

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278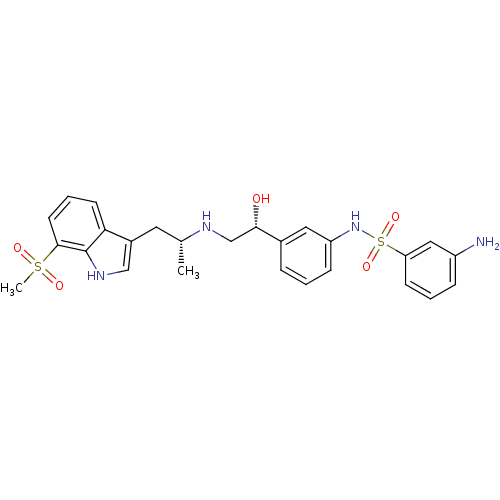 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267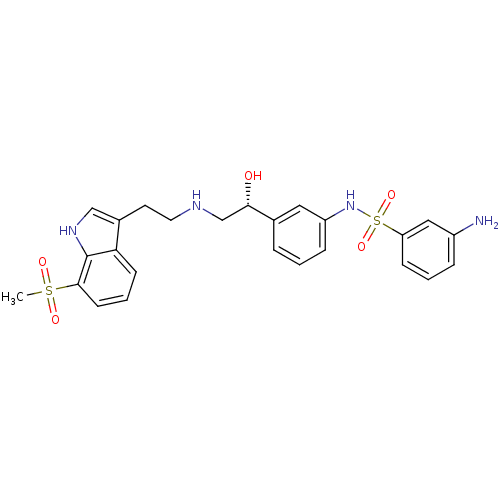 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring cAMP accumulation in CHO cells expressing cloned human Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250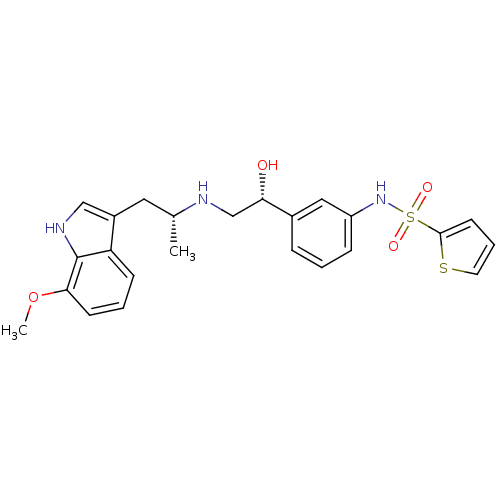 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257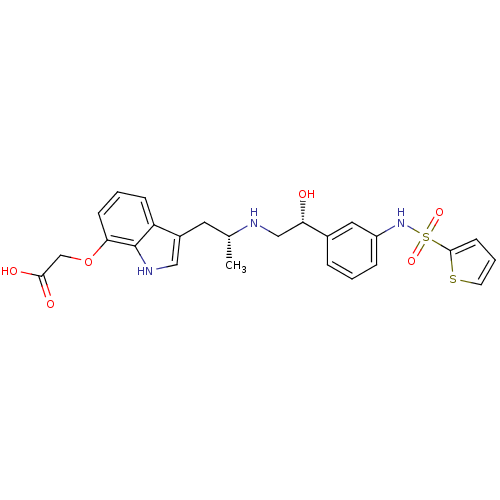 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252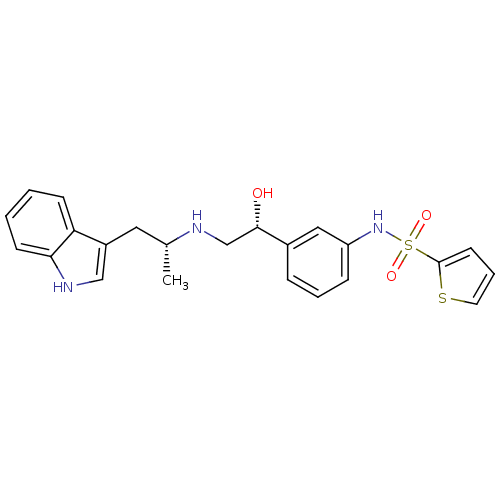 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270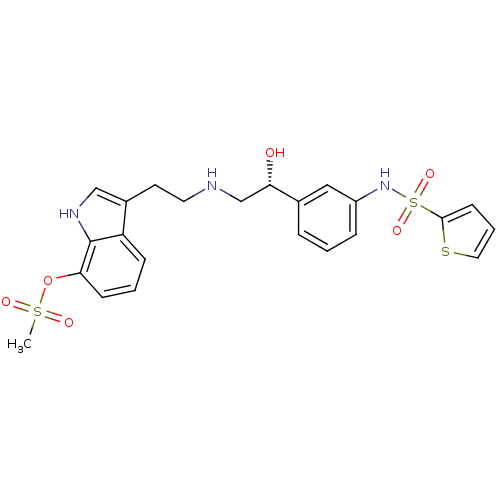 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112041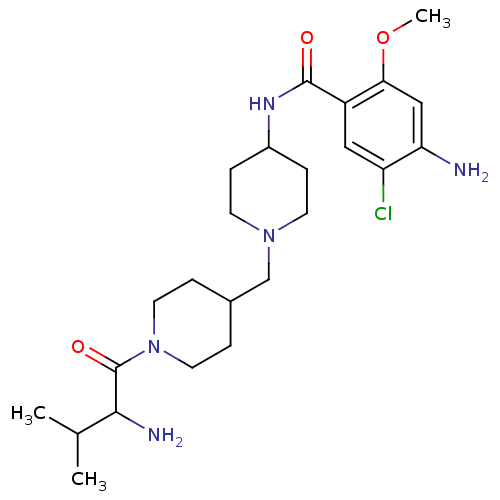 (4-Amino-N-{1-[1-(2-amino-3-methyl-butyryl)-piperid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112038 (4-Amino-N-{1-[1-(2-amino-2-methyl-propionyl)-piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112039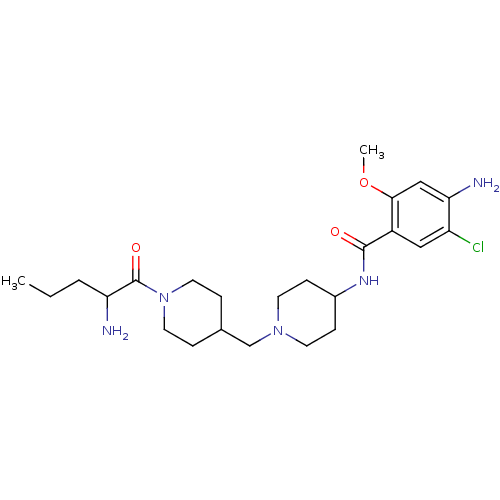 (4-Amino-N-{1-[1-(2-amino-pentanoyl)-piperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112046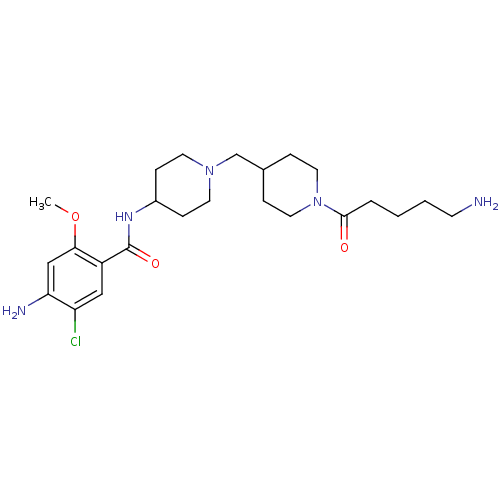 (4-Amino-N-{1-[1-(5-amino-pentanoyl)-piperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112042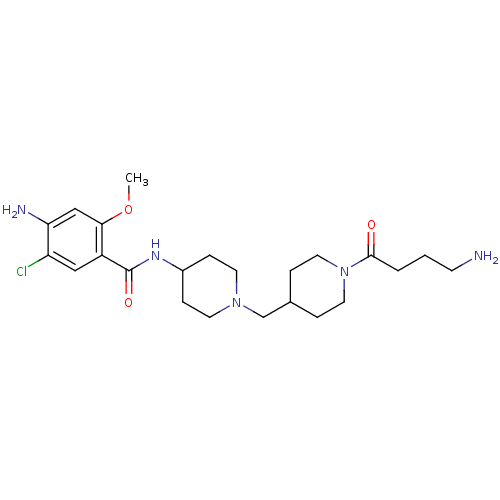 (4-Amino-N-{1-[1-(4-amino-butyryl)-piperidin-4-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112045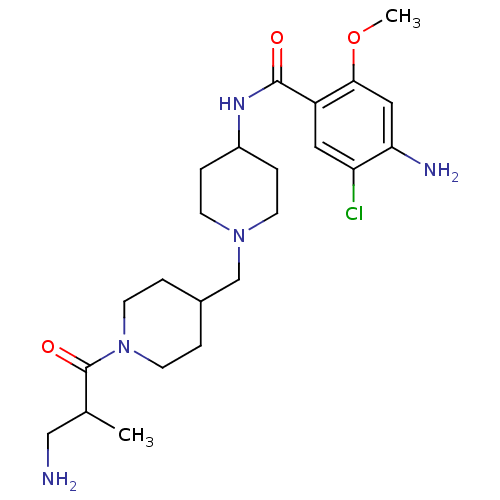 (4-Amino-N-{1-[1-(3-amino-2-methyl-propionyl)-piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112031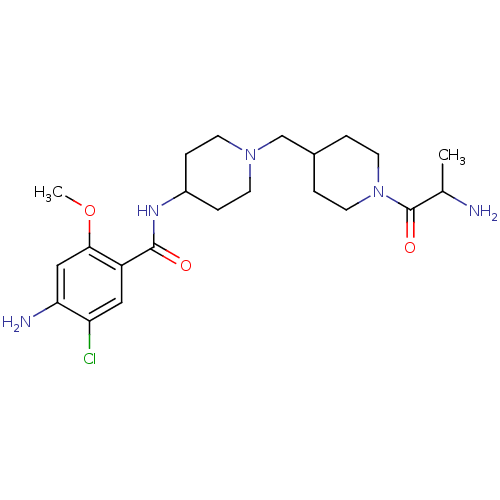 (4-Amino-N-{1-[1-(2-amino-propionyl)-piperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112029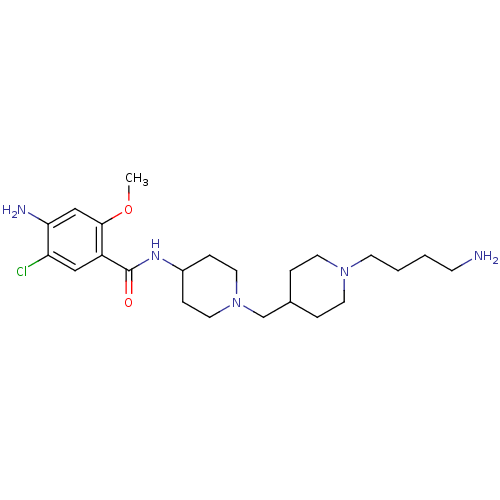 (4-Amino-N-{1-[1-(4-amino-butyl)-piperidin-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112044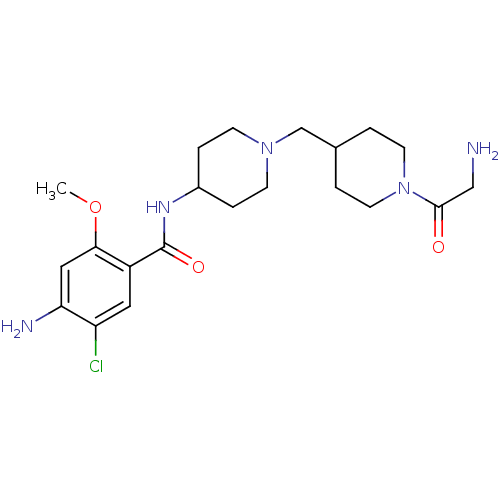 (4-Amino-N-{1-[1-(2-amino-acetyl)-piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112037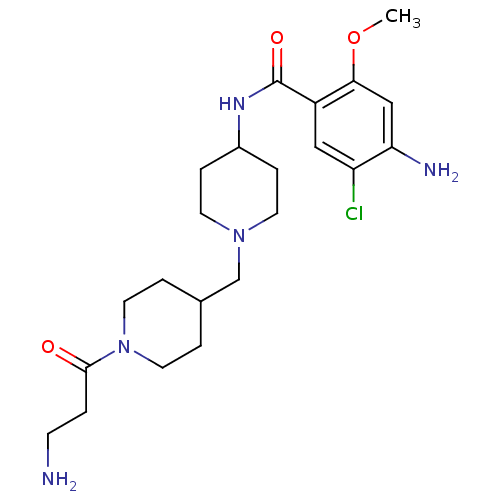 (4-Amino-N-{1-[1-(3-amino-propionyl)-piperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112033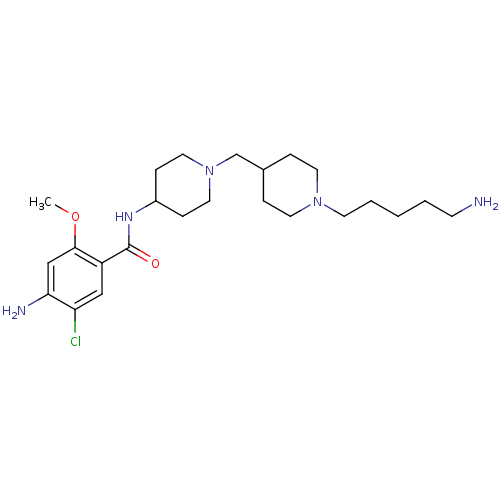 (4-Amino-N-{1-[1-(5-amino-pentyl)-piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112040 (4-Amino-N-{1-[1-(3-amino-propyl)-piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112036 (4-Amino-N-{1-[1-(2-amino-ethyl)-piperidin-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112035 (4-Amino-N-{1-[1-(3-amino-2-hydroxy-propyl)-piperid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112043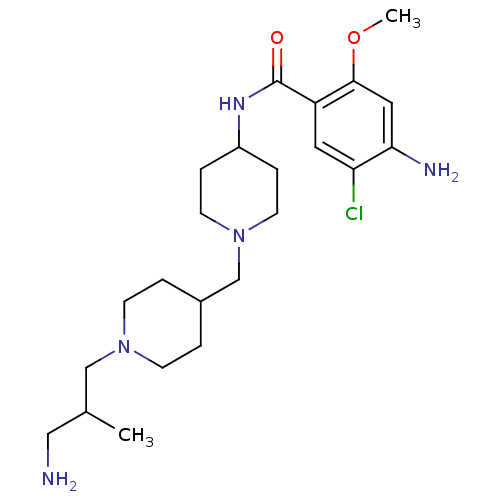 (4-Amino-N-{1-[1-(3-amino-2-methyl-propyl)-piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112032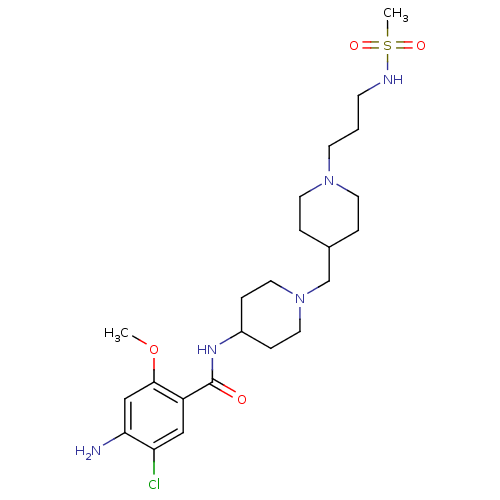 (4-Amino-5-chloro-N-{1-[1-(3-methanesulfonylamino-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112034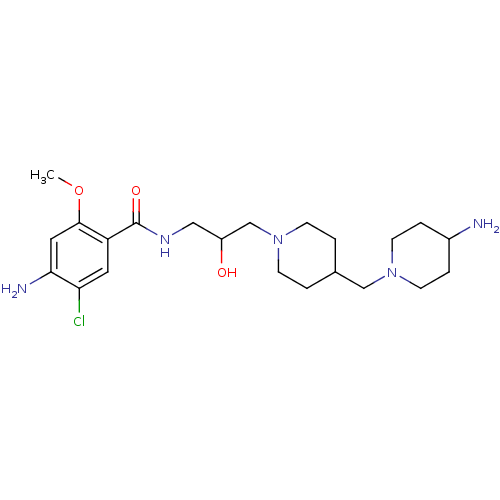 (4-Amino-N-{3-[4-(4-amino-piperidin-1-ylmethyl)-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM94630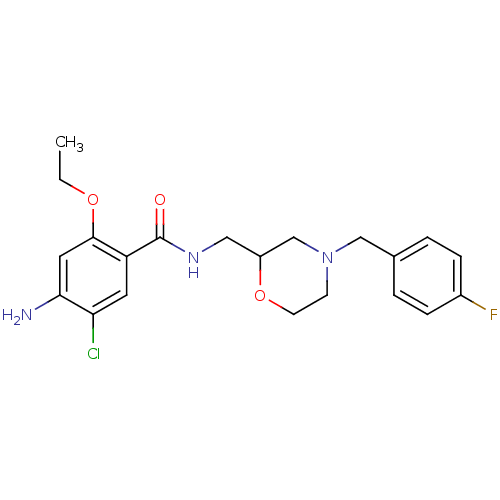 (4-Amino-5-chloro-2-ethoxy-N-[4-(4-fluoro-benzyl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50112030 (4-Amino-N-{1-[1-(4-amino-butyryl)-piperidine-4-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 4 receptor of guinea pig striatum using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 12: 967-70 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553465 (CHEMBL4779549) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553470 (CHEMBL4749343) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553471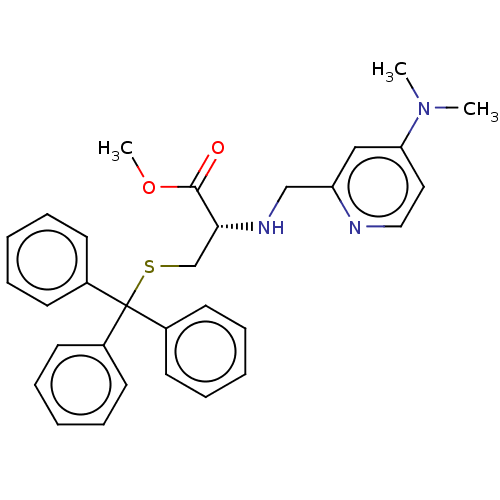 (CHEMBL4751387) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553468 (CHEMBL4743384) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM23802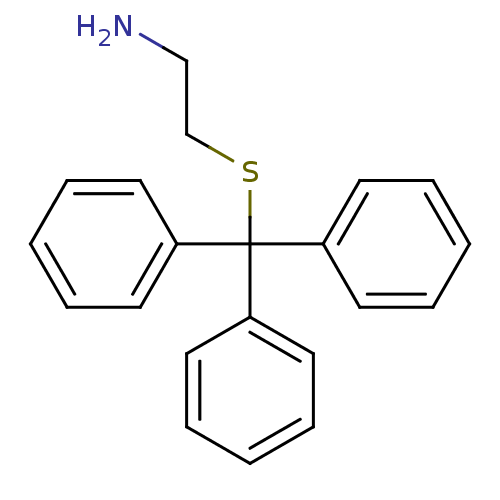 (2-[(triphenylmethyl)sulfanyl]ethan-1-amine | Triph...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553466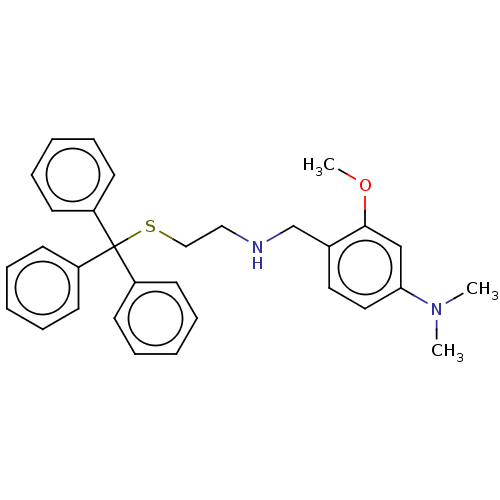 (CHEMBL4799578) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50553467 (CHEMBL4782892) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant SIRT2 (unknown origin) using FAM-labeled RHKK(Ac) LM peptide as substrate incubated for 60 mins by electrophoretic mobility... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127458 BindingDB Entry DOI: 10.7270/Q2TM7FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 160 total ) | Next | Last >> |