 Found 409 hits with Last Name = 'taborn' and Initial = 'k'
Found 409 hits with Last Name = 'taborn' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM154061
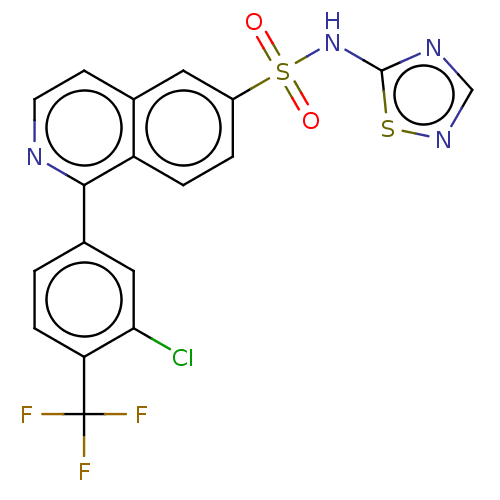
(US9012443, 57)Show SMILES FC(F)(F)c1ccc(cc1Cl)-c1nccc2cc(ccc12)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C18H10ClF3N4O2S2/c19-15-8-11(1-4-14(15)18(20,21)22)16-13-3-2-12(7-10(13)5-6-23-16)30(27,28)26-17-24-9-25-29-17/h1-9H,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533547

(CHEMBL4537339)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(Oc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C14H9Cl2F2NO4S/c1-24(21,22)19-14(20)8-5-12(18)13(6-11(8)17)23-7-2-3-9(15)10(16)4-7/h2-6H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50272533
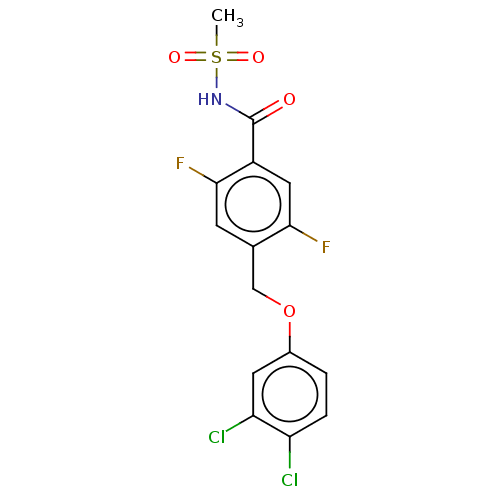
(CHEMBL4129030)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(COc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C15H11Cl2F2NO4S/c1-25(22,23)20-15(21)10-6-13(18)8(4-14(10)19)7-24-9-2-3-11(16)12(17)5-9/h2-6H,7H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533554

(CHEMBL4470763)Show SMILES CC(C)COc1ncc(cc1Cl)-c1cc(F)c(cc1F)C(=O)NS(C)(=O)=O Show InChI InChI=1S/C17H17ClF2N2O4S/c1-9(2)8-26-17-13(18)4-10(7-21-17)11-5-15(20)12(6-14(11)19)16(23)22-27(3,24)25/h4-7,9H,8H2,1-3H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533553

(CHEMBL4445237)Show SMILES CS(=O)(=O)NC(=O)c1cc(F)c(cc1F)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H9Cl2F2NO3S/c1-23(21,22)19-14(20)9-6-12(17)8(5-13(9)18)7-2-3-10(15)11(16)4-7/h2-6H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM329203
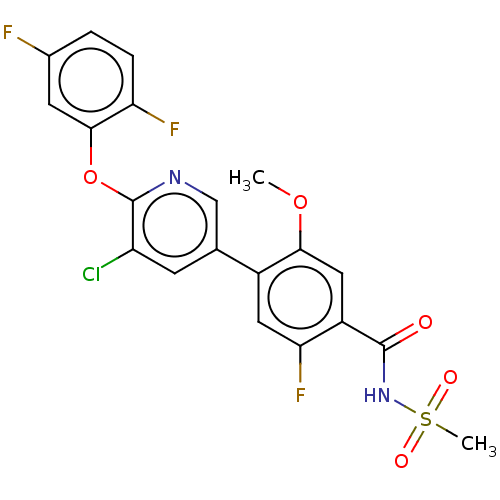
(4-(5-Chloro-6-((1-Methylcyclopropyl)Methoxy)Pyridi...)Show SMILES COc1cc(C(=O)NS(C)(=O)=O)c(F)cc1-c1cnc(Oc2cc(F)ccc2F)c(Cl)c1 Show InChI InChI=1S/C20H14ClF3N2O5S/c1-30-17-8-13(19(27)26-32(2,28)29)16(24)7-12(17)10-5-14(21)20(25-9-10)31-18-6-11(22)3-4-15(18)23/h3-9H,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533549
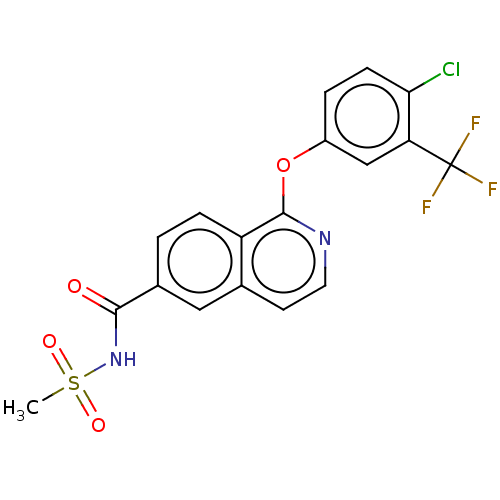
(CHEMBL4579742)Show SMILES CS(=O)(=O)NC(=O)c1ccc2c(Oc3ccc(Cl)c(c3)C(F)(F)F)nccc2c1 Show InChI InChI=1S/C18H12ClF3N2O4S/c1-29(26,27)24-16(25)11-2-4-13-10(8-11)6-7-23-17(13)28-12-3-5-15(19)14(9-12)18(20,21)22/h2-9H,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533546

