1S/C51H73N13O10S2/c1-29(2)43-49(73)61-36(25-40(52)65)46(70)62-37(50(74)64-22-12-20-38(64)48(72)59-33(19-11-21-56-51(54)55)44(68)57-27-41(53)66)28-75-76-39(32-17-9-10-18-32)26-42(67)58-34(23-30-13-5-3-6-14-30)45(69)60-35(47(71)63-43)24-31-15-7-4-8-16-31/h3-8,13-16,29,32-39,43H,9-12,17-28H2,1-2H3,(H2,52,65)(H2,53,66)(H,57,68)(H,58,67)(H,59,72)(H,60,69)(H,61,73)(H,62,70)(H,63,71)(H4,54,55,56)/t33-,34-,35-,36-,37-,38-,39?,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation |
J Med Chem 31: 742-4 (1988)
BindingDB Entry DOI: 10.7270/Q2VD6XFS |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020700
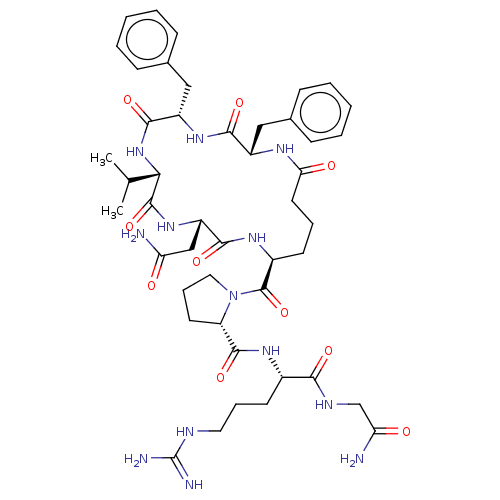
(1-(2,5-Dibenzyl-11-carbamoylmethyl-8-isopropyl-3,6...)Show SMILES [H][C@@]([#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])([#7]-[#6](=O)[C@]1([H])[#6]-[#6]-[#6]-[#7]1-[#6](=O)[C@]1([H])[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7][C@@]([H])([#6]-[#6](-[#7])=O)[#6](=O)-[#7]1)[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O10/c1-26(2)38-44(68)57-33(24-35(47)60)41(65)55-30(45(69)59-21-11-18-34(59)43(67)54-29(17-10-20-51-46(49)50)39(63)52-25-36(48)61)16-9-19-37(62)53-31(22-27-12-5-3-6-13-27)40(64)56-32(42(66)58-38)23-28-14-7-4-8-15-28/h3-8,12-15,26,29-34,38H,9-11,16-25H2,1-2H3,(H2,47,60)(H2,48,61)(H,52,63)(H,53,62)(H,54,67)(H,55,65)(H,56,64)(H,57,68)(H,58,66)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation |
J Med Chem 31: 742-4 (1988)
BindingDB Entry DOI: 10.7270/Q2VD6XFS |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020699
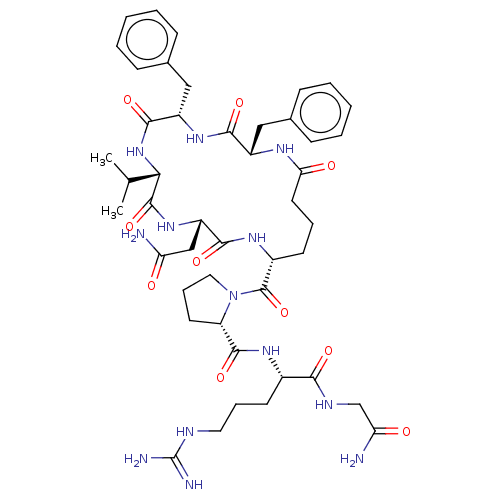
(1-(2,5-Dibenzyl-11-carbamoylmethyl-8-isopropyl-3,6...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCC[C@@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N13O10/c1-26(2)38-44(68)57-33(24-35(47)60)41(65)55-30(45(69)59-21-11-18-34(59)43(67)54-29(17-10-20-51-46(49)50)39(63)52-25-36(48)61)16-9-19-37(62)53-31(22-27-12-5-3-6-13-27)40(64)56-32(42(66)58-38)23-28-14-7-4-8-15-28/h3-8,12-15,26,29-34,38H,9-11,16-25H2,1-2H3,(H2,47,60)(H2,48,61)(H,52,63)(H,53,62)(H,54,67)(H,55,65)(H,56,64)(H,57,68)(H,58,66)(H4,49,50,51)/t29-,30+,31-,32-,33-,34-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation |
J Med Chem 31: 742-4 (1988)
BindingDB Entry DOI: 10.7270/Q2VD6XFS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12972

