

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]sulpiride from dopamine D2 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]pirenzepine from muscarinic M1 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50064176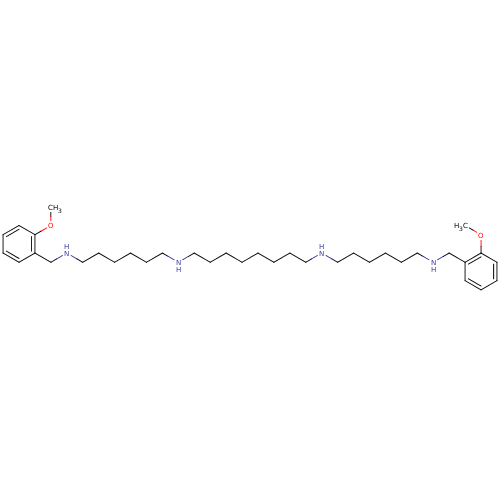 (CHEMBL27673 | CHEMBL500996 | METHOCTRAMINE | N,N''...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]AF-DX384 from muscarinic M2 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50292408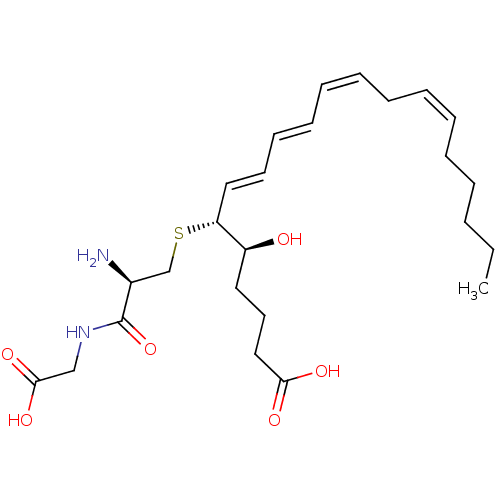 ((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LTD4 from LTD4 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAGO from mu opioid receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50292411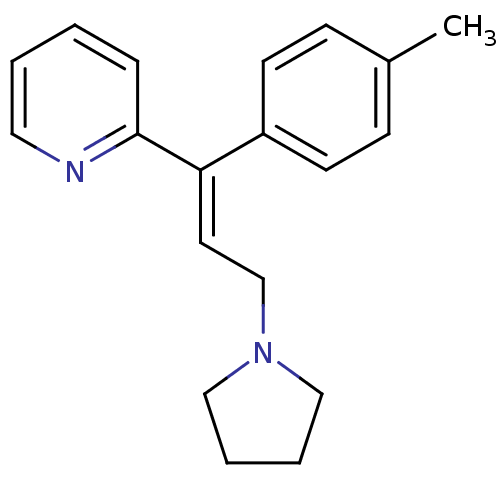 ((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM31046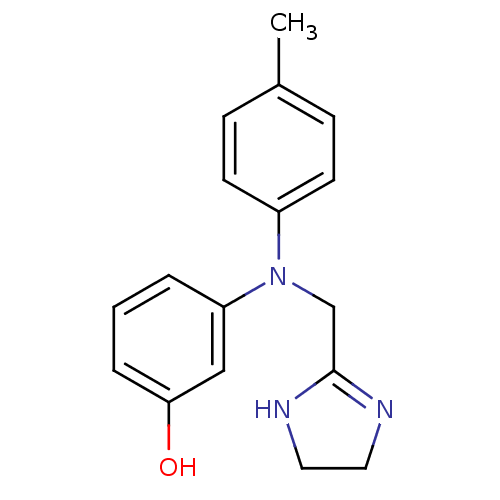 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]RX781094 from alpha2 adrenergic receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889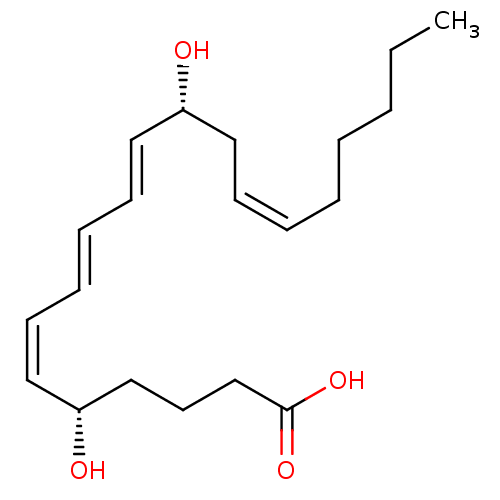 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LTB4 from LTB4R | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50292409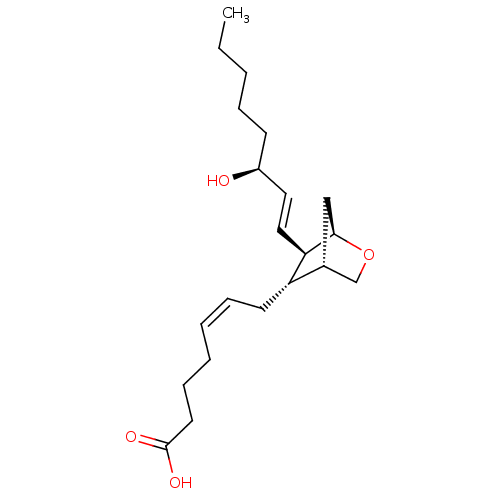 ((Z)-7-((1R,4S,5S,6R)-6-((S,E)-3-hydroxyoct-1-enyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]SQ29548 from thromboxane A2 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50008369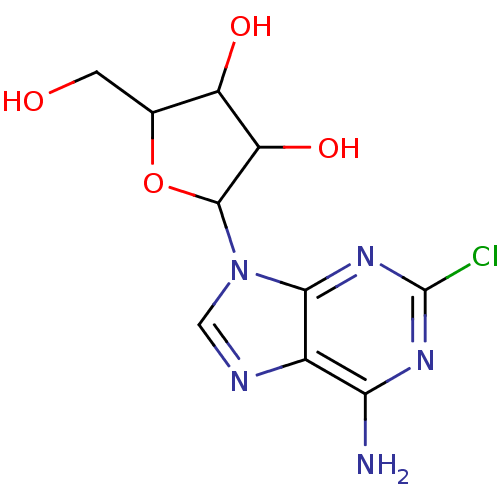 ((2R,3R,4S,5R)-2-(6-amino-2-chloro-9H-purin-9-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CPX from adenosine A1 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50010859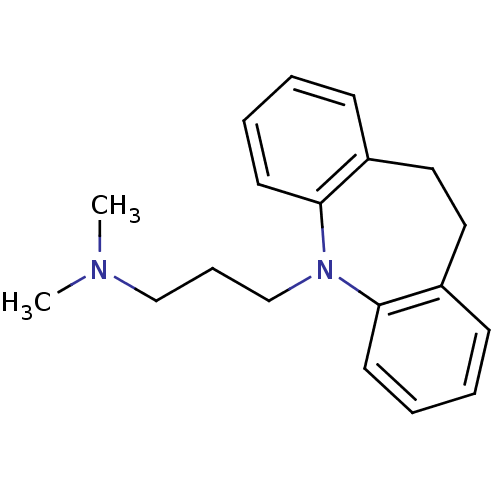 (CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from serotonin transporter | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50005548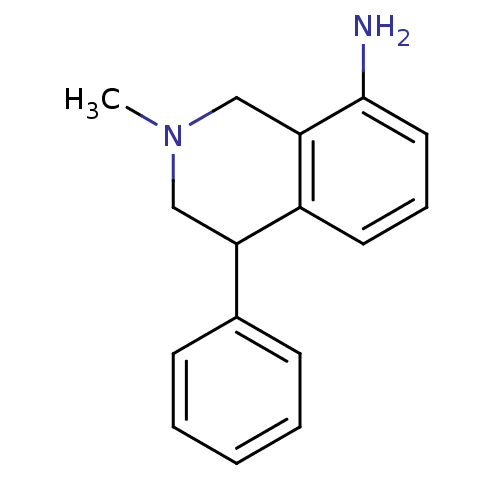 ((+)-2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinol...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]WIN-from cocaine site of dopamine transporter | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50292410 ((-)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DMI from norepinephrine transporter | J Nat Prod 56: 441-455 (1993) Article DOI: 10.1021/np50094a001 BindingDB Entry DOI: 