(CHEMBL4462738)Show SMILES CC(C)COc1ncc(Oc2nccc3cc(ccc23)C(=O)NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C20H20ClN3O5S/c1-12(2)11-28-20-17(21)9-15(10-23-20)29-19-16-5-4-14(8-13(16)6-7-22-19)18(25)24-30(3,26)27/h4-10,12H,11H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533552
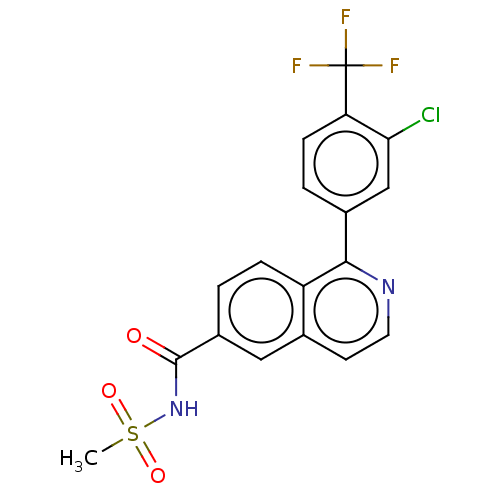
(CHEMBL4450471)Show SMILES CS(=O)(=O)NC(=O)c1ccc2c(nccc2c1)-c1ccc(c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C18H12ClF3N2O3S/c1-28(26,27)24-17(25)12-2-4-13-10(8-12)6-7-23-16(13)11-3-5-14(15(19)9-11)18(20,21)22/h2-9H,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50533541
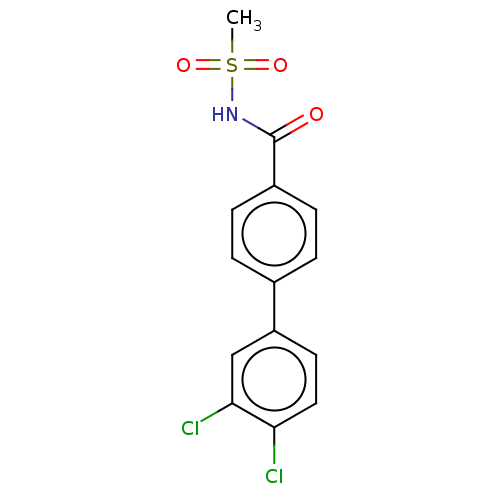
(CHEMBL4559824)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H11Cl2NO3S/c1-21(19,20)17-14(18)10-4-2-9(3-5-10)11-6-7-12(15)13(16)8-11/h2-8H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting analysis |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217933

(US9212182, 674)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16F3N3O5S/c1-35-23-12-17(14-2-5-18(26)20(28)11-14)19(27)13-22(23)31-21-6-4-16(10-15(21)3-7-25(31)32)37(33,34)30-24-8-9-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217798
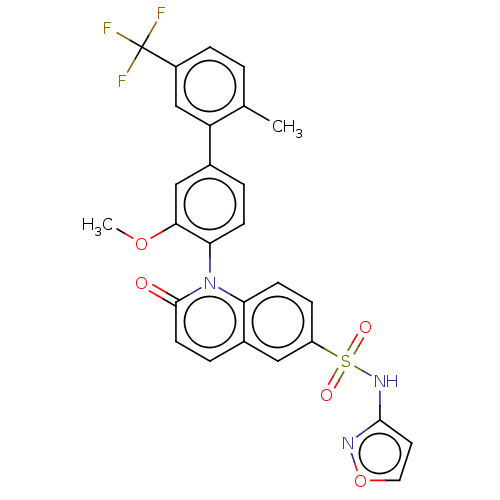
(US9212182, 1053)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(ccc1C)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-9.24,;,-12.32,;-1.33,-13.09,;.77,-13.65,;-.77,-10.99,)| Show InChI InChI=1S/C27H20F3N3O5S/c1-16-3-6-19(27(28,29)30)15-21(16)17-4-8-23(24(14-17)37-2)33-22-9-7-20(13-18(22)5-10-26(33)34)39(35,36)32-25-11-12-38-31-25/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217696
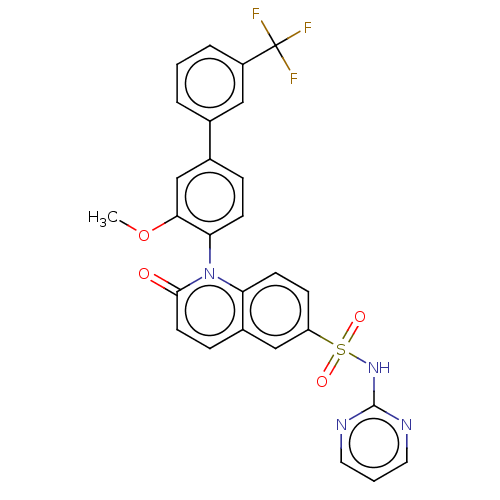
(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217799
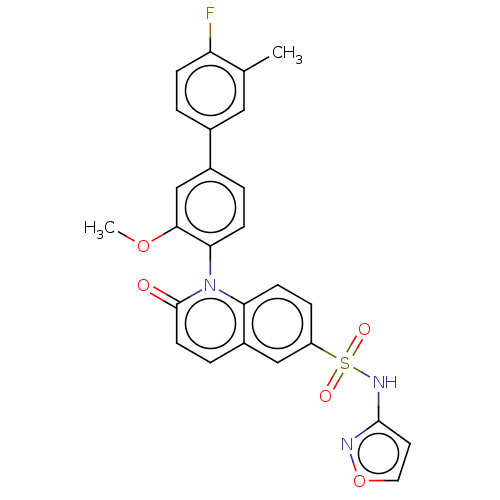
(US9212182, 1054)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H20FN3O5S/c1-16-13-17(3-7-21(16)27)18-4-8-23(24(15-18)34-2)30-22-9-6-20(14-19(22)5-10-26(30)31)36(32,33)29-25-11-12-35-28-25/h3-15H,1-2H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444954