((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C32H41BrN8O5S/c1-18(2)26(29(45)39-24(5-4-14-37-31(34)35)27(43)30-36-15-16-47-30)41-28(44)25(17-20-6-12-23(42)13-7-20)40-32(46)38-19(3)21-8-10-22(33)11-9-21/h6-13,15-16,18-19,24-26,42H,4-5,14,17H2,1-3H3,(H,39,45)(H,41,44)(H4,34,35,37)(H2,38,40,46)/t19-,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12973

((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C29H44BrN11O4S/c1-16(2)22(25(44)39-20(6-4-12-36-27(31)32)23(42)26-35-14-15-46-26)41-24(43)21(7-5-13-37-28(33)34)40-29(45)38-17(3)18-8-10-19(30)11-9-18/h8-11,14-17,20-22H,4-7,12-13H2,1-3H3,(H,39,44)(H,41,43)(H4,31,32,36)(H4,33,34,37)(H2,38,40,45)/t17-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12974
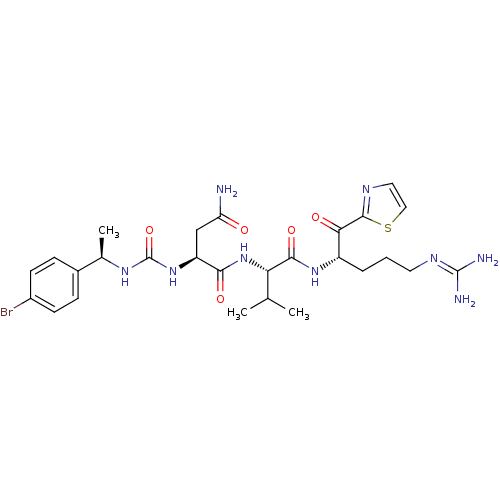
((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#7]-[#6@H](-[#6])-c1ccc(Br)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nccs1 |r| Show InChI InChI=1S/C27H38BrN9O5S/c1-14(2)21(24(41)35-18(5-4-10-33-26(30)31)22(39)25-32-11-12-43-25)37-23(40)19(13-20(29)38)36-27(42)34-15(3)16-6-8-17(28)9-7-16/h6-9,11-12,14-15,18-19,21H,4-5,10,13H2,1-3H3,(H2,29,38)(H,35,41)(H,37,40)(H4,30,31,33)(H2,34,36,42)/t15-,18+,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED)
| Assay Description
Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... |
J Med Chem 49: 7781-91 (2006)
Article DOI: 10.1021/jm060978s
BindingDB Entry DOI: 10.7270/Q2SJ1HTM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607802