10.7270/Q2VT1S4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001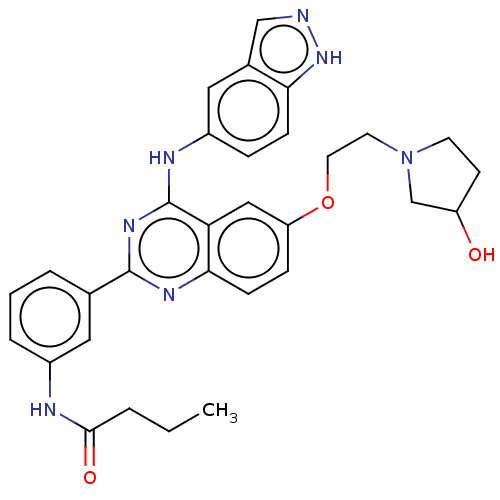 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999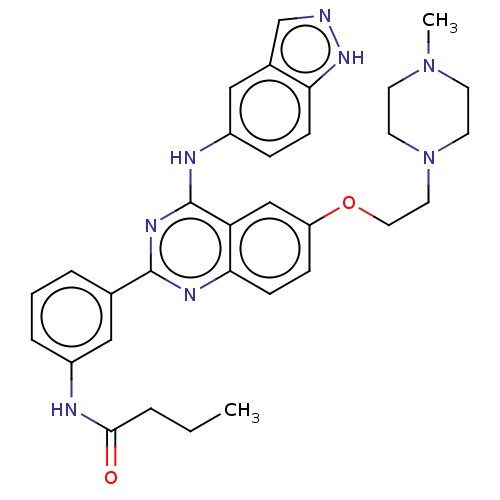 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996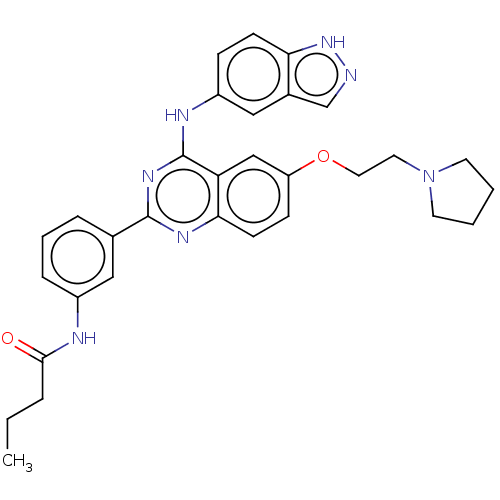 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM50471710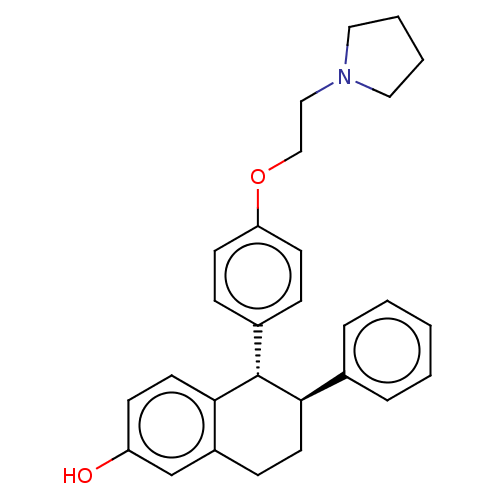 (CHEMBL317748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141005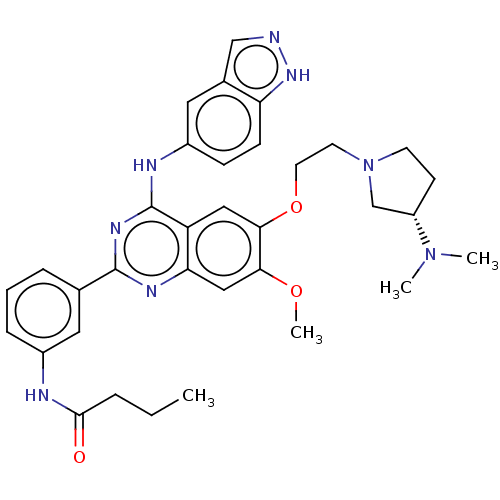 (US10570123, Example 210 | US8916576, 210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141005 (US10570123, Example 210 | US8916576, 210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140998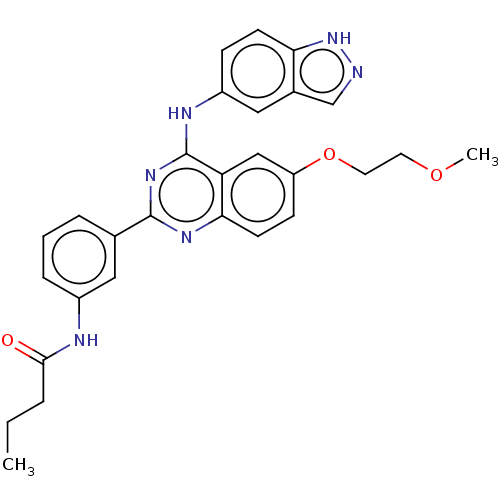 (US10570123, Example 201 | US8916576, 201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM50471711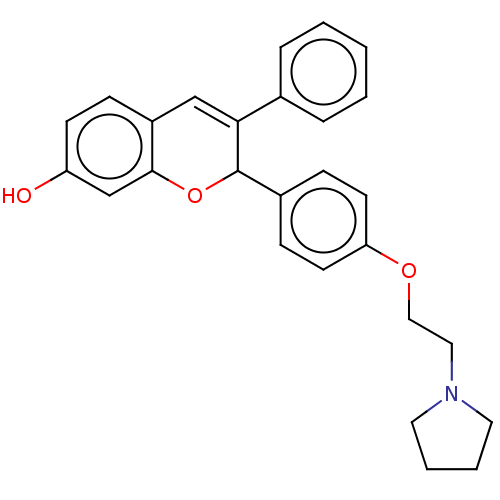 (CHEMBL98892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM20606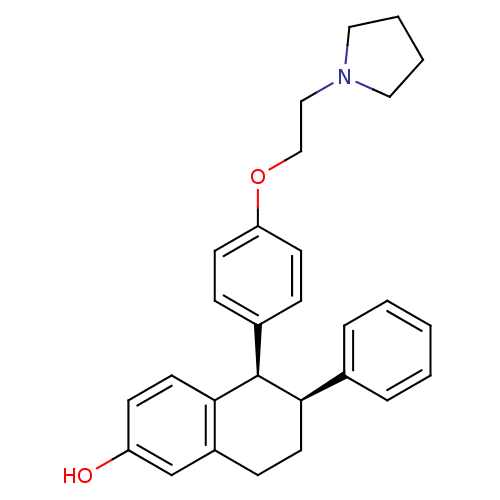 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM20606 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140997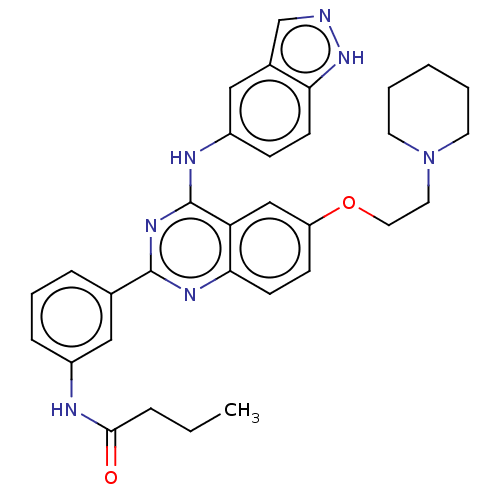 (US10570123, Example 200 | US8916576, 200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140997 (US10570123, Example 200 | US8916576, 200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140997 (US10570123, Example 200 | US8916576, 200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140999 (US10570123, Example 203 | US8916576, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140996 (US10570123, Example 199 | US8916576, 199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM140997 (US10570123, Example 200 | US8916576, 200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Surface Logix, Inc. US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US8916576 (2014) BindingDB Entry DOI: 10.7270/Q2P55M6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM141001 (US10570123, Example 205 | US8916576, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SURFACE LOGIX, LLC US Patent | Assay Description ROCK-II inhibitory activity can be measured using the ROCK-II Assay Kit (Molecular Devices, inc.; Sunnyvale, Calif.). | US Patent US10570123 (2020) BindingDB Entry DOI: 10.7270/Q2542R1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 226 total ) | Next | Last >> |