(6-((2,4'-difluoro-5-methoxy-3'-(trifluoromethoxy)-...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1cccnn1)c1cc(F)c(cc1OC)-c1ccc(F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C25H19F5N6O5S/c1-31-24(37)36(23-8-6-15(13-32-23)42(38,39)35-22-4-3-9-33-34-22)19-12-18(27)16(11-21(19)40-2)14-5-7-17(26)20(10-14)41-25(28,29)30/h3-13H,1-2H3,(H,31,37)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217725
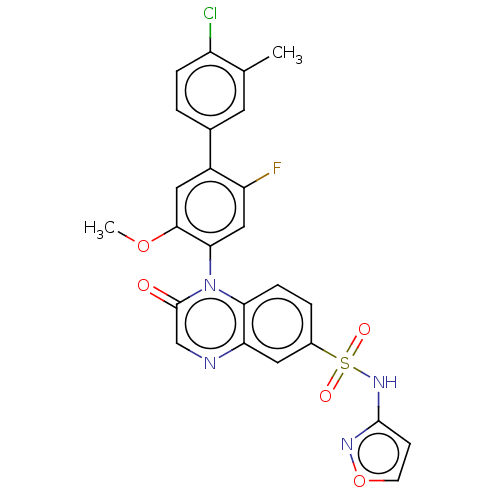
(US9212182, 652 | US9212182, 653 | US9212182, 654)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ncc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H18ClFN4O5S/c1-14-9-15(3-5-18(14)26)17-11-23(35-2)22(12-19(17)27)31-21-6-4-16(10-20(21)28-13-25(31)32)37(33,34)30-24-7-8-36-29-24/h3-13H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217447
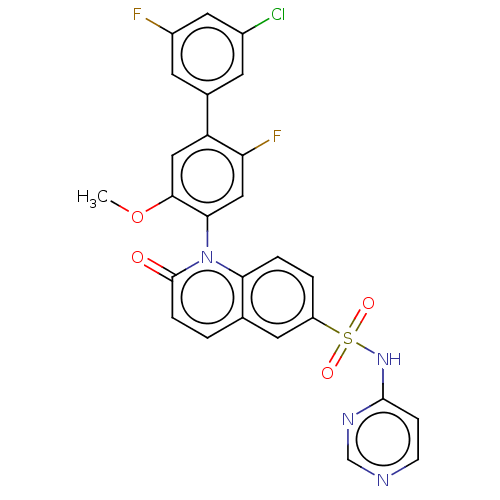
(US9212182, 540 | US9212182, 541)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H17ClF2N4O4S/c1-37-24-12-20(16-8-17(27)11-18(28)9-16)21(29)13-23(24)33-22-4-3-19(10-15(22)2-5-26(33)34)38(35,36)32-25-6-7-30-14-31-25/h2-14H,1H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444965

(6-((1-(2-chloro-5-methoxy-3'-(trifluoromethyl)-[1,...)Show SMILES COc1cc(c(Cl)cc1N(C(N)=O)c1ccc(cn1)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H17ClF3N5O5S/c1-36-19-10-16(13-3-2-4-14(9-13)23(25,26)27)17(24)11-18(19)32(22(28)33)21-6-5-15(12-29-21)38(34,35)31-20-7-8-37-30-20/h2-12H,1H3,(H2,28,33)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217544

