

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50063266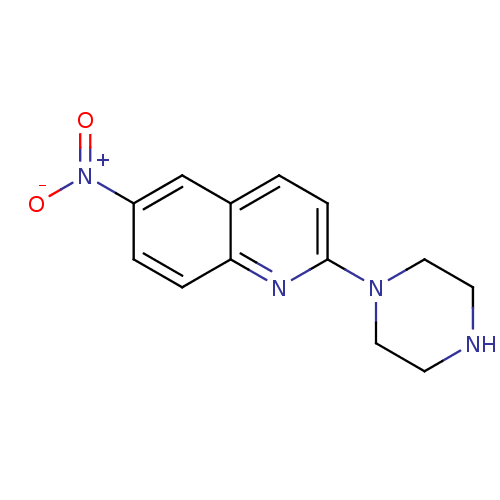 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human SERT | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105212 (2-(4-Nonyl-piperazin-1-yl)-6-nitroquinoline (9)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105213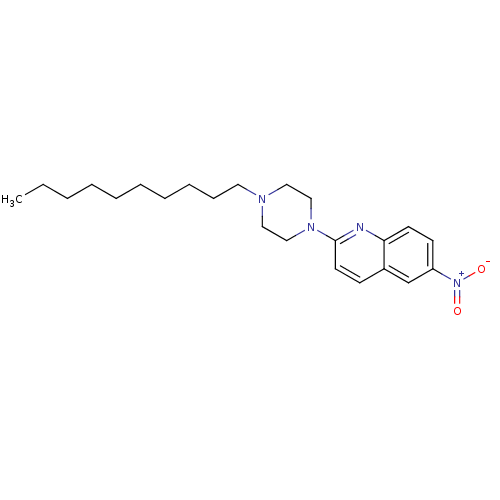 (2-(4-Decyl-piperazin-1-yl)-6-nitroquinoline (10)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50315962 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity at 5HT1A receptor | Bioorg Med Chem Lett 20: 2465-8 (2010) Article DOI: 10.1016/j.bmcl.2010.03.012 BindingDB Entry DOI: 10.7270/Q2736R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301431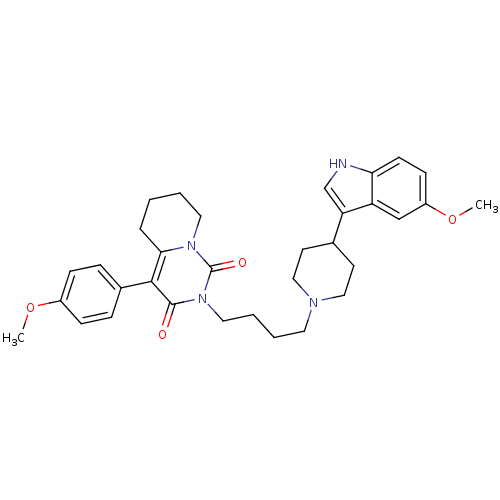 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25876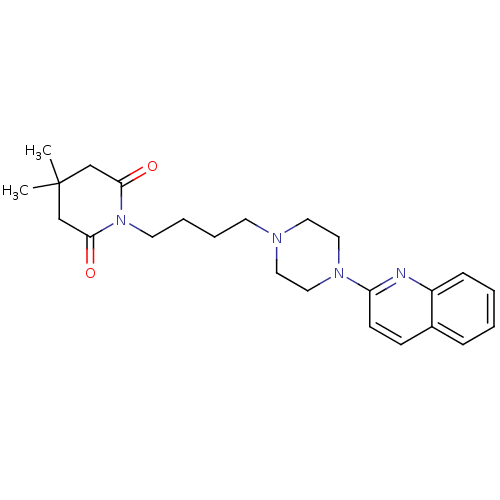 (4,4-dimethyl-1-{4-[4-(quinolin-2-yl)piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321113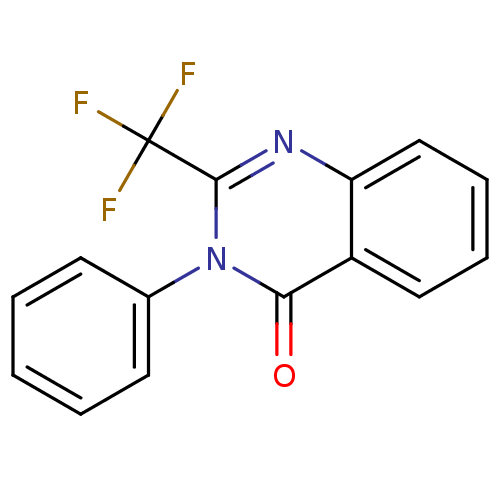 (3-Phenyl-2-(trifluoromethyl)quinazolin-4(3H)-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105214 (2-(4-Undecyl-piperazin-1-yl)-6-nitroquinoline (11)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.6 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25869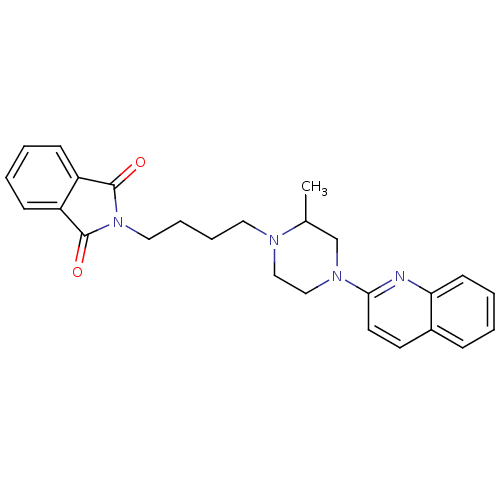 (2-{4-[2-methyl-4-(quinolin-2-yl)piperazin-1-yl]but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301442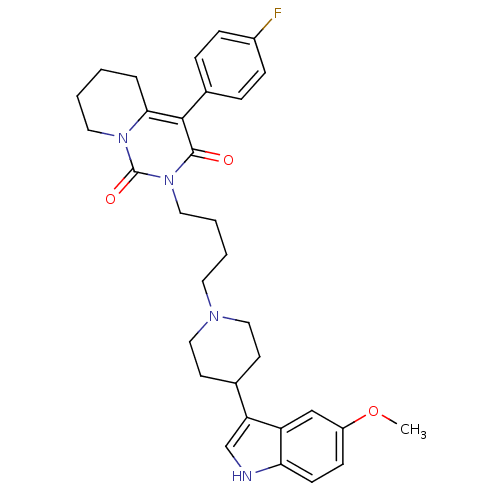 (4-(4-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301429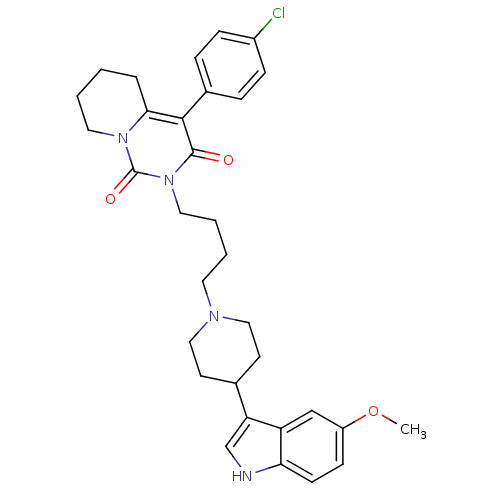 (4-(4-Chloro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50363815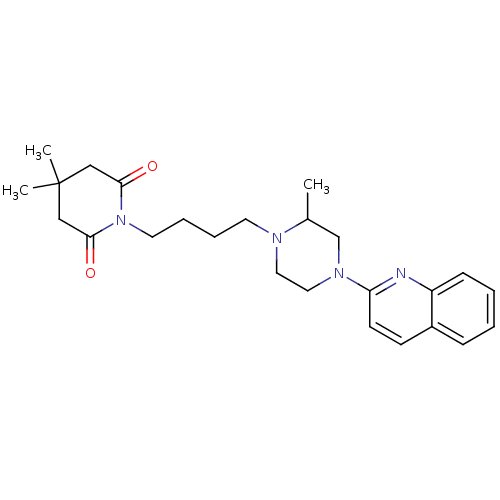 (CHEMBL1945686) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105215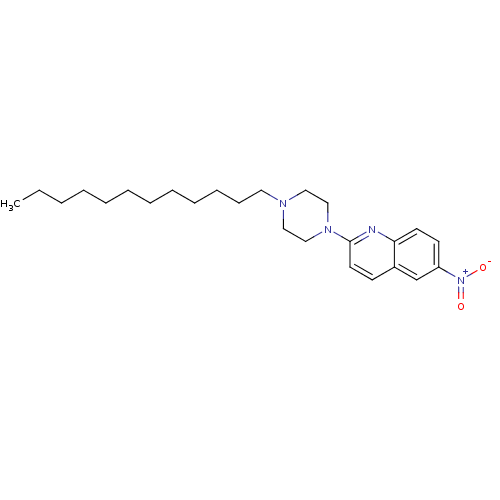 (2-(4-Dodecyl-piperazin-1-yl)-6-nitroquinoline (12)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16.1 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301432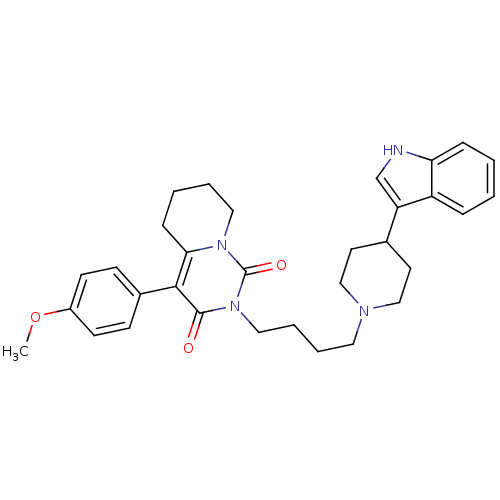 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105211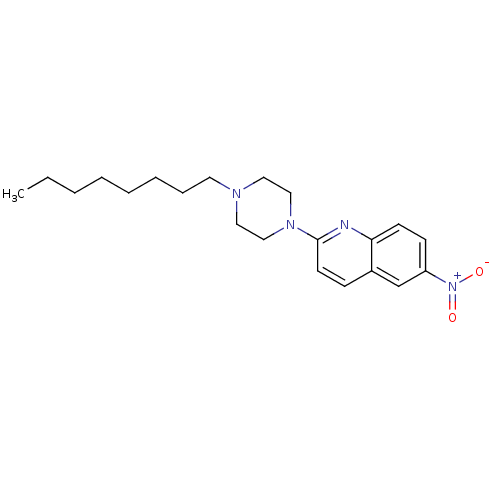 (2-(4-Octyl-piperazin-1-yl)-6-nitroquinoline (8)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20.8 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301446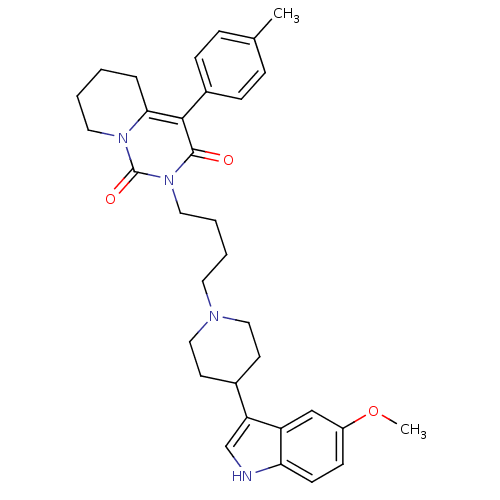 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105207 (2-(4-Butyl-piperazin-1-yl)-6-nitroquinoline (4)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25873 (8-{4-[2-methyl-4-(quinolin-2-yl)piperazin-1-yl]but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301445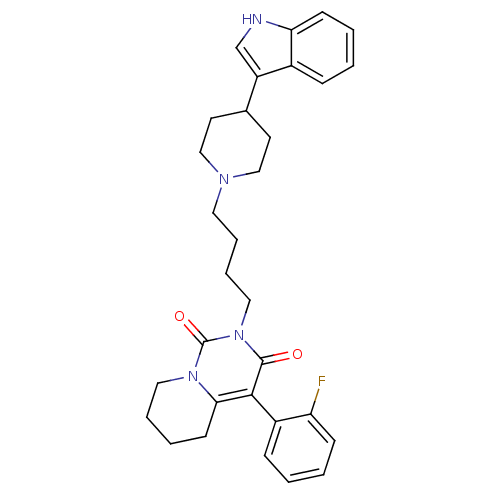 (4-(2-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50014407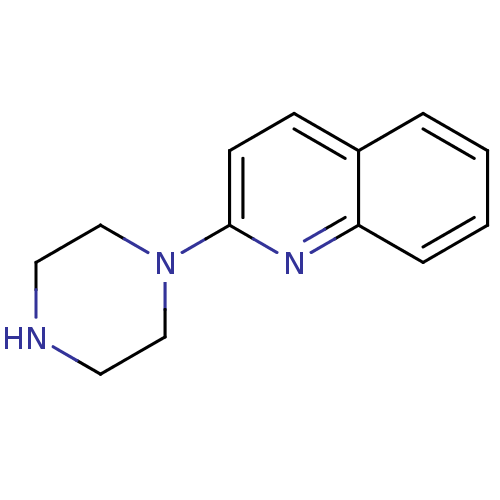 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human SERT | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50315963 (1-(2-chlorobenzyloxy)-3-((2,3-dihydrobenzo[b][1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity at 5HT1A receptor | Bioorg Med Chem Lett 20: 2465-8 (2010) Article DOI: 10.1016/j.bmcl.2010.03.012 BindingDB Entry DOI: 10.7270/Q2736R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301441 (4-(2-Chloro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25871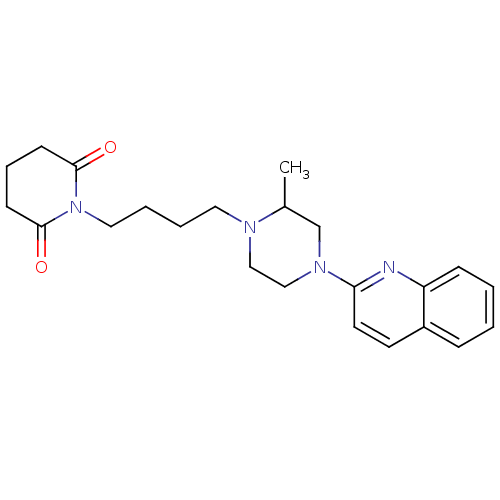 (1-{4-[2-methyl-4-(quinolin-2-yl)piperazin-1-yl]but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25875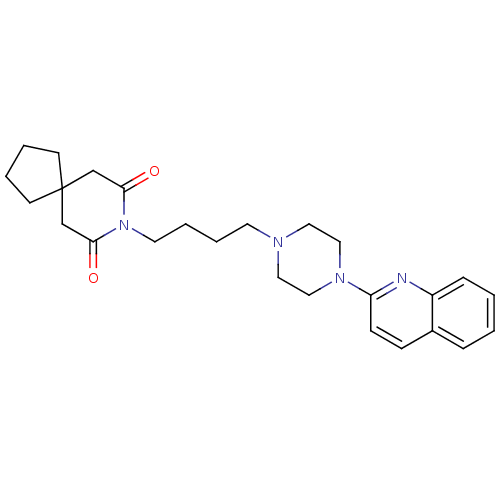 (8-{4-[4-(quinolin-2-yl)piperazin-1-yl]butyl}-8-aza...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM25874 (2-{4-[4-(quinolin-2-yl)piperazin-1-yl]butyl}-2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301430 (4-(2-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301428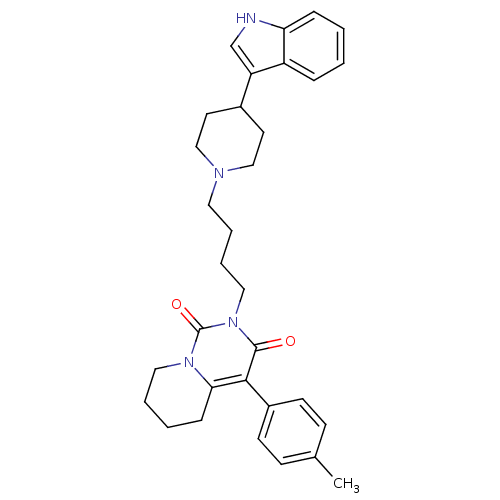 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301443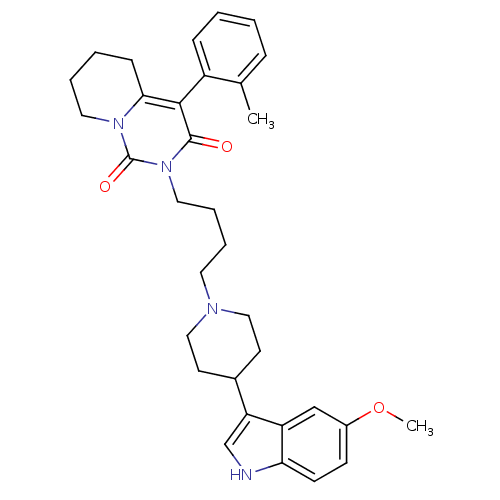 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301444 