

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559562 (CHEMBL4762834) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559561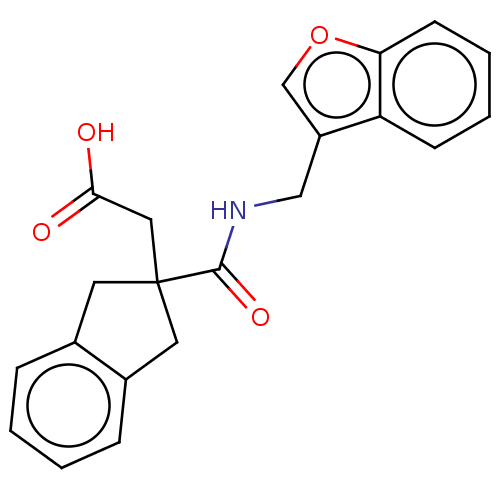 (CHEMBL4760006) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559558 (CHEMBL4764936) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559555 (CHEMBL4745988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559556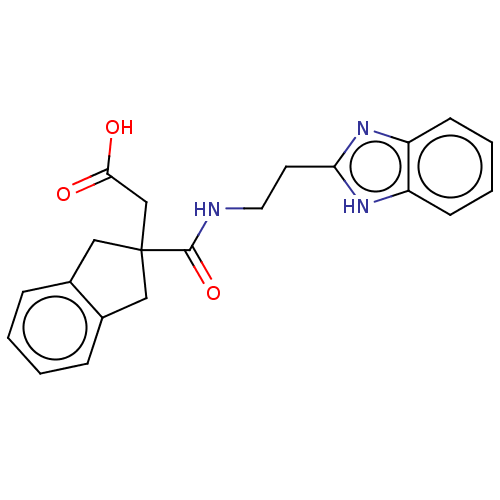 (CHEMBL4749889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559557 (CHEMBL4794689) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559559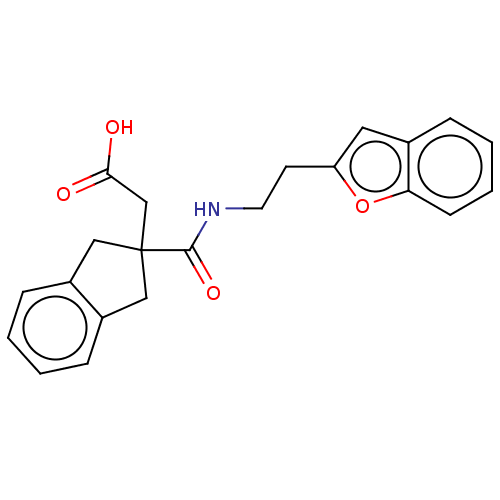 (CHEMBL4746129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559560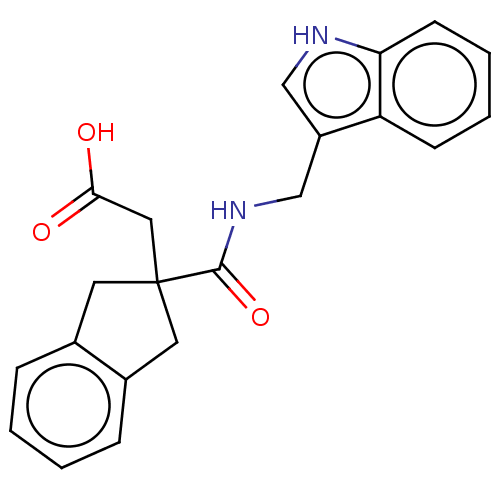 (CHEMBL4756804) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559552 (CHEMBL4776002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559563 (CHEMBL4776454) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559564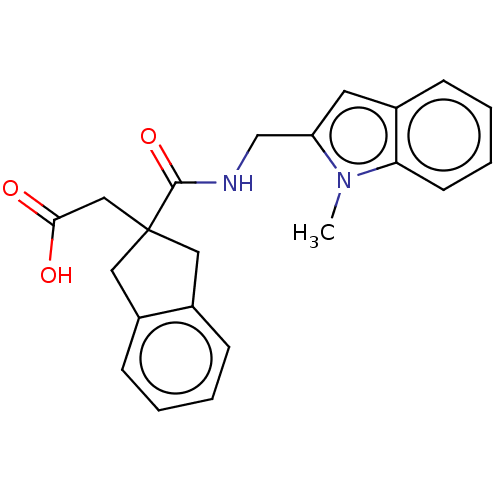 (CHEMBL4746167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559565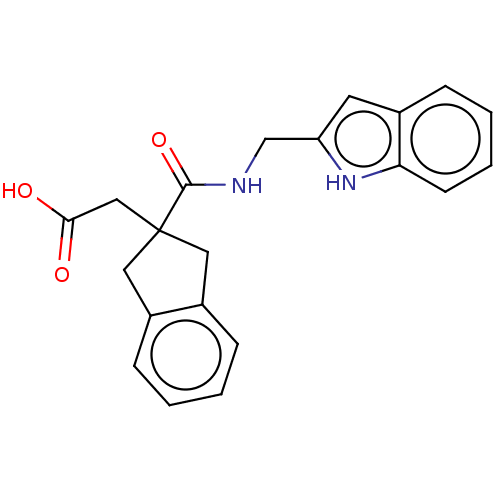 (CHEMBL4776453) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559566 (CHEMBL4749721) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559567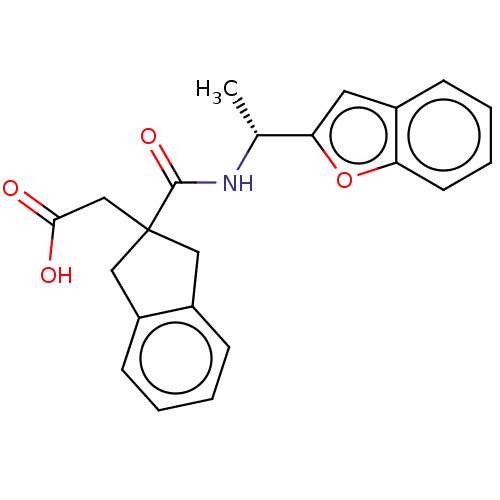 (CHEMBL4760989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559568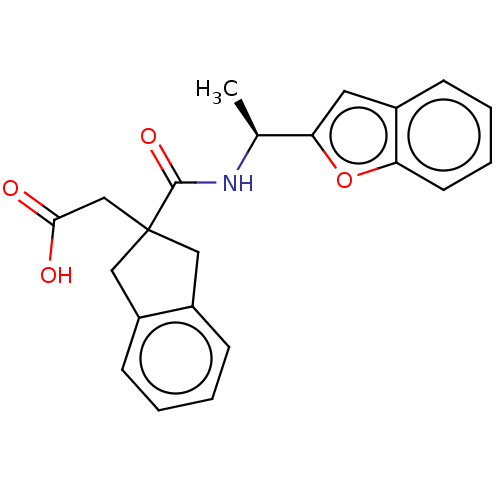 (CHEMBL4748733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559569 (CHEMBL4761199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559570 (CHEMBL4760832) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559571 (CHEMBL4793389) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559572 (CHEMBL4747047) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559573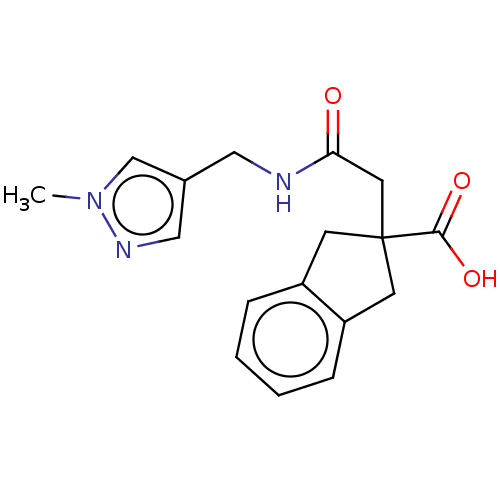 (CHEMBL4741361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559574 (CHEMBL4785438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559575 (CHEMBL4798439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559576 (CHEMBL4794850) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559577 (CHEMBL4797456) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559554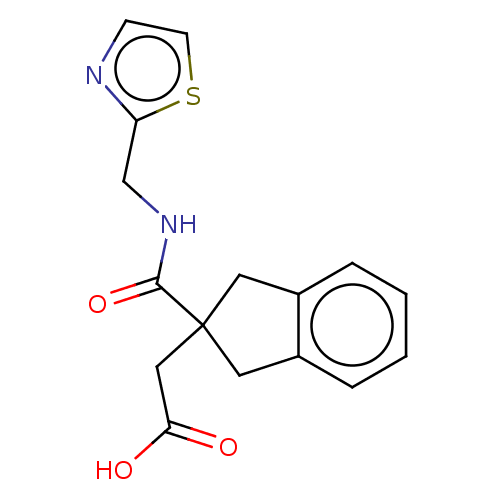 (CHEMBL4751454) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559578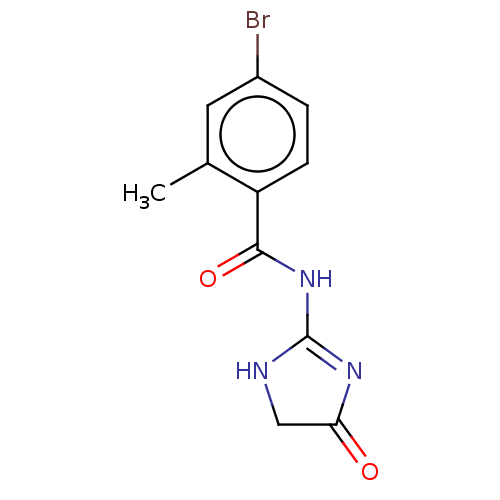 (CHEMBL4751618) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559551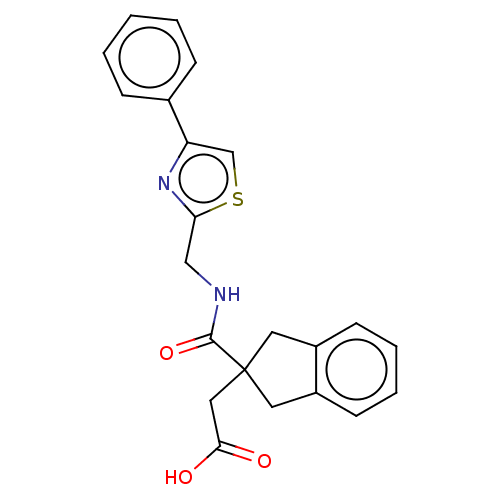 (CHEMBL4758612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50559553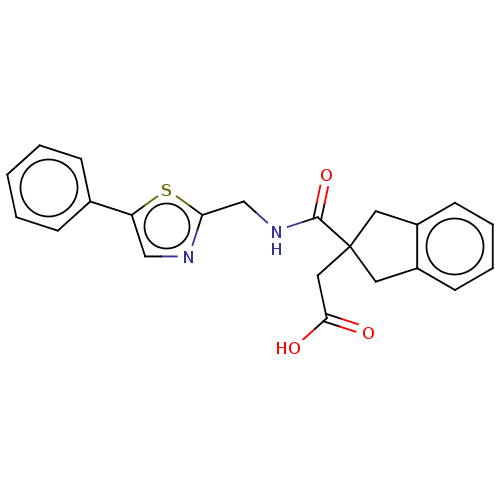 (CHEMBL4745918) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00554 BindingDB Entry DOI: 10.7270/Q2X63RNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50421360 (CHEMBL358119 | SB-207058) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM82505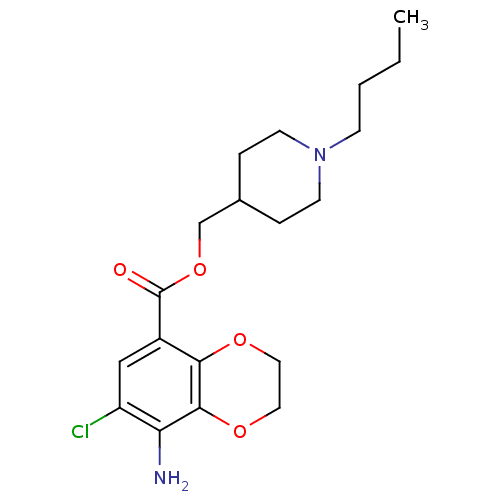 (CAS_121881 | NSC_121881 | SB204070) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50422085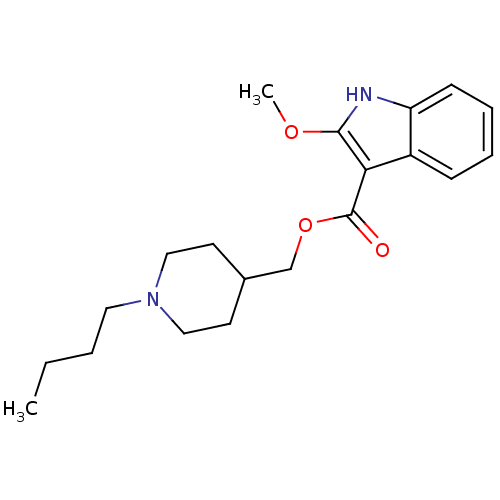 (CHEMBL142490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50421359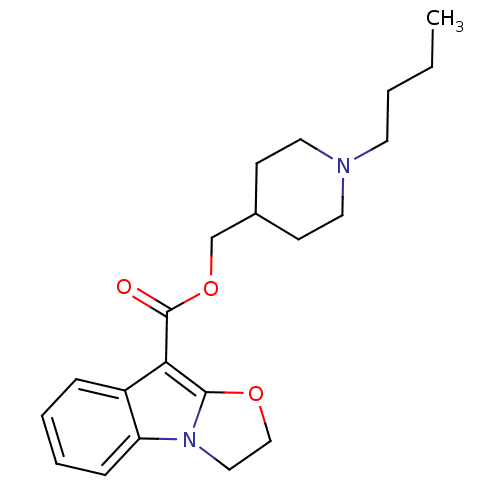 (CHEMBL145075) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50421362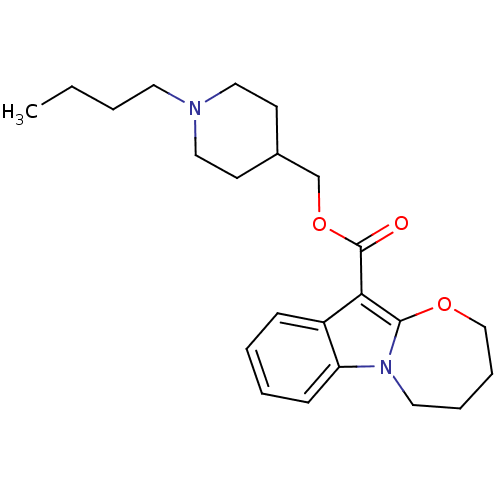 (CHEMBL143862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473678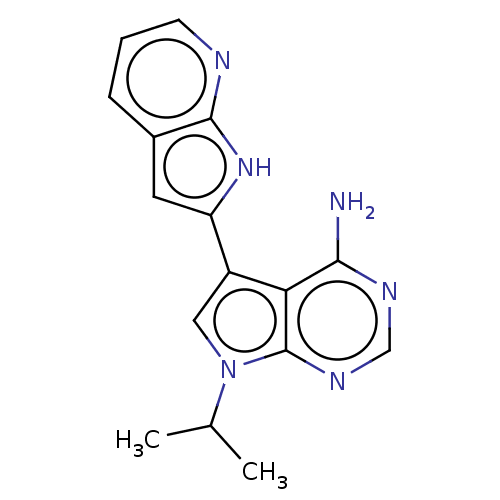 (7-Isopropyl-5-(1H-pyrrolo[2,3-b]pyridin-2- yl)pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4TVP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473678 (7-Isopropyl-5-(1H-pyrrolo[2,3-b]pyridin-2- yl)pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description