(US9212182, 508 | US9212182, 509)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(c1)C#N |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,)| Show InChI InChI=1S/C26H16ClFN4O5S/c1-36-24-12-19(15-2-5-20(27)17(10-15)14-29)21(28)13-23(24)32-22-6-4-18(11-16(22)3-7-26(32)33)38(34,35)31-25-8-9-37-30-25/h2-13H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444937
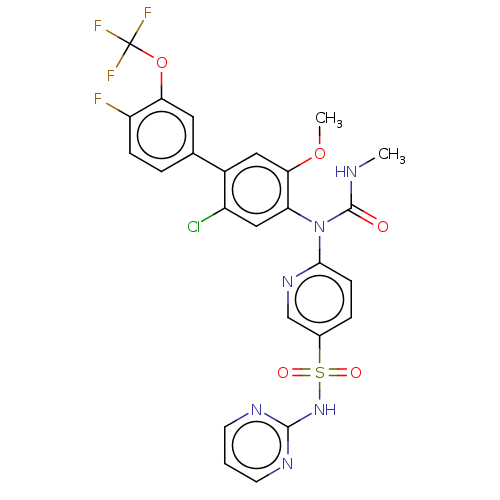
(6-((2-chloro-4'-fluoro-5-methoxy-3'-(trifluorometh...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ncccn1)c1cc(Cl)c(cc1OC)-c1ccc(F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C25H19ClF4N6O5S/c1-31-24(37)36(22-7-5-15(13-34-22)42(38,39)35-23-32-8-3-9-33-23)19-12-17(26)16(11-21(19)40-2)14-4-6-18(27)20(10-14)41-25(28,29)30/h3-13H,1-2H3,(H,31,37)(H,32,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217465
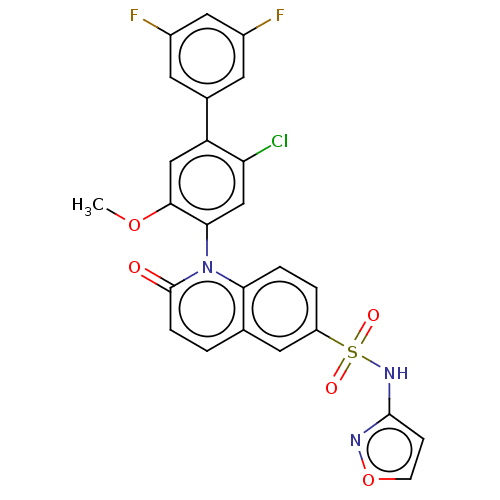
(US9212182, 457 | US9212182, 458)Show SMILES COc1cc(c(Cl)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(27)11-17(28)9-15)20(26)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217422

(US9212182, 419 | US9212182, 420)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;6.1,-10.99,;4.56,-13.65,)| Show InChI InChI=1S/C27H20F3N3O5S/c1-16-12-23(24(37-2)15-21(16)17-4-3-5-19(13-17)27(28,29)30)33-22-8-7-20(14-18(22)6-9-26(33)34)39(35,36)32-25-10-11-38-31-25/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217656
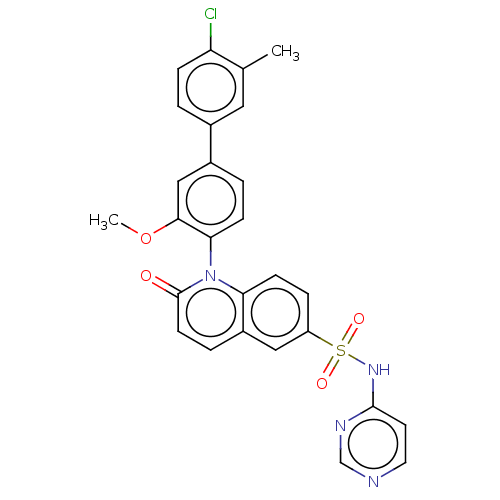
(US9212182, 658)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H21ClN4O4S/c1-17-13-18(3-7-22(17)28)19-4-8-24(25(15-19)36-2)32-23-9-6-21(14-20(23)5-10-27(32)33)37(34,35)31-26-11-12-29-16-30-26/h3-16H,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217455
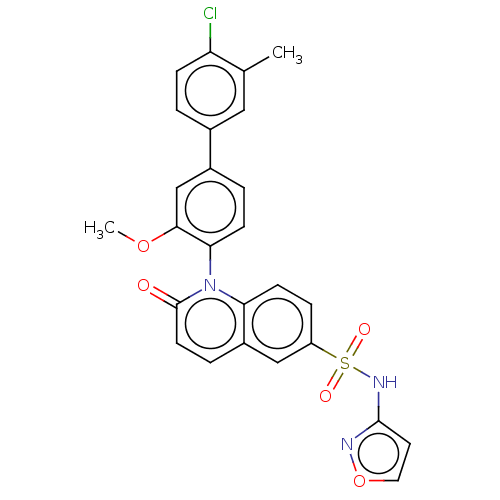
(US9212182, 431)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H20ClN3O5S/c1-16-13-17(3-7-21(16)27)18-4-8-23(24(15-18)34-2)30-22-9-6-20(14-19(22)5-10-26(30)31)36(32,33)29-25-11-12-35-28-25/h3-15H,1-2H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217481
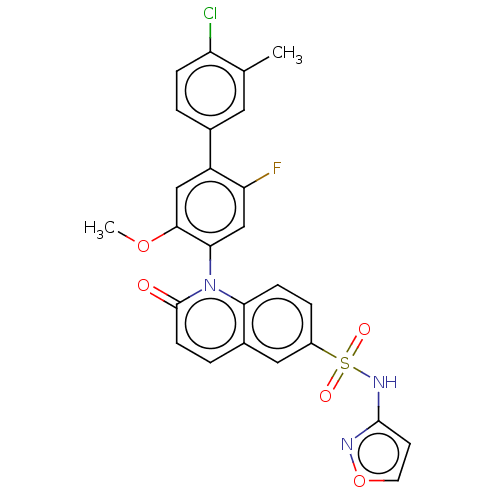
(US9212182, 469 | US9212182, 470)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H19ClFN3O5S/c1-15-11-16(3-6-20(15)27)19-13-24(35-2)23(14-21(19)28)31-22-7-5-18(12-17(22)4-8-26(31)32)37(33,34)30-25-9-10-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217481

