

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153690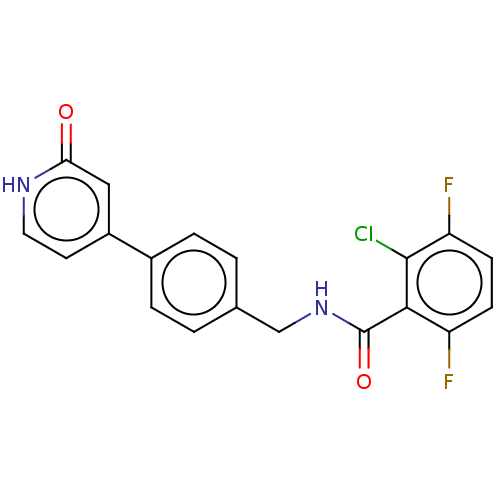 (US10507215, Compound 7 | US9000015, 7 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153687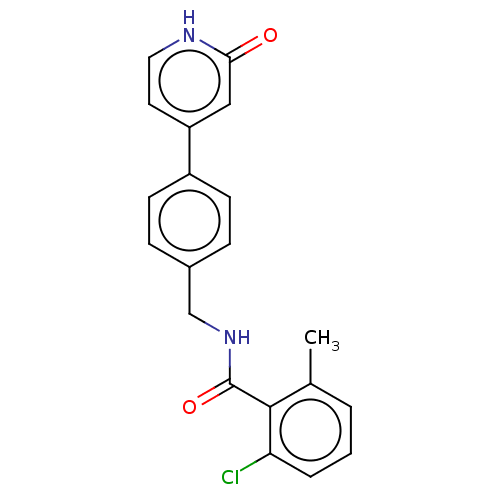 (US10507215, Compound 4 | US9000015, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153687 (US10507215, Compound 4 | US9000015, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153696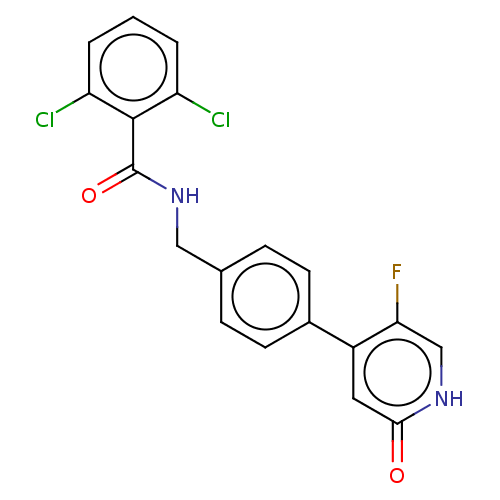 (US10507215, Compound 13 | US9000015, 13 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153696 (US10507215, Compound 13 | US9000015, 13 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153696 (US10507215, Compound 13 | US9000015, 13 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153684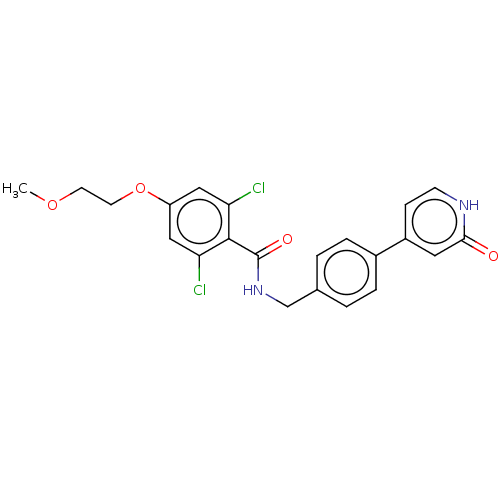 (US10507215, Compound 1 | US9000015, 1 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153684 (US10507215, Compound 1 | US9000015, 1 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153684 (US10507215, Compound 1 | US9000015, 1 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153685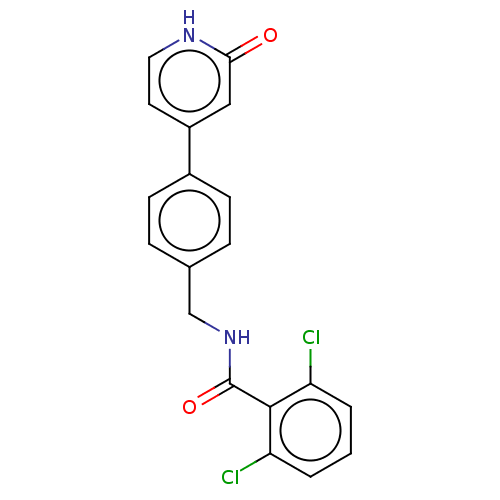 (US10507215, Compound 2 | US9000015, 2 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153685 (US10507215, Compound 2 | US9000015, 2 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153685 (US10507215, Compound 2 | US9000015, 2 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153688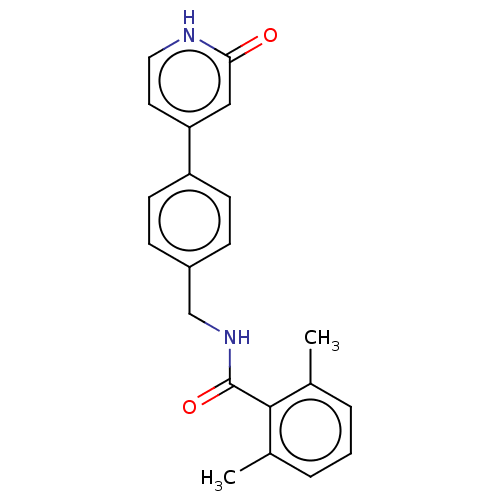 (US10507215, Compound 5 | US9000015, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153697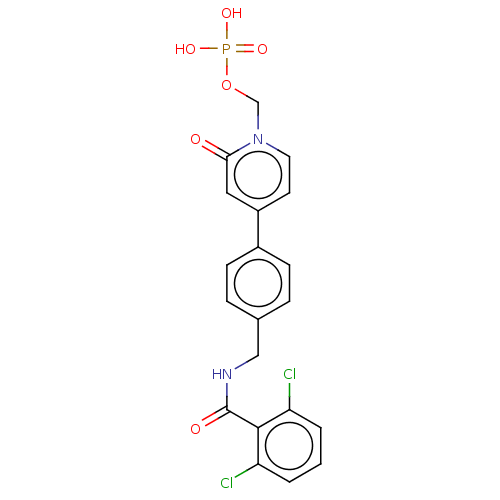 (US10507215, Compound 14 | US9000015, 14 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153688 (US10507215, Compound 5 | US9000015, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153686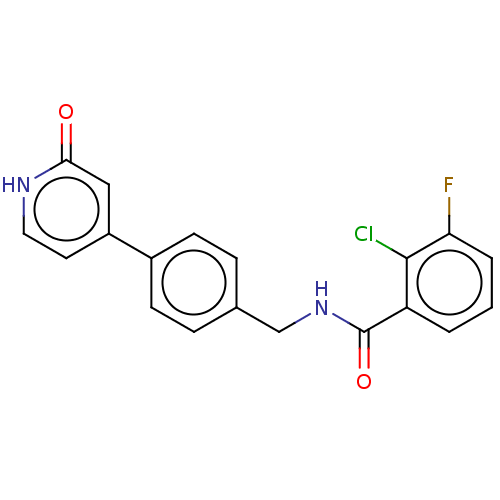 (US10507215, Compound 3 | US9000015, 3 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153686 (US10507215, Compound 3 | US9000015, 3 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 215 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153686 (US10507215, Compound 3 | US9000015, 3 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153695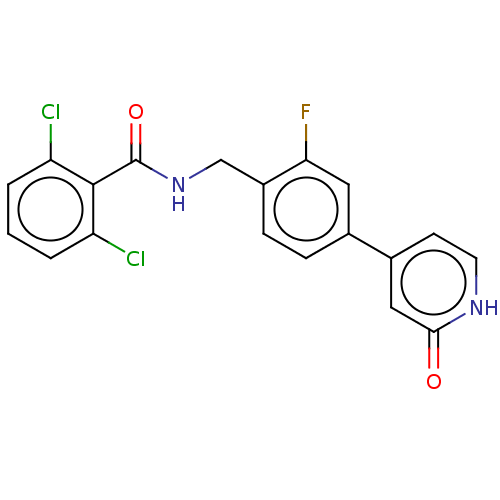 (US10507215, Compound 12 | US9000015, 12 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153695 (US10507215, Compound 12 | US9000015, 12 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153695 (US10507215, Compound 12 | US9000015, 12 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153694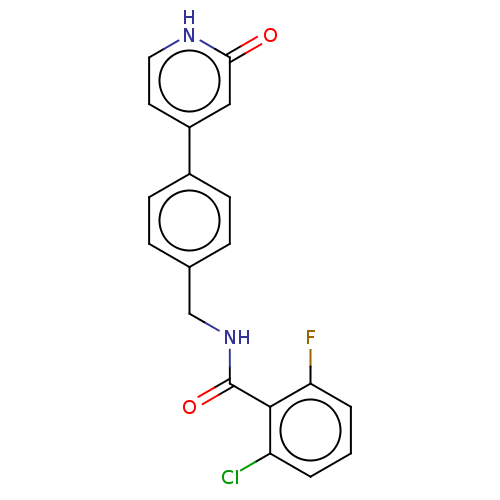 (US10507215, Compound 11 | US9000015, 11 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 379 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153694 (US10507215, Compound 11 | US9000015, 11 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153694 (US10507215, Compound 11 | US9000015, 11 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447466 (CHEMBL3115191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153690 (US10507215, Compound 7 | US9000015, 7 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153690 (US10507215, Compound 7 | US9000015, 7 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 464 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153690 (US10507215, Compound 7 | US9000015, 7 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153691 (US10507215, Compound 8 | US9000015, 8 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153691 (US10507215, Compound 8 | US9000015, 8 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153691 (US10507215, Compound 8 | US9000015, 8 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447469 (CHEMBL3115185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447467 (CHEMBL3115188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447472 (CHEMBL3115177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153693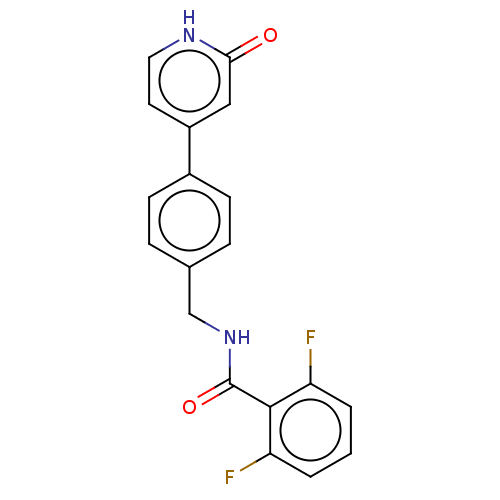 (US10507215, Compound 10 | US9000015, 10 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153693 (US10507215, Compound 10 | US9000015, 10 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153693 (US10507215, Compound 10 | US9000015, 10 | US961029...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM314091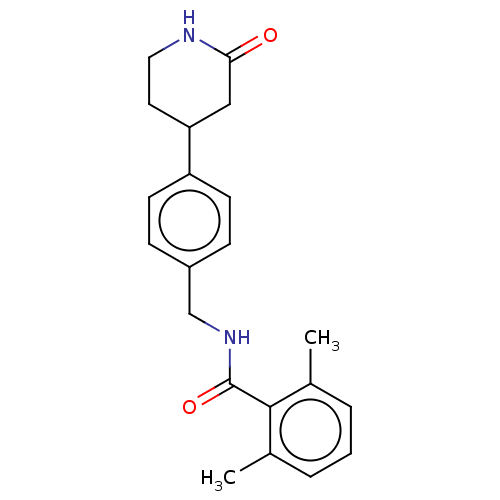 (2,6-dimethyl-N-(4-(2-oxopiperidin-4-yl)benzyl)benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153689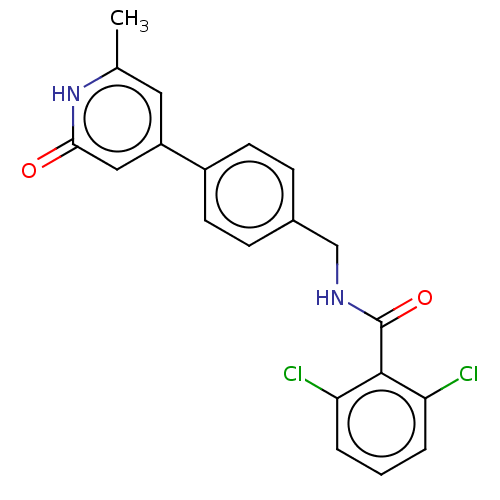 (US10507215, Compound 6 | US9000015, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153689 (US10507215, Compound 6 | US9000015, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50447470 (CHEMBL3115180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447468 (CHEMBL3115187) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153692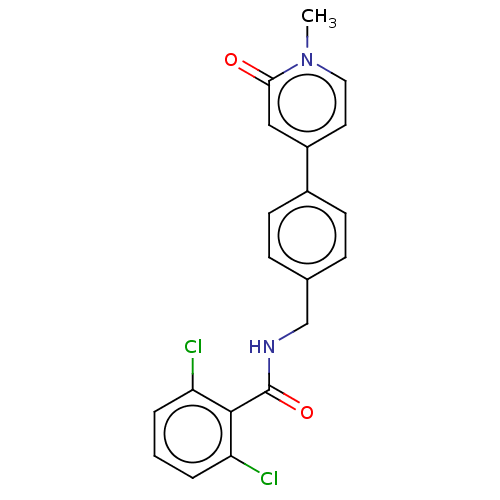 (US10507215, Compound 9 | US9000015, 9 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9610299 (2017) BindingDB Entry DOI: 10.7270/Q2FQ9ZPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153692 (US10507215, Compound 9 | US9000015, 9 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US9000015 (2015) BindingDB Entry DOI: 10.7270/Q28S4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM153692 (US10507215, Compound 9 | US9000015, 9 | US9610299,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amygdala Neurosciences, Inc. US Patent | Assay Description Standard ALDH2 reaction mixtures contained 150 uM formaldehyde, 2.5 mM NAD+, 10 mM MgCl2 and 10 nM recombinant human ALDH2 in 50 mM Hepes buffer, pH ... | US Patent US10507215 (2019) BindingDB Entry DOI: 10.7270/Q2FF3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50447466 (CHEMBL3115191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50447469 (CHEMBL3115185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50447470 (CHEMBL3115180) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50447469 (CHEMBL3115185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50447466 (CHEMBL3115191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | Bioorg Med Chem Lett 24: 995-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.057 BindingDB Entry DOI: 10.7270/Q2P270M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |