

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287890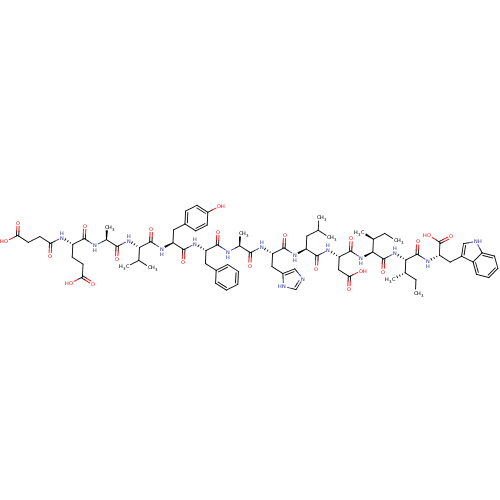 (CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287885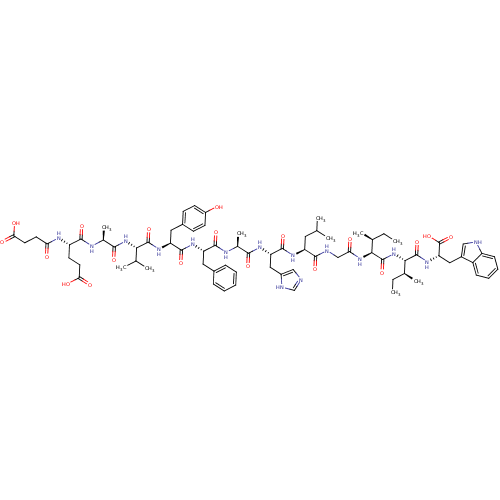 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287883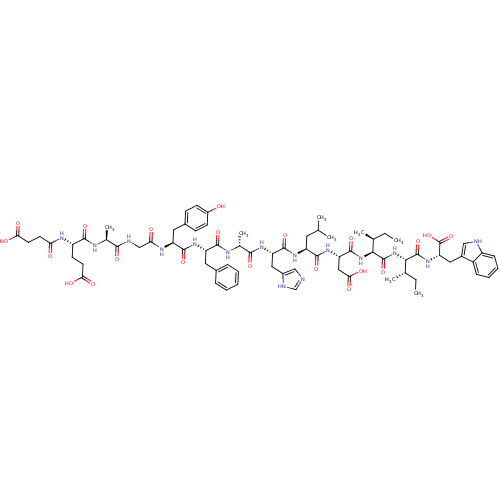 (CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071433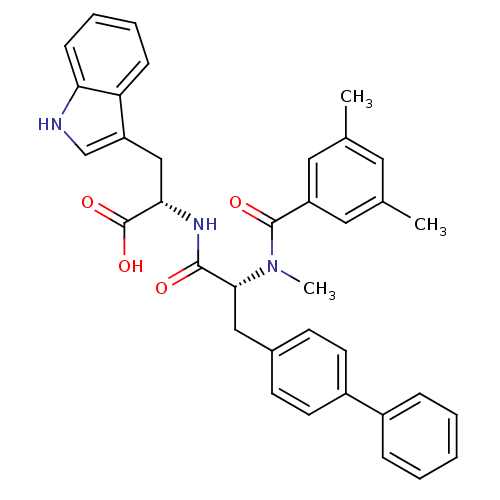 ((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287882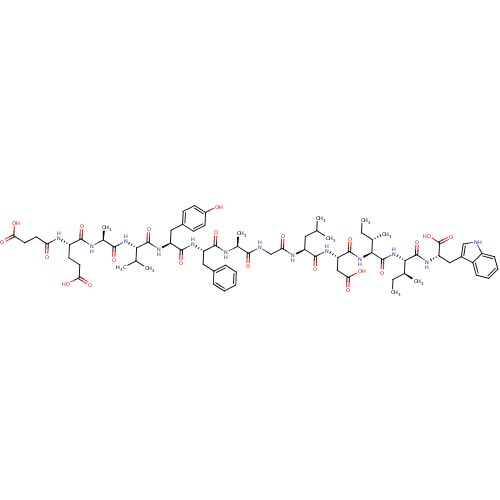 (CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287878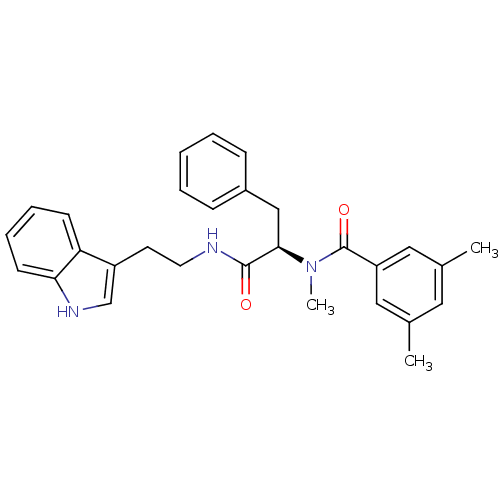 (CGP-49941 | CHEMBL305615 | N-{(R)-1-[2-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287888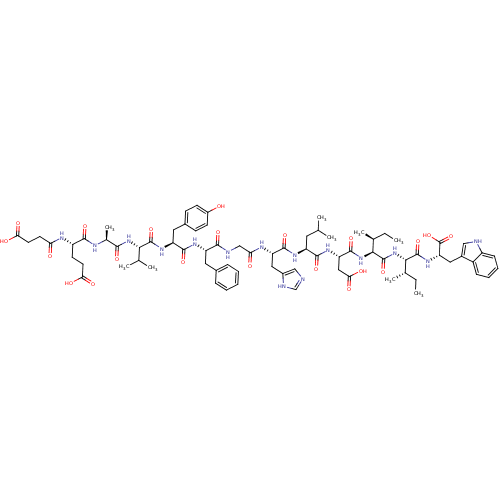 (CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287886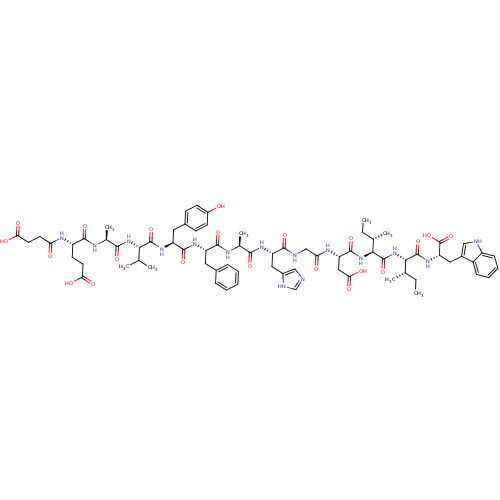 (CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287887 (CHEMBL413604 | Suc-Glu-Ala-Val-Gly-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287880 ((S)-3-(1H-Indol-3-yl)-2-[(2-phenyl-cyclopropanecar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071431 ((S)-2-{(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287885 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287884 (CHEMBL411399 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287882 (CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287889 (CHEMBL412002 | Suc-Glu-Ala-Val-Tyr-Gly-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50071433 ((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287890 (CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287883 (CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287888 (CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287887 (CHEMBL413604 | Suc-Glu-Ala-Val-Gly-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287879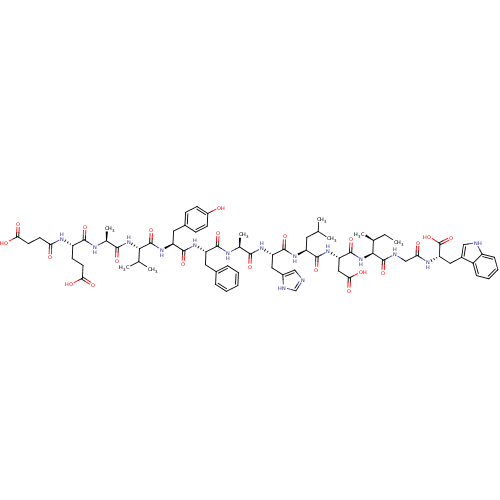 (CHEMBL405753 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287881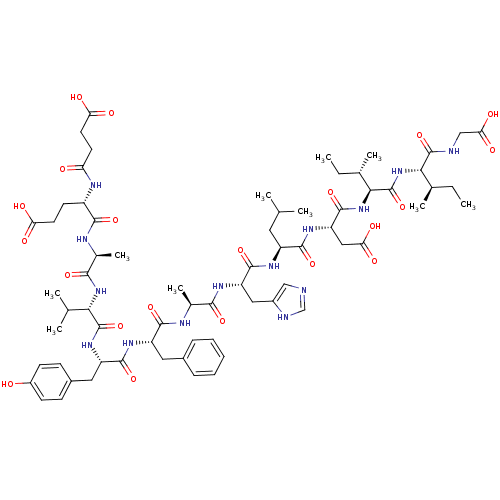 (CHEMBL216772 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287889 (CHEMBL412002 | Suc-Glu-Ala-Val-Tyr-Gly-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287881 (CHEMBL216772 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287886 (CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287879 (CHEMBL405753 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287884 (CHEMBL411399 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50071431 ((S)-2-{(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15459 