(US9212182, 469 | US9212182, 470)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H19ClFN3O5S/c1-15-11-16(3-6-20(15)27)19-13-24(35-2)23(14-21(19)28)31-22-7-5-18(12-17(22)4-8-26(31)32)37(33,34)30-25-9-10-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217548
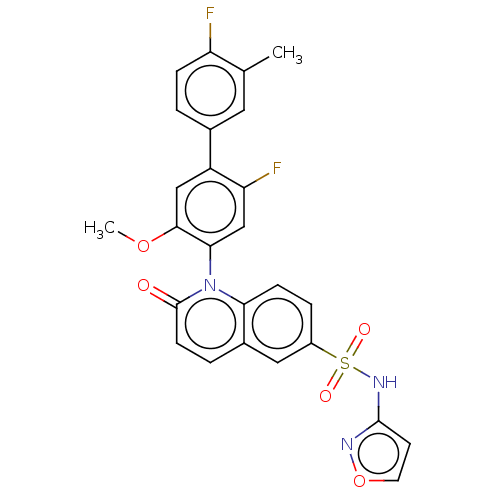
(US9212182, 512 | US9212182, 513)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H19F2N3O5S/c1-15-11-16(3-6-20(15)27)19-13-24(35-2)23(14-21(19)28)31-22-7-5-18(12-17(22)4-8-26(31)32)37(33,34)30-25-9-10-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444876
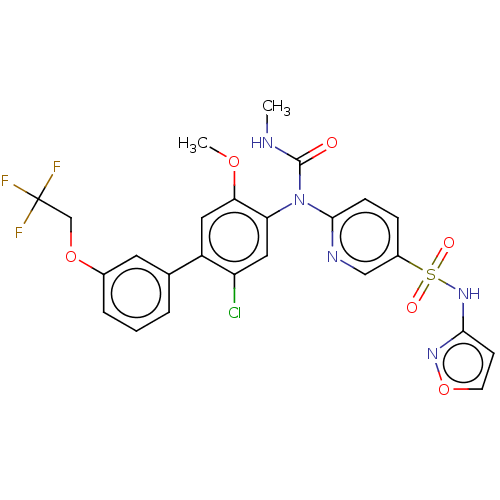
(6-((2-chloro-5-methoxy-3'-(2,2,2-trifluoroethoxy)-...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ccon1)c1cc(Cl)c(cc1OC)-c1cccc(OCC(F)(F)F)c1 Show InChI InChI=1S/C25H21ClF3N5O6S/c1-30-24(35)34(23-7-6-17(13-31-23)41(36,37)33-22-8-9-40-32-22)20-12-19(26)18(11-21(20)38-2)15-4-3-5-16(10-15)39-14-25(27,28)29/h3-13H,14H2,1-2H3,(H,30,35)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444875

(6-((2-chloro-3',5-dimethoxy-5'-(trifluoromethyl)-4...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ccon1)c1cc(Cl)c(cc1OC)-c1cc(OC)cc(c1)C(F)(F)F Show InChI InChI=1S/C25H21ClF3N5O6S/c1-30-24(35)34(23-5-4-17(13-31-23)41(36,37)33-22-6-7-40-32-22)20-12-19(26)18(11-21(20)39-3)14-8-15(25(27,28)29)10-16(9-14)38-2/h4-13H,1-3H3,(H,30,35)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217457
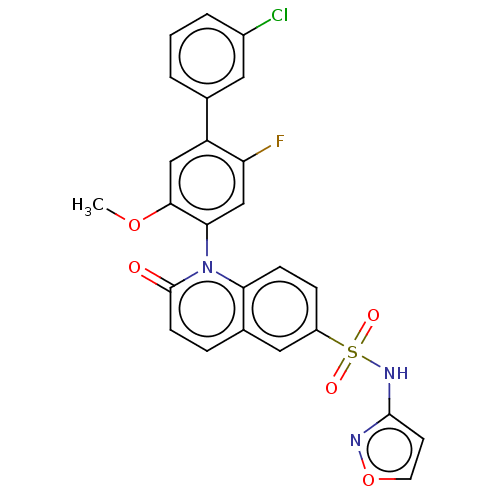
(US9212182, 475 | US9212182, 476)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H17ClFN3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50237989

(CHEMBL4084372)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(45.59,-10.99,;44.26,-11.77,;44.27,-13.31,;45.6,-14.07,;45.61,-15.62,;44.28,-16.39,;44.29,-17.93,;42.95,-15.63,;42.94,-14.09,;41.61,-13.33,;41.6,-11.78,;42.92,-11.01,;42.92,-9.48,;41.58,-8.72,;40.26,-9.49,;40.27,-11.01,;38.94,-11.78,;38.94,-13.33,;40.27,-14.1,;40.27,-15.64,;41.57,-7.17,;40.08,-6.77,;41.17,-5.68,;42.9,-6.4,;42.9,-4.86,;41.65,-3.96,;42.13,-2.49,;43.67,-2.49,;44.15,-3.96,;46.95,-16.38,;46.96,-17.92,;48.29,-18.69,;49.62,-17.91,;50.96,-18.67,;49.61,-16.36,;50.94,-15.58,;48.27,-15.61,)| Show InChI InChI=1S/C27H22FN3O5S/c1-16-13-24(25(35-3)15-21(16)18-4-7-22(28)17(2)12-18)31-23-8-6-20(14-19(23)5-9-27(31)32)37(33,34)30-26-10-11-36-29-26/h4-15H,1-3H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217758