(US11691962, Compound 1-200)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1ccc(F)c(C)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607803
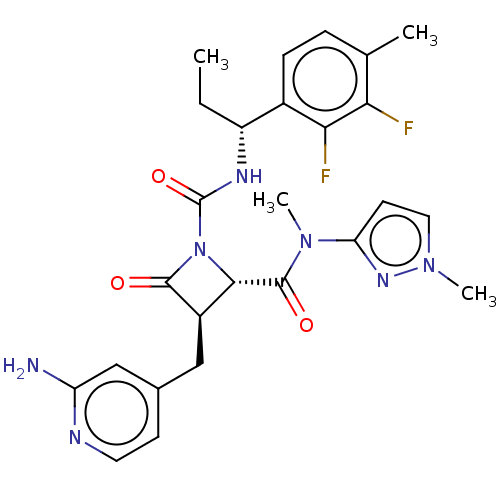
(US11691962, Compound 1-201)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccn(C)n1)c1ccc(C)c(F)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607804
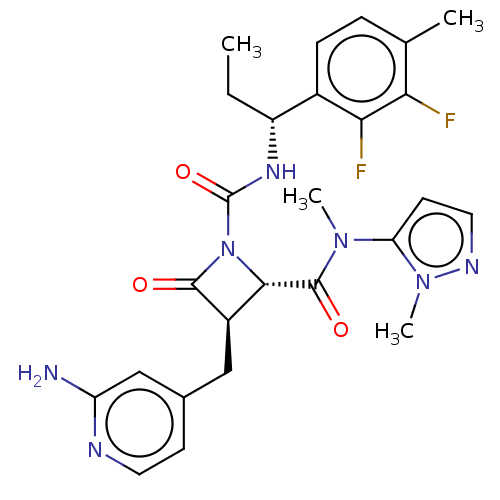
(US11691962, Compound 1-202)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1ccc(C)c(F)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607805
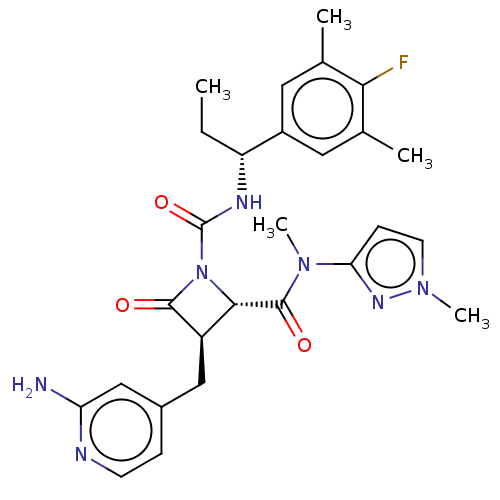
(US11691962, Compound 1-203)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccn(C)n1)c1cc(C)c(F)c(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607806

(US11691962, Compound 1-204)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1cc(F)c(F)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607807
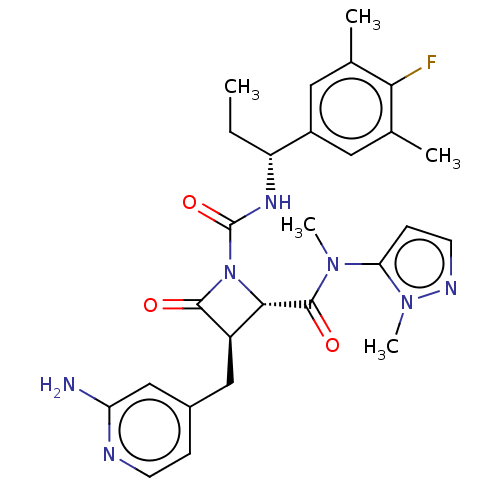
(US11691962, Compound 1-205)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1cc(C)c(F)c(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607808

(US11691962, Compound 1-206)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1cnn(C)c1)c1cc(C)c(F)c(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607809
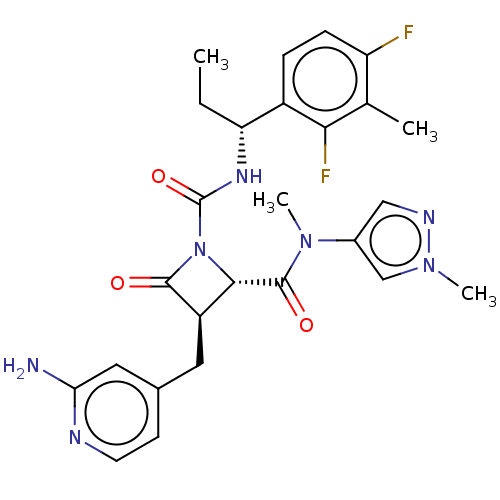
(US11691962, Compound 1-207)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1cnn(C)c1)c1ccc(F)c(C)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607810
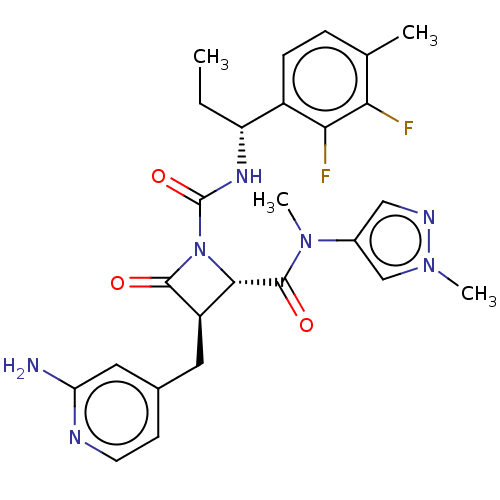
(US11691962, Compound 1-208)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1cnn(C)c1)c1ccc(C)c(F)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607811

(US11691962, Compound 1-209)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(C)c(C)c1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607812
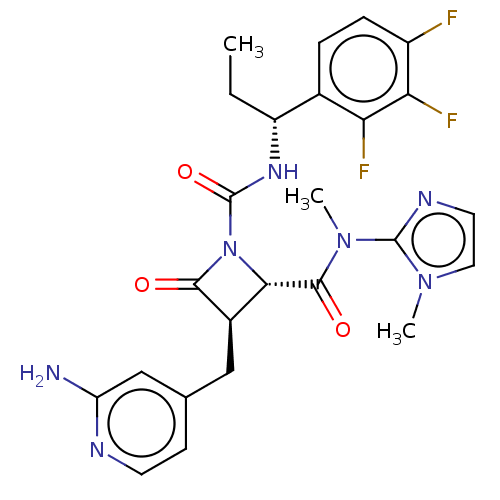
(US11691962, Compound 1-210)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(F)c(F)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607813

(US11691962, Compound 1-211)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(F)c(C)c1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607814

(US11691962, Compound 1-212)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(F)c(F)c1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607815

(US11691962, Compound 1-213)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1cccc(F)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607816

(US11691962, Compound 1-214)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1ccc(F)c(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607817

(US11691962, Compound 1-215)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1ccc(F)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607818

(US11691962, Compound 1-216)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1ccc(C)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607819
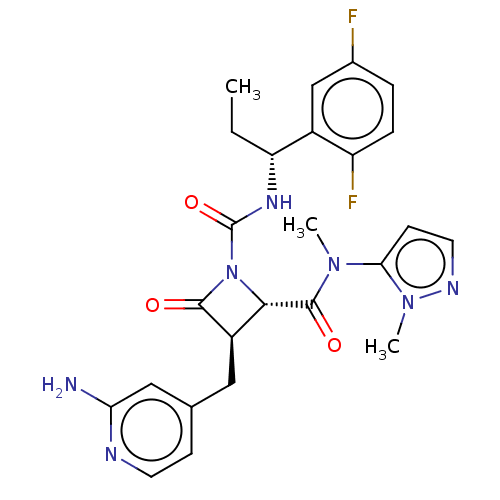
(US11691962, Compound 1-217)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1cc(F)ccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607820

(US11691962, Compound 1-218)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(C)c(C)c1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607821

(US11691962, Compound 1-219)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccnn1C)c1cc(F)ccc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607716
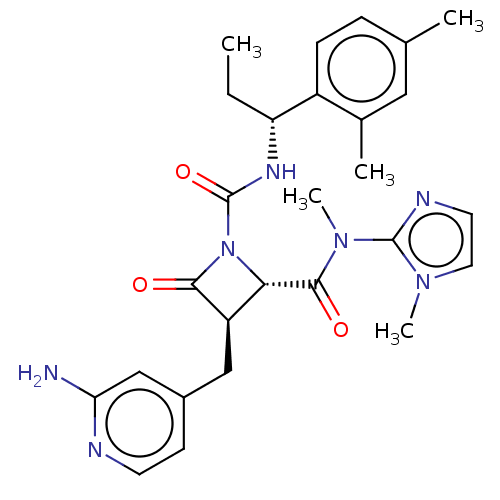
(US11691962, Compound 1-114)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(C)cc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607717

(US11691962, Compound 1-115)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(F)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607718
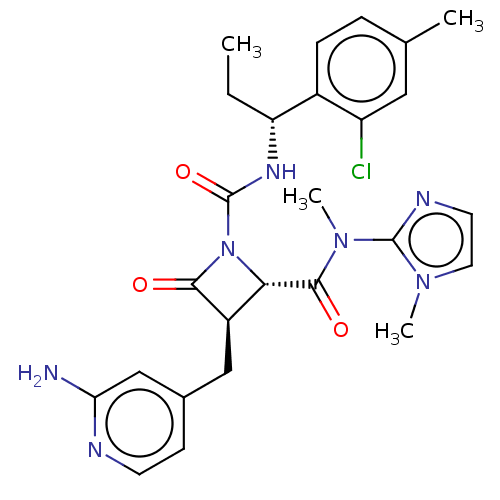
(US11691962, Compound 1-116)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(C)cc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607719