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15448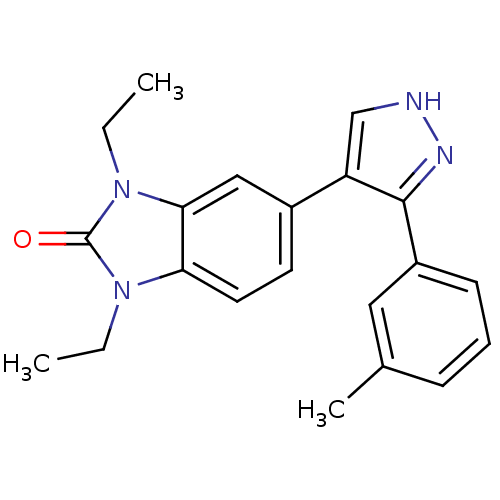 (1,3-diethyl-5-[3-(3-methylphenyl)-1H-pyrazol-4-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15442 (1,3-diethyl-5-[3-(4-fluoro-3-methylphenyl)-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13336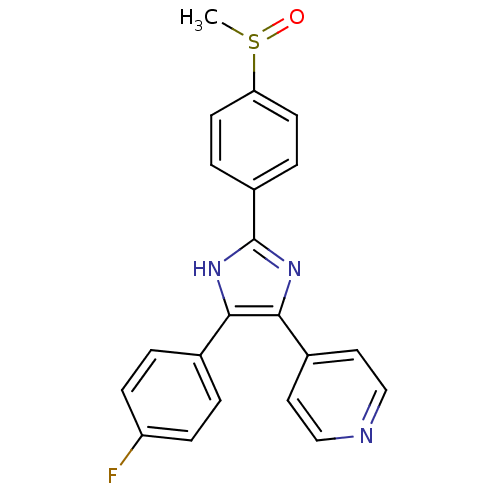 (4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15458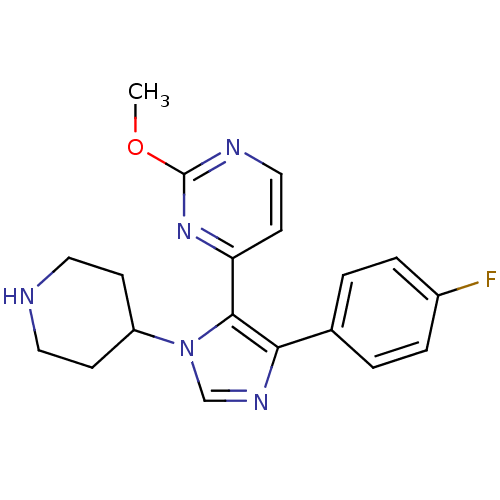 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15419 (1,3-diethyl-5-[4-(3-methylphenyl)-1H-imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15457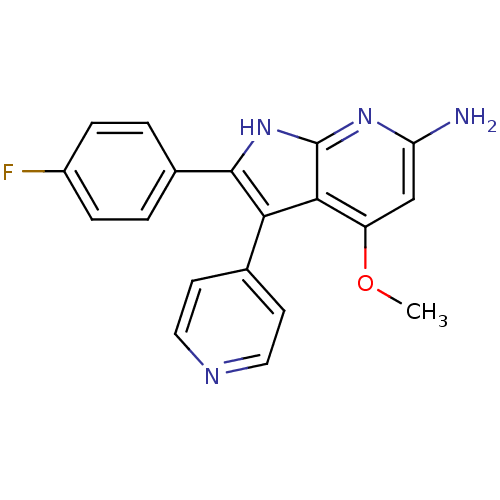 (2-(4-fluorophenyl)-4-methoxy-3-(pyridin-4-yl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15443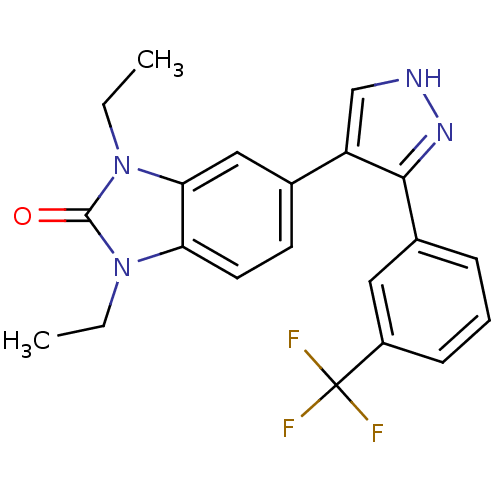 (1,3-diethyl-5-{3-[3-(trifluoromethyl)phenyl]-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15438 (1,3-diethyl-5-[4-(3-methylphenyl)-2-(pyridin-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15439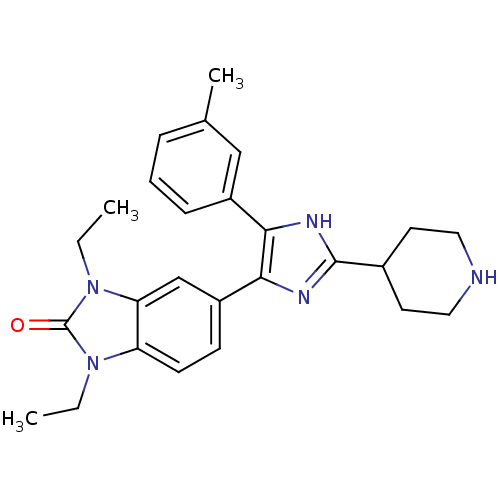 (1,3-diethyl-5-[4-(3-methylphenyl)-2-(piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15461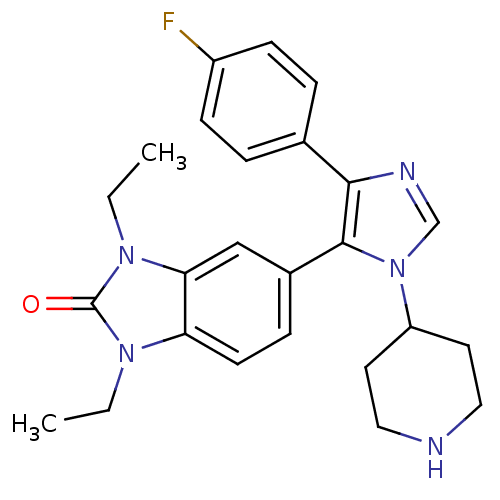 (1,3-diethyl-5-[4-(4-fluorophenyl)-1-(piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15418 (1,3-dimethyl-5-[4-(3-methylphenyl)-1H-imidazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15421 (1,3-diethyl-5-[4-(4-fluorophenyl)-1H-imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15420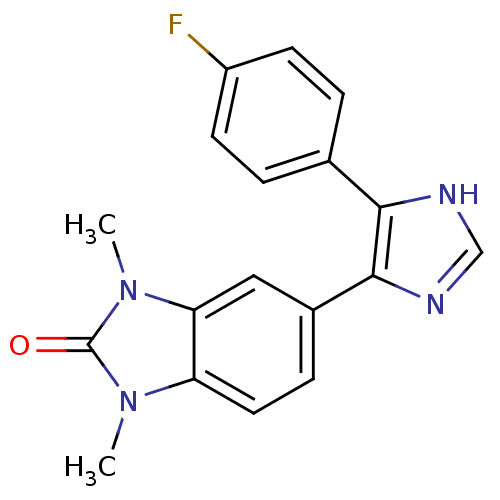 (5-[4-(4-fluorophenyl)-1H-imidazol-5-yl]-1,3-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15417 (1,3-diethyl-5-(4-phenyl-1H-imidazol-5-yl)-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15432 (1,3-dimethyl-5-[4-(3-methylphenyl)-2-(thiophen-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15446 (1,3-diethyl-5-(3-phenyl-1H-pyrazol-4-yl)-2,3-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15437 (1,3-dimethyl-5-[4-(3-methylphenyl)-2-(pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15444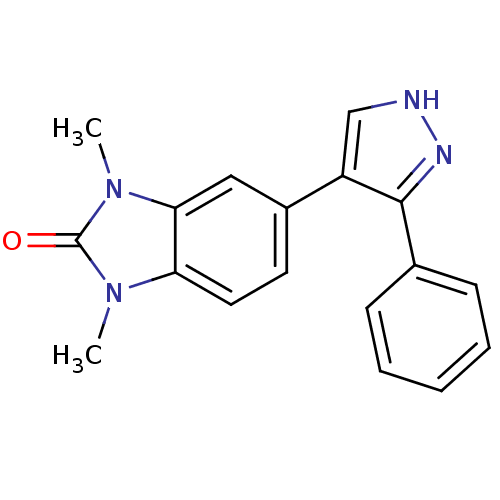 (1,3-dimethyl-5-(3-phenyl-1H-pyrazol-4-yl)-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15422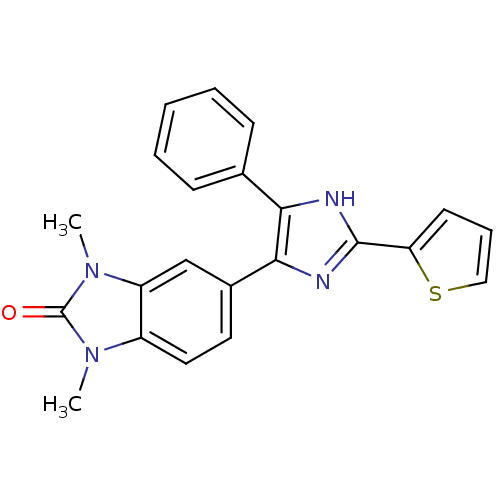 (1,3-dimethyl-5-[4-phenyl-2-(thiophen-2-yl)-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 14: 919-23 (2004) Article DOI: 10.1016/j.bmcl.2003.12.023 BindingDB Entry DOI: 10.7270/Q28050V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 222 total ) | Next | Last >> |