

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258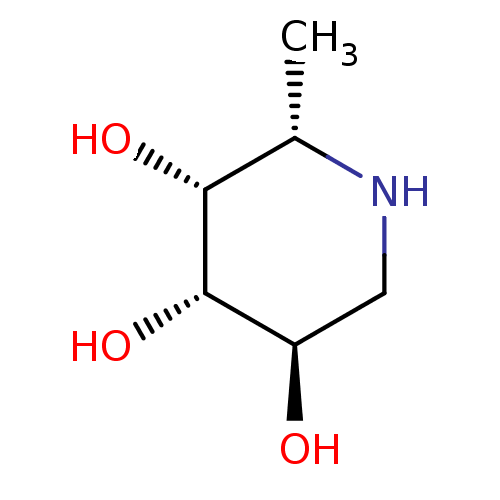 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50163439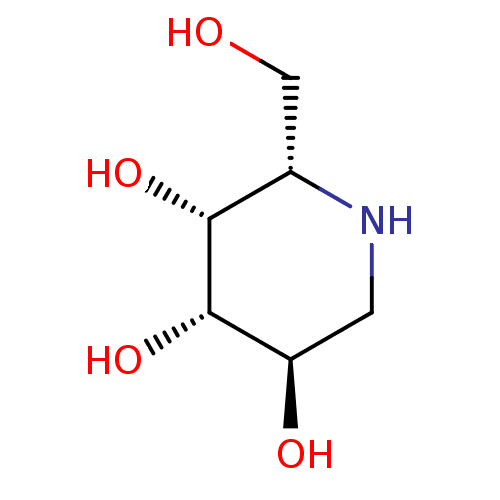 ((2S,3R,4S,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455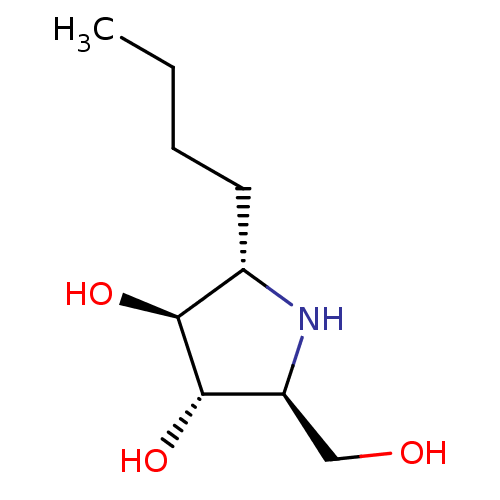 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50369362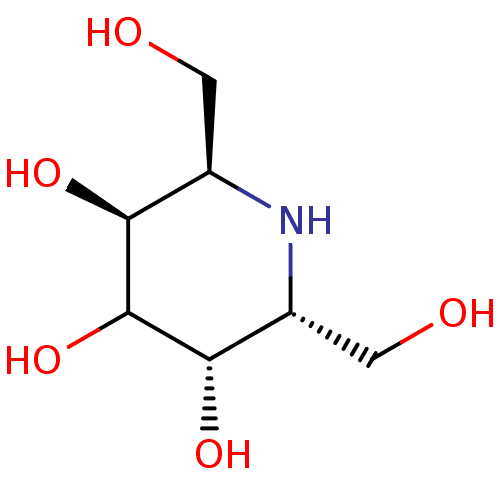 (CHEMBL1169500) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50242271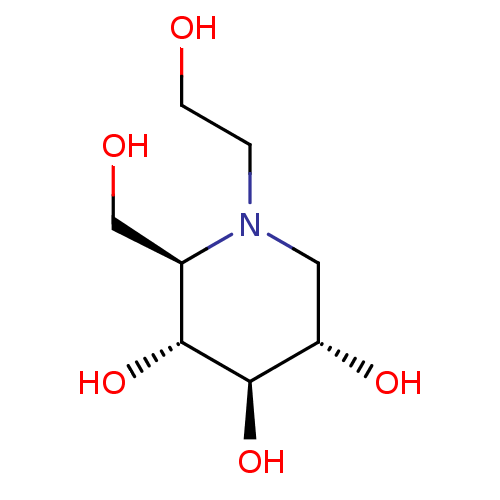 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50163446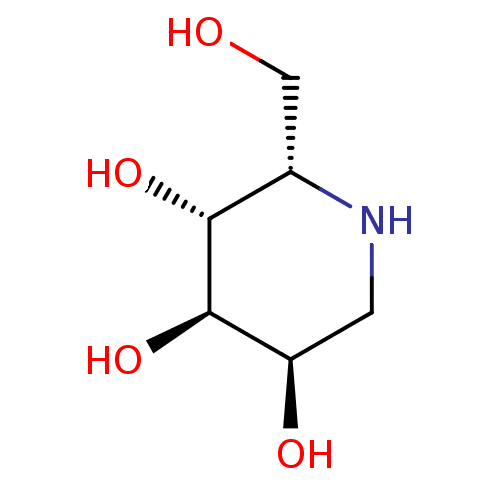 ((2S,3R,4R,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089157 ((3R,5S,7aR)-3-Heptyl-5-methyl-hexahydro-pyrrolizin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089159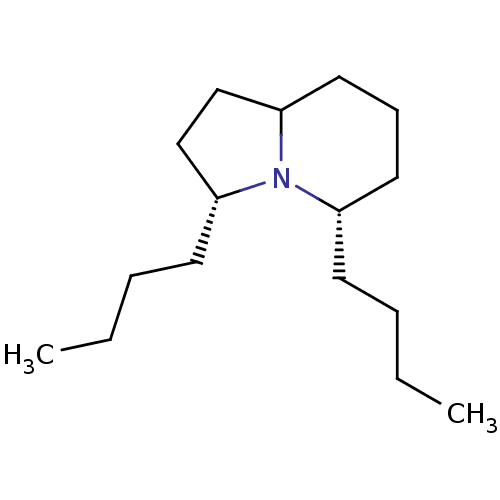 ((3R,5S)-3,5-Dibutyl-octahydro-indolizine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089158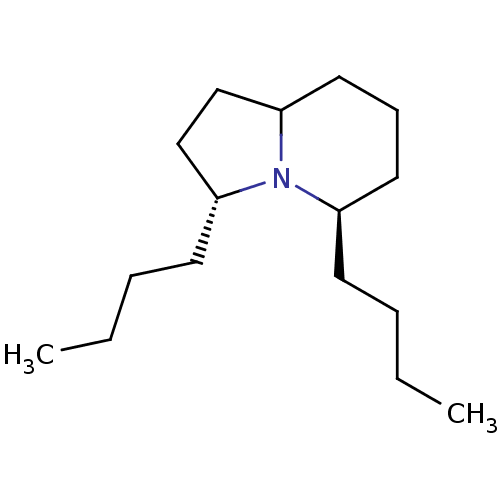 ((3R,5R)-3,5-Dibutyl-octahydro-indolizine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089162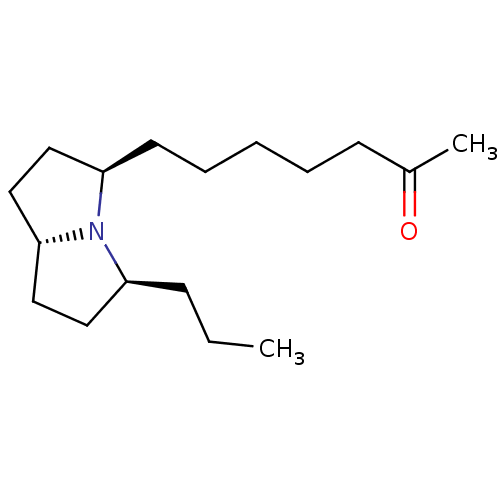 (7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089164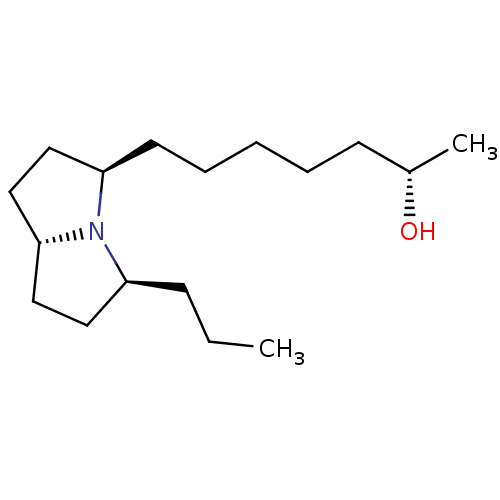 ((S)-7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089163 ((R)-7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089160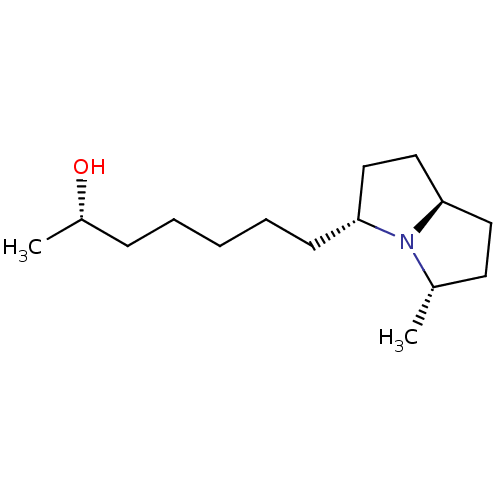 ((S)-7-((3R,5S,7aR)-5-Methyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089161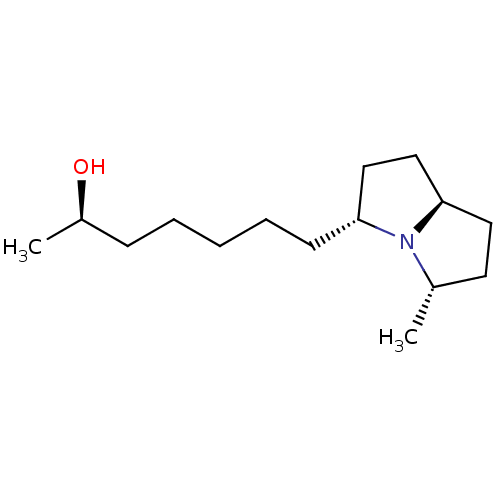 ((R)-7-((3R,5S,7aR)-5-Methyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048090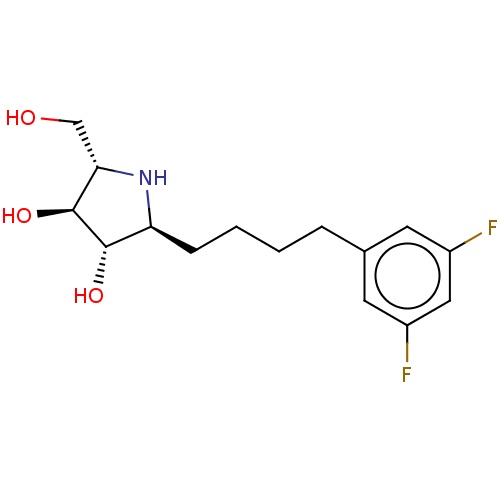 (CHEMBL3311519) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against rice alpha-glucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against human alpha-glucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048086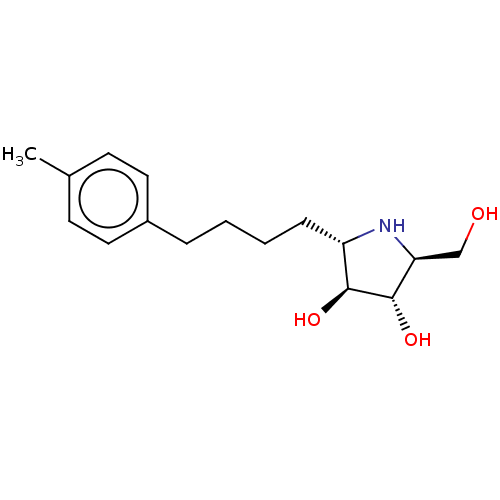 (CHEMBL3311515) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801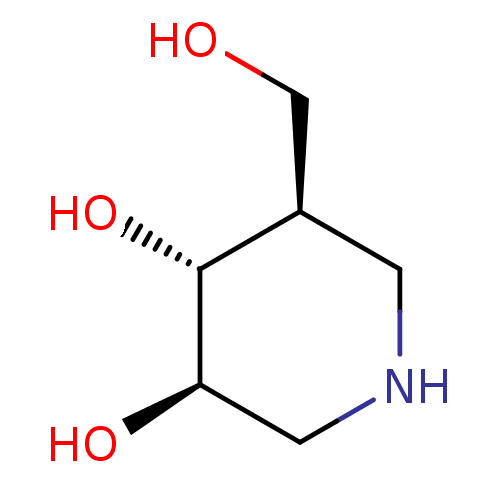 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucosidase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of maltase in human Caco-2 cell model system after 2 hrs | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human lysosome beta-glucosidase assessed as production of 4-methylumbelliferone using 4-methylumbelliferyl beta-D-glucoside as substrat... | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50263044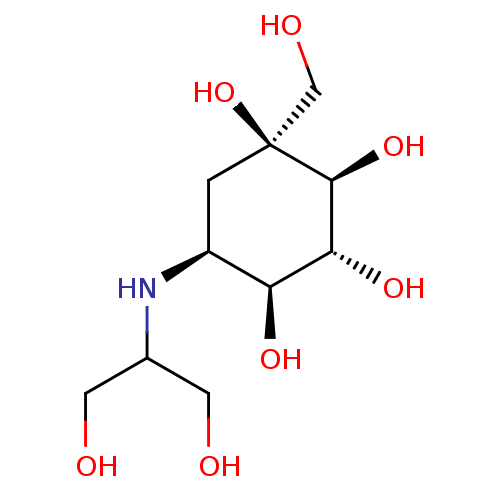 (CHEMBL476960 | Voglibose) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane sucrase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of maltase in human Caco-2 cell model system after 2 hrs | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50163440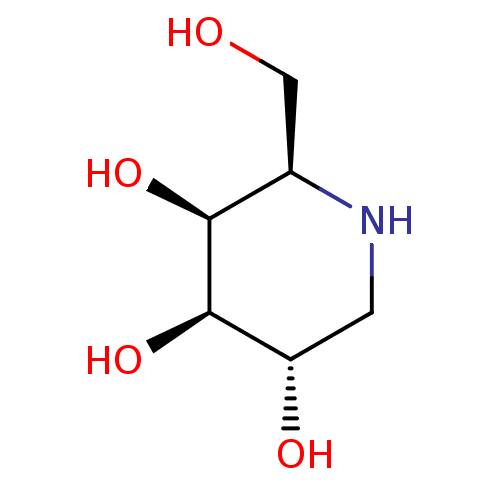 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against human alpha-galactosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50259956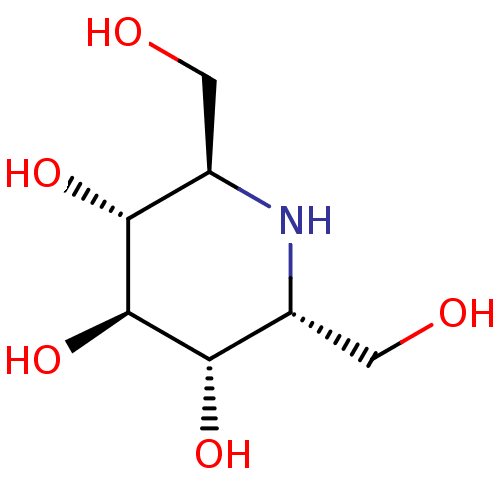 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane maltase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50259956 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of maltase in human Caco-2 cell model system after 2 hrs | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane maltase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50242271 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane sucrase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048088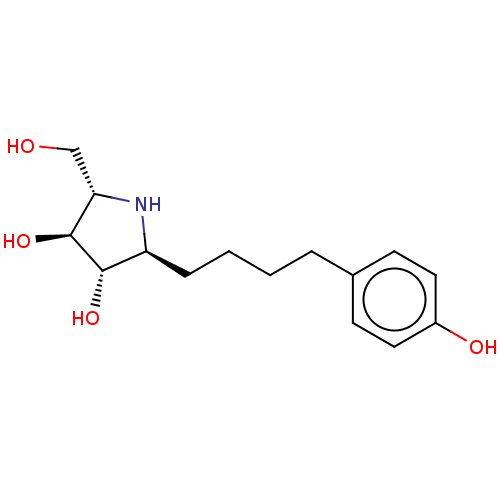 (CHEMBL3311517) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen debranching enzyme (Oryctolagus cuniculus) | BDBM50259956 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rabbit muscle amylo-1,6-glucosidase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457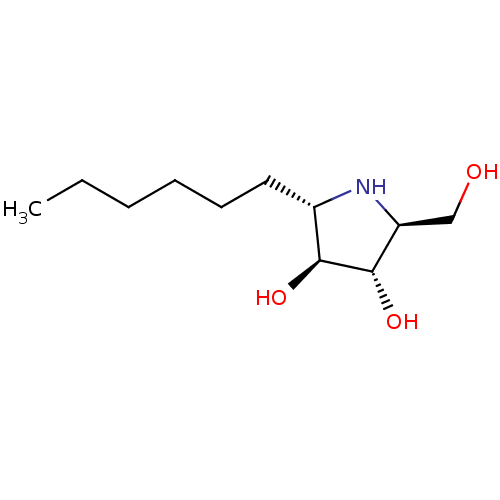 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048087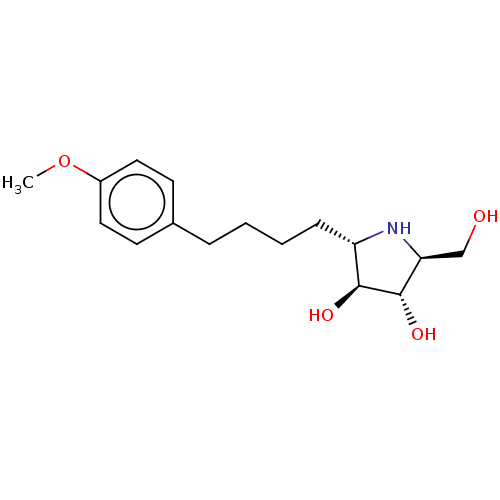 (CHEMBL3311516) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactase/phlorizin hydrolase (Rattus norvegicus) | BDBM50182801 