(US11691962, Compound 1-117)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(F)c(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607720
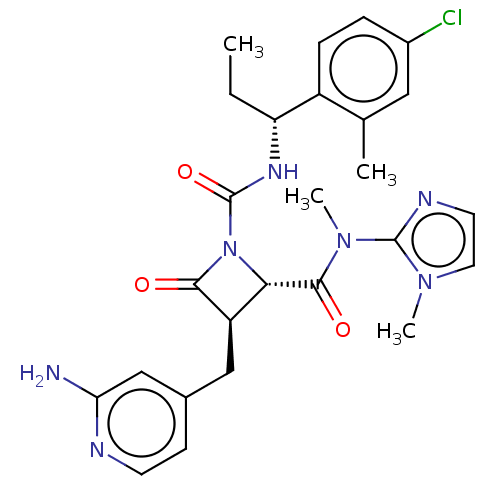
(US11691962, Compound 1-118)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(Cl)cc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607721
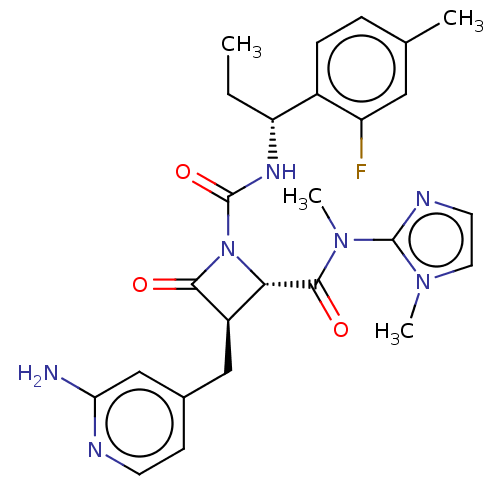
(US11691962, Compound 1-119)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1ccc(C)cc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607722
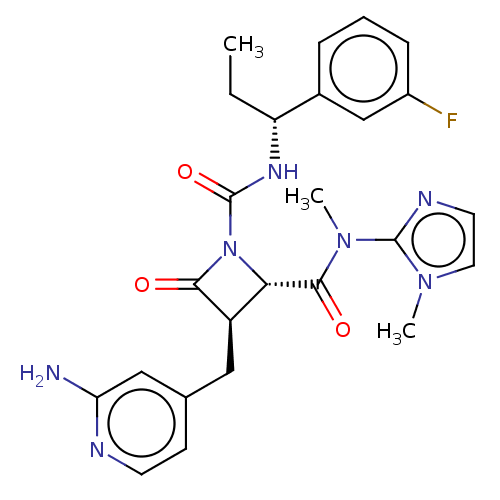
(US11691962, Compound 1-120)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1nccn1C)c1cccc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607725

(US11691962, Compound 1-123)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccn(C)n1)c1cc(F)ccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607726
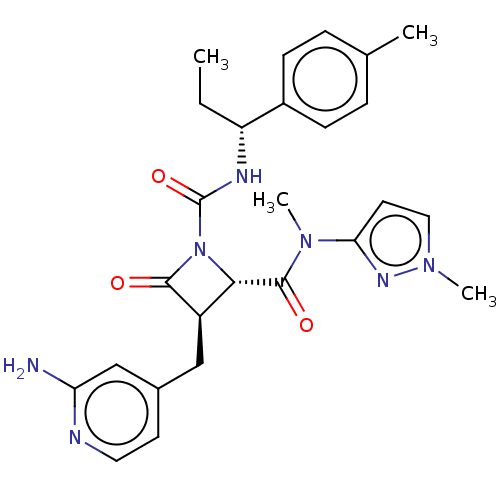
(US11691962, Compound 1-124)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccn(C)n1)c1ccc(C)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM607727

(US11691962, Compound 1-125)Show SMILES CC[C@@H](NC(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(=O)N(C)c1ccn(C)n1)c1cc(C)ccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23R0XZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data