(US9212182, 696)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H20ClFN4O4S/c1-16-12-17(4-7-21(16)28)20-14-25(37-2)24(15-22(20)29)33-23-8-6-19(13-18(23)5-9-26(33)34)38(35,36)32-27-30-10-3-11-31-27/h3-15H,1-2H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444897

(6-((2-chloro-4'-fluoro-5-methoxy-3'-(trifluorometh...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ccon1)c1cc(Cl)c(cc1OC)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C24H18ClF4N5O5S/c1-30-23(35)34(22-6-4-14(12-31-22)40(36,37)33-21-7-8-39-32-21)19-11-17(25)15(10-20(19)38-2)13-3-5-18(26)16(9-13)24(27,28)29/h3-12H,1-2H3,(H,30,35)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217829
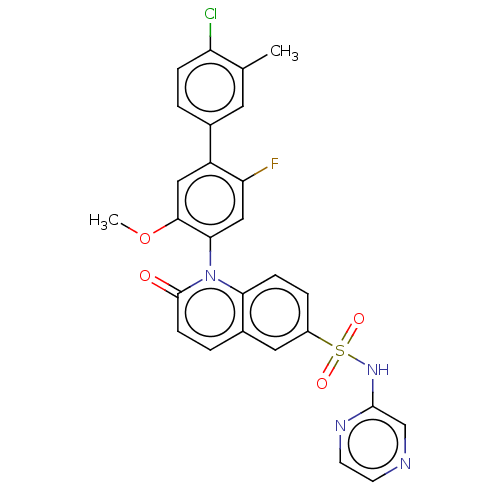
(US9212182, 691)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1cnccn1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H20ClFN4O4S/c1-16-11-17(3-6-21(16)28)20-13-25(37-2)24(14-22(20)29)33-23-7-5-19(12-18(23)4-8-27(33)34)38(35,36)32-26-15-30-9-10-31-26/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM329203

(4-(5-Chloro-6-((1-Methylcyclopropyl)Methoxy)Pyridi...)Show SMILES COc1cc(C(=O)NS(C)(=O)=O)c(F)cc1-c1cnc(Oc2cc(F)ccc2F)c(Cl)c1 Show InChI InChI=1S/C20H14ClF3N2O5S/c1-30-17-8-13(19(27)26-32(2,28)29)16(24)7-12(17)10-5-14(21)20(25-9-10)31-18-6-11(22)3-4-15(18)23/h3-9H,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-6,6-fused heteroaryl-sulfonamide derivative from human Nav1.7 expressed in HEK293 cell membranes preincubated for 30 mins follow... |
J Med Chem 59: 7818-39 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00425
BindingDB Entry DOI: 10.7270/Q25Q50KX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444870
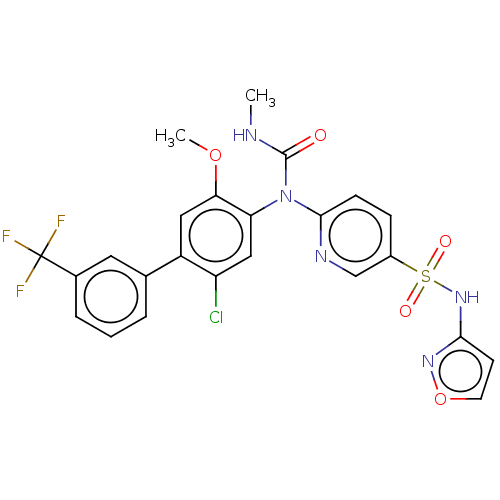
(6-((2-chloro-5-methoxy-3'-(trifluoromethyl)-4- | U...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ccon1)c1cc(Cl)c(cc1OC)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19ClF3N5O5S/c1-29-23(34)33(22-7-6-16(13-30-22)39(35,36)32-21-8-9-38-31-21)19-12-18(25)17(11-20(19)37-2)14-4-3-5-15(10-14)24(26,27)28/h3-13H,1-2H3,(H,29,34)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM342904
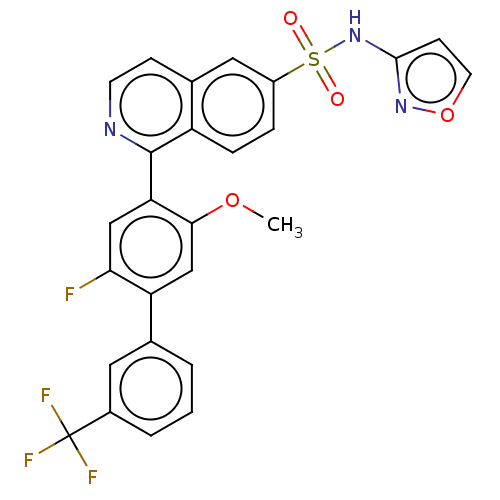
(1-(2-fluoro-5-methoxy-3'-(trifluoromethyl)-4-biphe...)Show SMILES COc1cc(c(F)cc1-c1nccc2cc(ccc12)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C26H17F4N3O4S/c1-36-23-14-20(15-3-2-4-17(11-15)26(28,29)30)22(27)13-21(23)25-19-6-5-18(12-16(19)7-9-31-25)38(34,35)33-24-8-10-37-32-24/h2-14H,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells assessed as reduction in peak inward current by ionworks quattro electrophysiology assay |
J Med Chem 60: 5969-5989 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01851
BindingDB Entry DOI: 10.7270/Q2RJ4MS7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217517
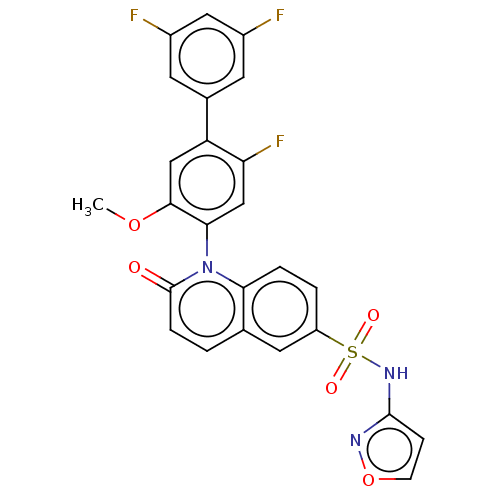
(US9212182, 363 | US9212182, 364)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C25H16F3N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217817

(US9212182, 683)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H21ClN4O4S/c1-17-14-18(4-8-22(17)28)19-5-9-24(25(16-19)36-2)32-23-10-7-21(15-20(23)6-11-26(32)33)37(34,35)31-27-29-12-3-13-30-27/h3-16H,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217889

(US9212182, 632)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(OC(F)F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;5.33,-13.86,;6.67,-14.63,;4,-14.63,;4,-10.01,)| Show InChI InChI=1S/C26H18F3N3O6S/c1-36-23-13-19(15-3-2-4-17(11-15)38-26(28)29)20(27)14-22(23)32-21-7-6-18(12-16(21)5-8-25(32)33)39(34,35)31-24-9-10-37-30-24/h2-14,26H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM444926
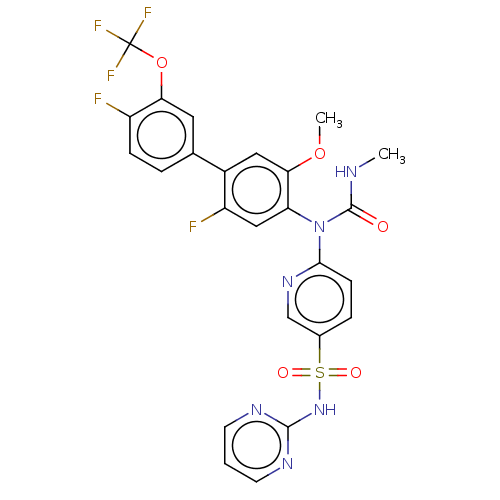
(6-((2,4'-difluoro-5-methoxy-3'-(trifluoromethoxy)-...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ncccn1)c1cc(F)c(cc1OC)-c1ccc(F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C25H19F5N6O5S/c1-31-24(37)36(22-7-5-15(13-34-22)42(38,39)35-23-32-8-3-9-33-23)19-12-18(27)16(11-21(19)40-2)14-4-6-17(26)20(10-14)41-25(28,29)30/h3-13H,1-2H3,(H,31,37)(H,32,33,35) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444926

(6-((2,4'-difluoro-5-methoxy-3'-(trifluoromethoxy)-...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ncccn1)c1cc(F)c(cc1OC)-c1ccc(F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C25H19F5N6O5S/c1-31-24(37)36(22-7-5-15(13-34-22)42(38,39)35-23-32-8-3-9-33-23)19-12-18(27)16(11-21(19)40-2)14-4-6-17(26)20(10-14)41-25(28,29)30/h3-13H,1-2H3,(H,31,37)(H,32,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217872

(US9212182, 673)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(cc1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;2.67,-12.32,;1.33,-11.55,;1.33,-10.01,;2.67,-13.86,;2.67,-15.4,;1.13,-13.86,;4.21,-13.86,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-2-5-17(6-3-15)26(28,29)30)20(27)14-22(23)33-21-8-7-18(12-16(21)4-9-25(33)34)39(35,36)32-24-10-11-38-31-24/h2-14H,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 1-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM444866

(6-((2,4'-difluoro-5-methoxy-3'-(trifluoromethoxy)-...)Show SMILES CNC(=O)N(c1ccc(cn1)S(=O)(=O)Nc1ccon1)c1cc(F)c(cc1OC)-c1ccc(F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C24H18F5N5O6S/c1-30-23(35)34(22-6-4-14(12-31-22)41(36,37)33-21-7-8-39-32-21)18-11-17(26)15(10-20(18)38-2)13-3-5-16(25)19(9-13)40-24(27,28)29/h3-12H,1-2H3,(H,30,35)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128892
BindingDB Entry DOI: 10.7270/Q29027RX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM231465
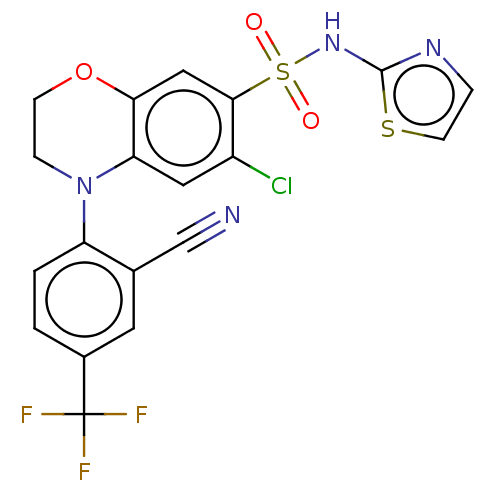
(US9346798, 212)Show SMILES FC(F)(F)c1ccc(N2CCOc3cc(c(Cl)cc23)S(=O)(=O)Nc2nccs2)c(c1)C#N Show InChI InChI=1S/C19H12ClF3N4O3S2/c20-13-8-15-16(9-17(13)32(28,29)26-18-25-3-6-31-18)30-5-4-27(15)14-2-1-12(19(21,22)23)7-11(14)10-24/h1-3,6-9H,4-5H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Amgen Inc., 360 Binney Street, Cambridge, MA 02142, United States. Electronic address: daniel.la@sagerx.com.
Curated by ChEMBL
| Assay Description
Displacement of (P)-1-(30-chloro-2-fluoro-5,50-dimethoxy-[1,10biphenyl]-4-yl)-N-(isoxazol-3-yl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide[50-methoxy-C... |
Bioorg Med Chem Lett 27: 3477-3485 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.070
BindingDB Entry DOI: 10.7270/Q2H134H4 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217696

(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM231284
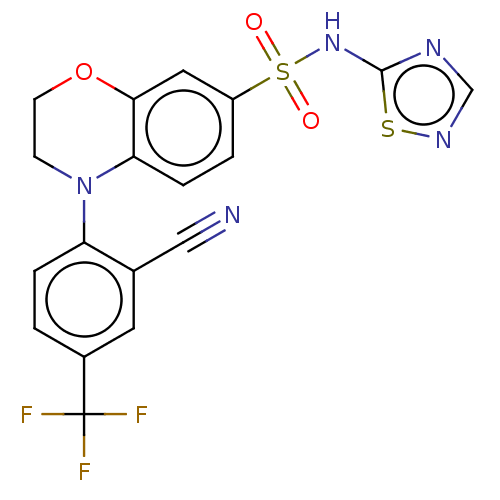
(US9346798, 31)Show SMILES FC(F)(F)c1ccc(N2CCOc3cc(ccc23)S(=O)(=O)Nc2ncns2)c(c1)C#N Show InChI InChI=1S/C18H12F3N5O3S2/c19-18(20,21)12-1-3-14(11(7-12)9-22)26-5-6-29-16-8-13(2-4-15(16)26)31(27,28)25-17-23-10-24-30-17/h1-4,7-8,10H,5-6H2,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Amgen Inc., 360 Binney Street, Cambridge, MA 02142, United States. Electronic address: daniel.la@sagerx.com.
Curated by ChEMBL
| Assay Description
Displacement of (P)-1-(30-chloro-2-fluoro-5,50-dimethoxy-[1,10biphenyl]-4-yl)-N-(isoxazol-3-yl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide[50-methoxy-C... |
Bioorg Med Chem Lett 27: 3477-3485 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.070
BindingDB Entry DOI: 10.7270/Q2H134H4 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217431
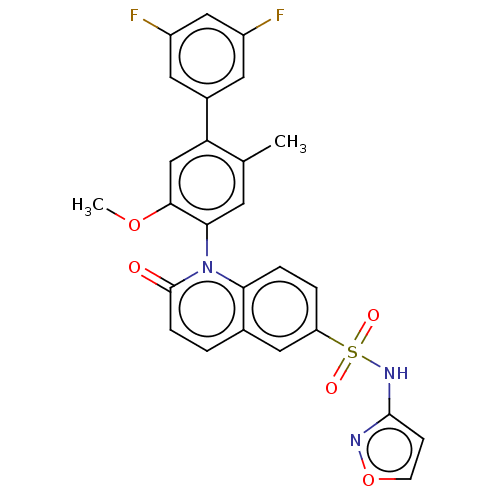
(US9212182, 423 | US9212182, 424)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C26H19F2N3O5S/c1-15-9-23(24(35-2)14-21(15)17-10-18(27)13-19(28)11-17)31-22-5-4-20(12-16(22)3-6-26(31)32)37(33,34)30-25-7-8-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM154311

(US9012443, 331)Show SMILES COc1cc(c(Cl)cc1-c1nccc2cc(ccc12)S(=O)(=O)Nc1ncns1)-c1cccc(F)c1 Show InChI InChI=1S/C24H16ClFN4O3S2/c1-33-22-12-19(14-3-2-4-16(26)9-14)21(25)11-20(22)23-18-6-5-17(10-15(18)7-8-27-23)35(31,32)30-24-28-13-29-34-24/h2-13H,1H3,(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells incubated for 3 to 5 mins at -125 mV holding potential by electrophysiology assay |
J Med Chem 60: 5969-5989 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01851
BindingDB Entry DOI: 10.7270/Q2RJ4MS7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217435

(US9212182, 323 | US9212182, 324)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;6.1,-10.99,;4.56,-13.65,)| Show InChI InChI=1S/C26H18F3N3O5S/c1-36-23-15-17(16-3-2-4-19(13-16)26(27,28)29)5-8-22(23)32-21-9-7-20(14-18(21)6-10-25(32)33)38(34,35)31-24-11-12-37-30-24/h2-15H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data