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal lactase assessed as production of p-nitrophenol by spectrophotometry | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18363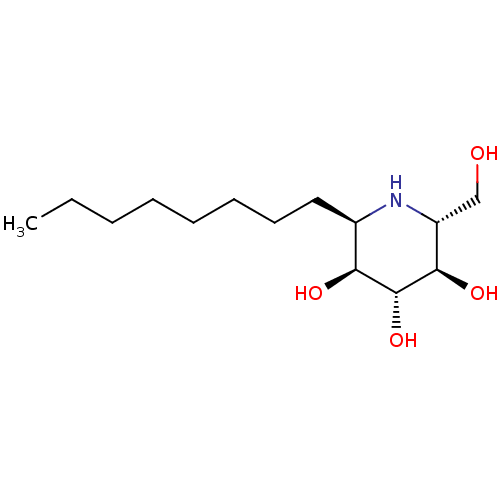 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048087 (CHEMBL3311516) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane maltase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane isomaltase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50263044 (CHEMBL476960 | Voglibose) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of rat intestinal brush border membrane isomaltase | Bioorg Med Chem 16: 7330-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.026 BindingDB Entry DOI: 10.7270/Q27D2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465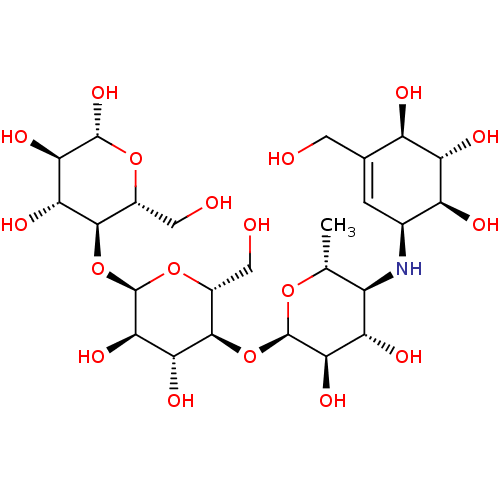 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine maltase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048088 (CHEMBL3311517) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |