

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668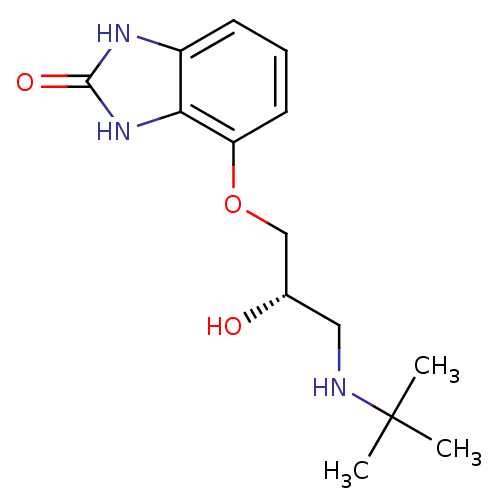 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50070156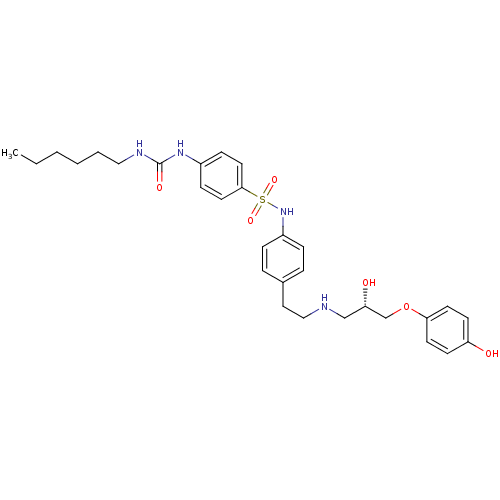 ((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098662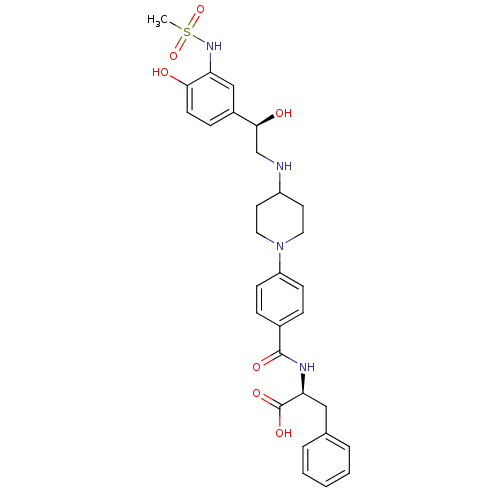 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50070156 ((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098659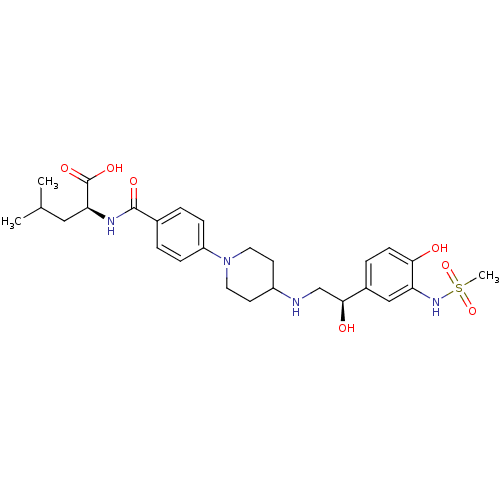 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098661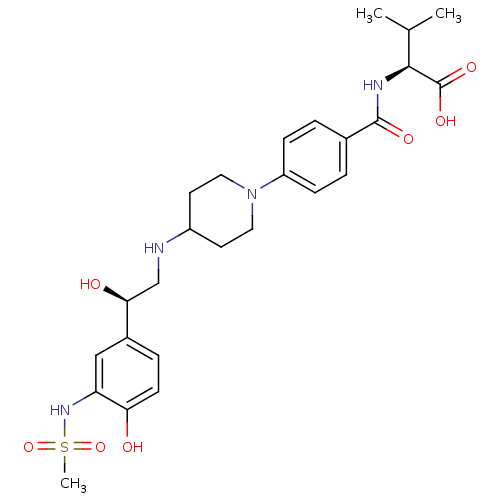 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098654 ((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134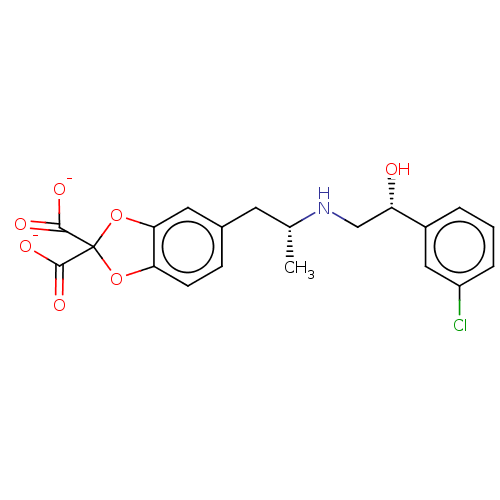 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098654 ((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098661 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098658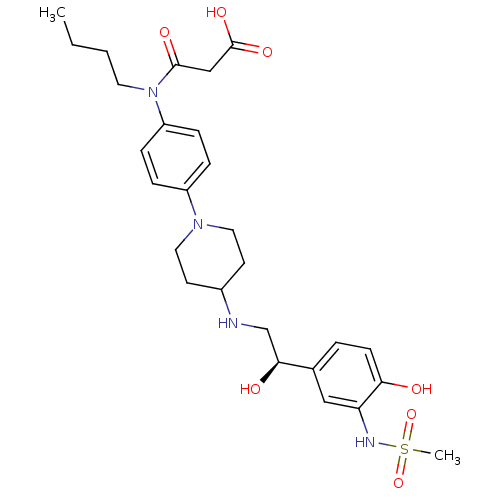 (CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098658 (CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098659 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098662 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191378 (CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065118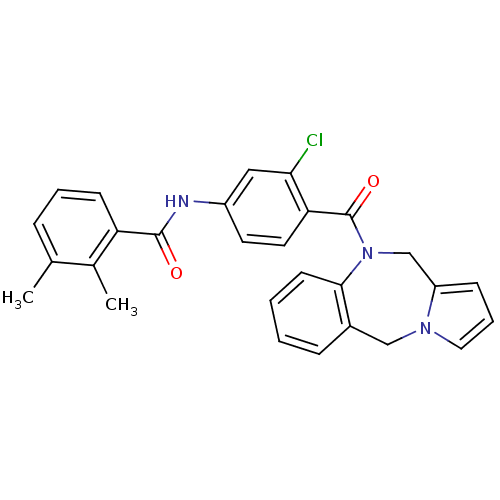 (CHEMBL311931 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065123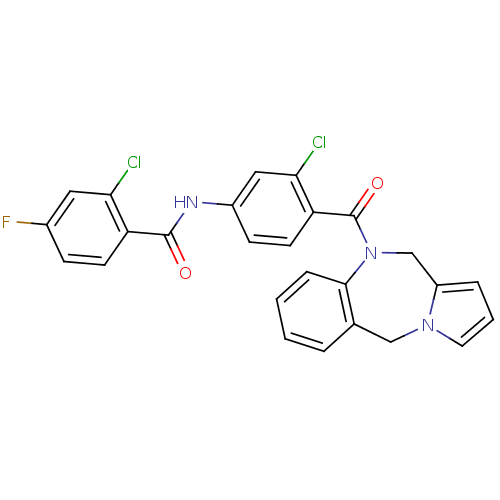 (CHEMBL420031 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191390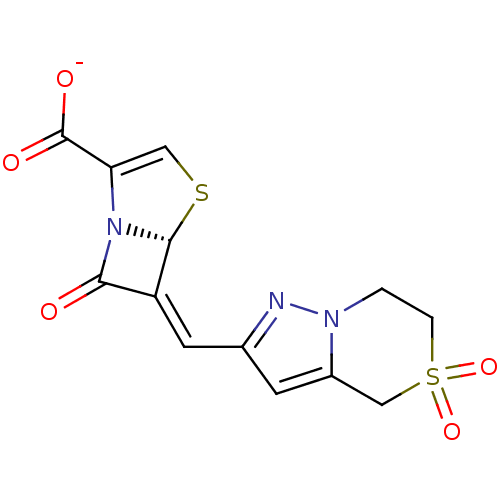 ((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191389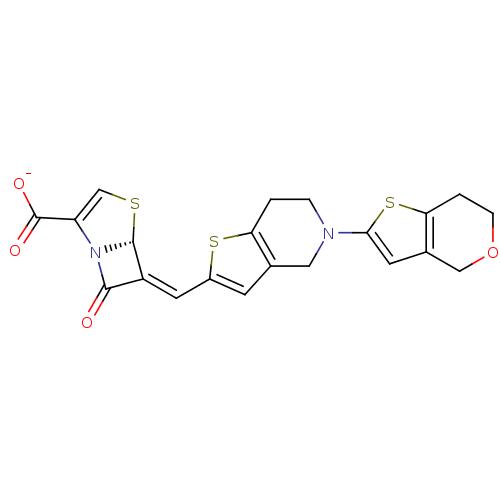 (6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191377 ((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191379 ((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191379 ((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065121 (CHEMBL310416 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065124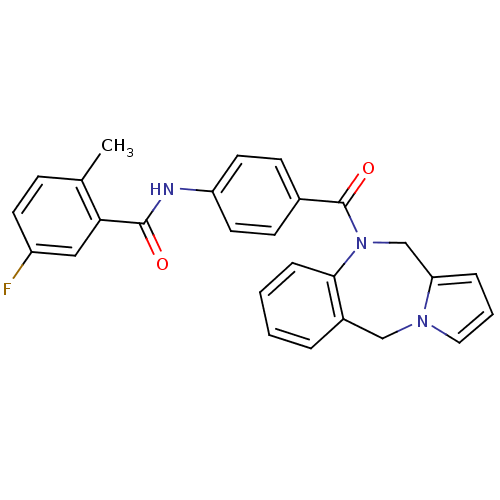 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065124 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065112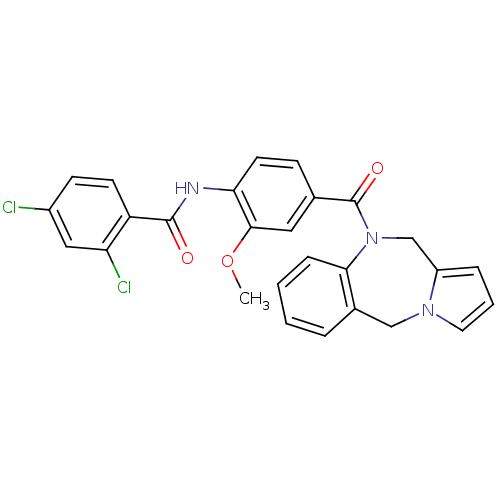 (CHEMBL81269 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065111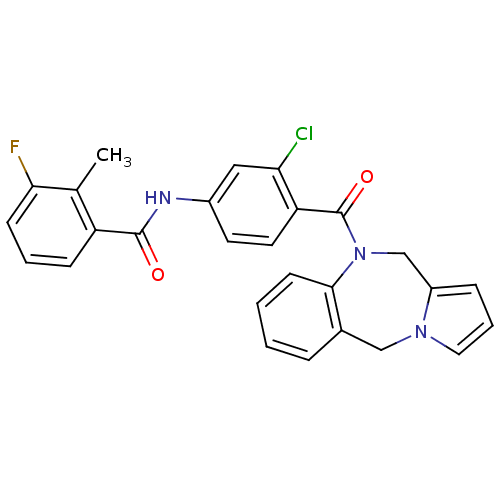 (CHEMBL80029 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191390 ((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065129 (CHEMBL312036 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191381 ((5R,6Z)-6-{[5-(4-methoxybenzyl)-4,5,6,7-tetrahydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191383 ((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129464 (CHEMBL71355 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191386 ((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065125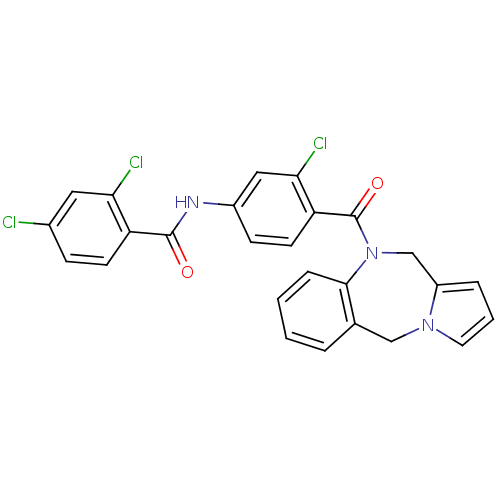 (CHEMBL311230 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065128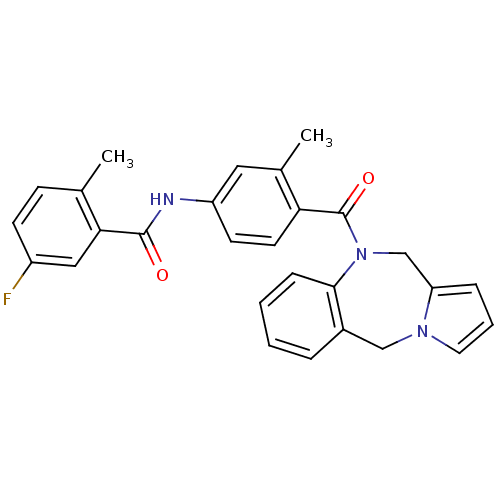 (CHEMBL81133 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191377 ((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191386 ((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191383 ((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191385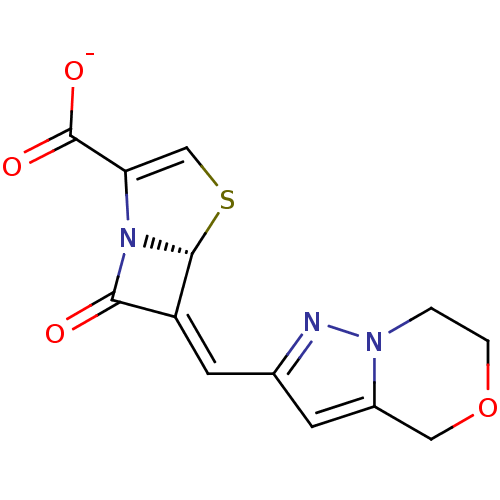 ((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191389 (6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50191387 ((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065116 (CHEMBL81755 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 392 total ) | Next | Last >> |