RET and KDR: Kinase activity was detected using CisBio HTRF kinEASE kit based on time-resolved fluorescence transfer (FRET). The assay was performed ... | US Patent US10844067 (2020) BindingDB Entry DOI: 10.7270/Q2HQ4308 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364156 (CHEMBL1951432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50421361 (CHEMBL143426) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Ability to antagonize 5-HT-evoked contractions mediated through 5-hydroxytryptamine 4 receptor activation in the guinea pig distal colon LMMP | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364171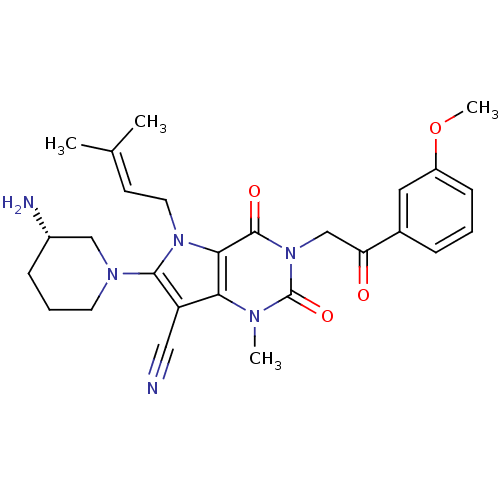 (CHEMBL1951598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026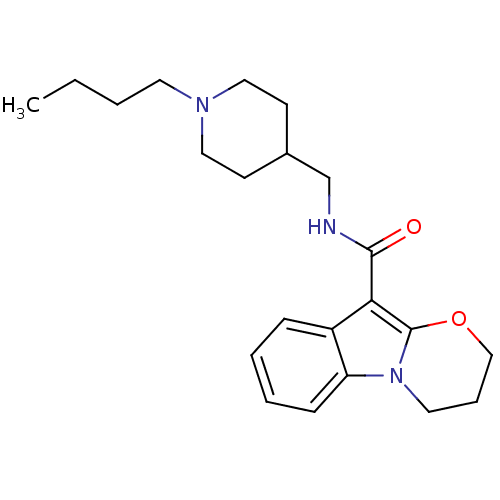 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity was determined against 5-hydroxytryptamine 3 receptor | J Med Chem 38: 4760-3 (1996) BindingDB Entry DOI: 10.7270/Q2B56M1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473666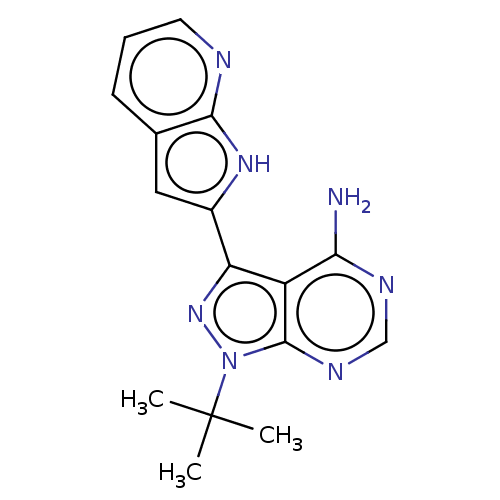 (1-tert-Butyl-3-{1H-pyrrolo[2,3-b]pyridin-2- yl}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description RET and KDR: Kinase activity was detected using