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105210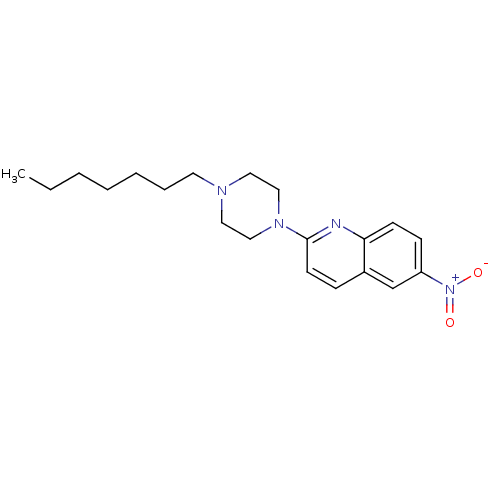 (2-(4-Heptyl-piperazin-1-yl)-6-nitroquinoline (7)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54.1 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301432 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105209 (2-(4-Hexyl-piperazin-1-yl)-6-nitroquinoline (6)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72.9 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301442 (4-(4-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301428 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105208 (2-(4-Pentyl-piperazin-1-yl)-6-nitroquinoline (5)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80.5 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301431 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM105205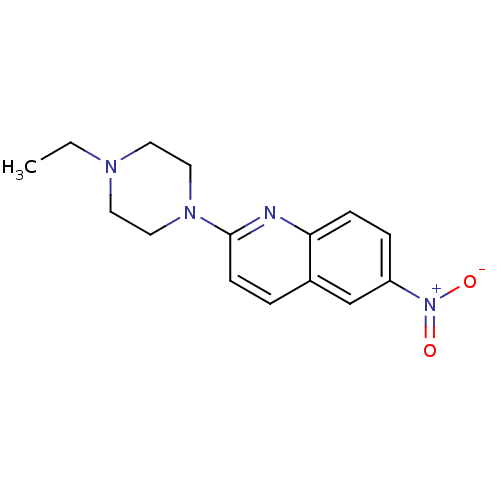 (2-(4-Ethyl-piperazin-1-yl)-6-nitroquinoline (2)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 86.1 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£ | Assay Description The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... | Chem Biol Drug Des 81: 695-706 (2013) Article DOI: 10.1111/cbdd.12116 BindingDB Entry DOI: 10.7270/Q2542M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301441 (4-(2-Chloro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301445 (4-(2-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301437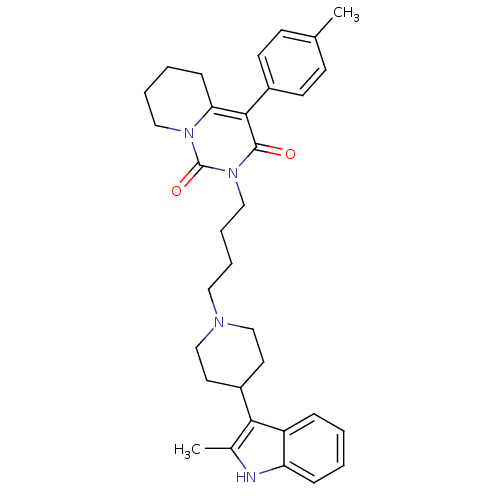 (2-{4-[4-(2-Methyl-1H-indol-3-yl)-piperidin-1-yl]-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301433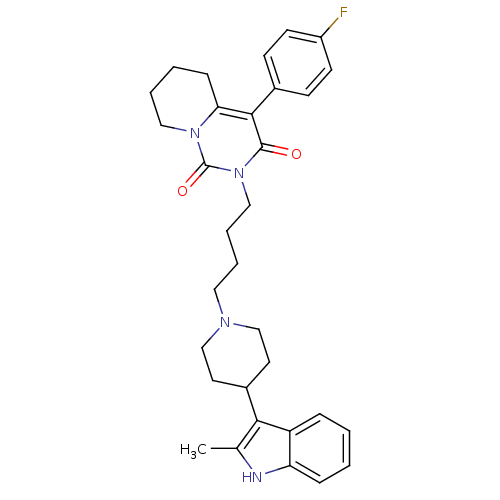 (4-(4-Fluoro-phenyl)-2-{4-[4-(2-methyl-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301444 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301443 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301446 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301430 (4-(2-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50315962 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor | Bioorg Med Chem Lett 20: 2465-8 (2010) Article DOI: 10.1016/j.bmcl.2010.03.012 BindingDB Entry DOI: 10.7270/Q2736R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301436 (4-(4-Methoxy-phenyl)-2-{4-[4-(2-methyl-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50363810 (CHEMBL1945681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301429 (4-(4-Chloro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50014407 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in cerebral cortex homogenates after 15 mins | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 264 total ) | Next | Last >> |