CisBio HTRF kinEASE kit based on time-resolved fluorescence transfer (FRET). The assay was performed ... | US Patent US10844067 (2020) BindingDB Entry DOI: 10.7270/Q2HQ4308 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473667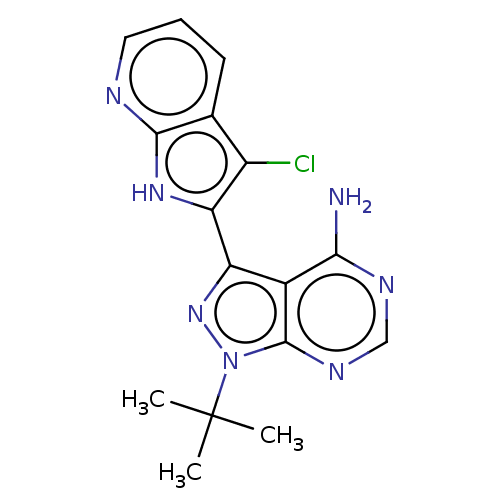 (1-tert-Butyl-3-{3-chloro-1H-pyrrolo[2,3- b]pyridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4TVP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473666 (1-tert-Butyl-3-{1H-pyrrolo[2,3-b]pyridin-2- yl}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4TVP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM473667 (1-tert-Butyl-3-{3-chloro-1H-pyrrolo[2,3- b]pyridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description RET and KDR: Kinase activity was detected using CisBio HTRF kinEASE kit based on time-resolved fluorescence transfer (FRET). The assay was performed ... | US Patent US10844067 (2020) BindingDB Entry DOI: 10.7270/Q2HQ4308 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM557389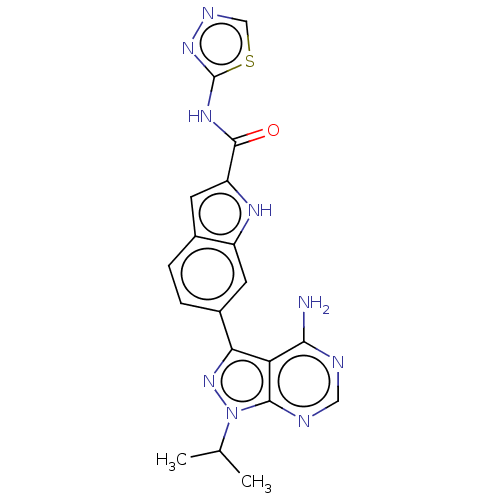 (US11352361, Example 39 | US11680068, Example 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.709 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kinase activity was detected using CisBio HTRF kinEASE kit based on time-resolved fluorescence transfer (FRET). The assay was performed in 384-well w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MW2MC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM557389 (US11352361, Example 39 | US11680068, Example 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.709 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q21Z48H4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM602904 (1-(4-(8-amino-3-ethyl-5-(4-(methylamino)cyclohex-1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3Q3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM602903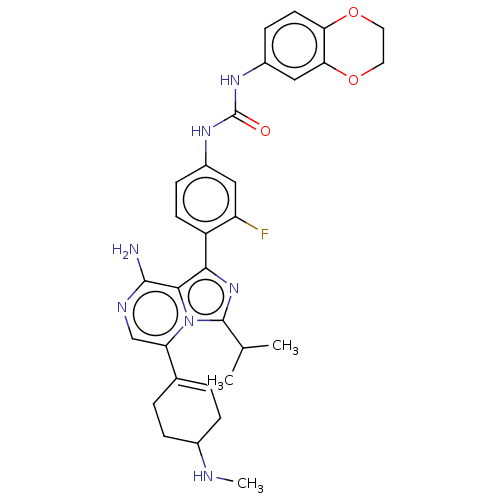 (1-(4-(8-amino-3-isopropyl-5-(4-(methylamino)cycloh...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3Q3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM602927 (1-(4-(8-amino-3-isopropyl-5-(4-(methylamino)cycloh...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3Q3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM602907 (1-(4-(8-amino-3-isopropyl-5-(4-(methylamino)cycloh...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3Q3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1833 total ) | Next | Last >> |