 Found 81 hits with Last Name = 'taylor' and Initial = 'er'
Found 81 hits with Last Name = 'taylor' and Initial = 'er' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM50143314
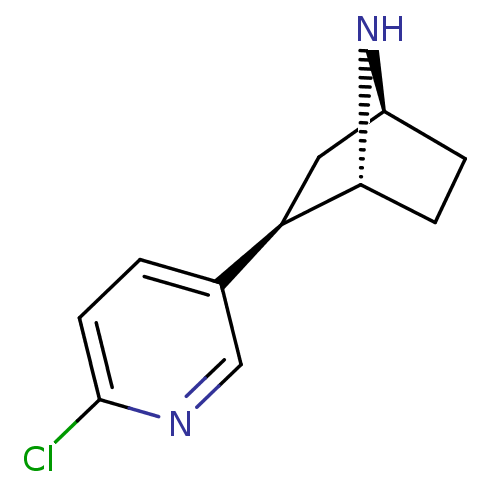
((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...)Show SMILES Clc1ccc(cn1)[C@H]1C[C@@H]2CC[C@H]1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8-,9+,10+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 7.40 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM91593
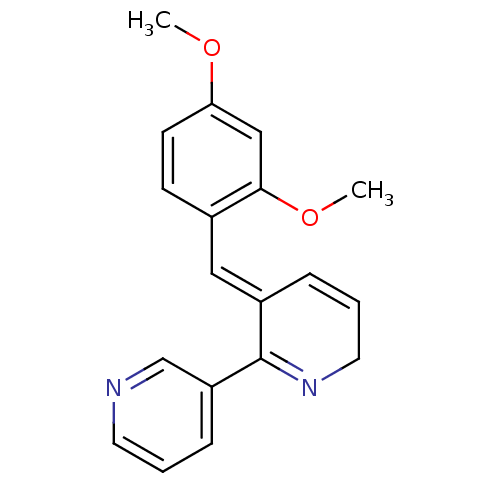
(DMXB-A)Show SMILES COc1ccc(\C=C2/C=CCN=C2c2cccnc2)c(OC)c1 |c:8,11| Show InChI InChI=1S/C19H18N2O2/c1-22-17-8-7-14(18(12-17)23-2)11-15-5-4-10-21-19(15)16-6-3-9-20-13-16/h3-9,11-13H,10H2,1-2H3/b15-11+ | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 100 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM91594
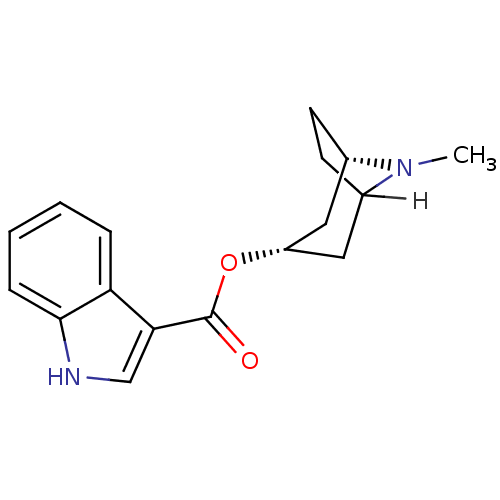
(Tropisetron)Show SMILES [H]C12CC[C@@H](C[C@H](C1)OC(=O)c1c[nH]c3ccccc13)N2C |TLB:8:6:20:2.3| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12?,13+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | 109 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM82070
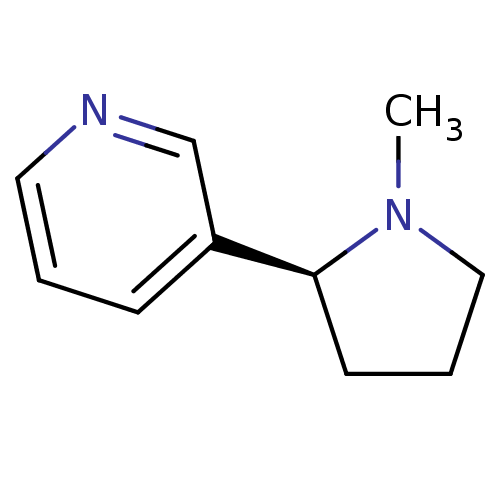
(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 280 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM50190786
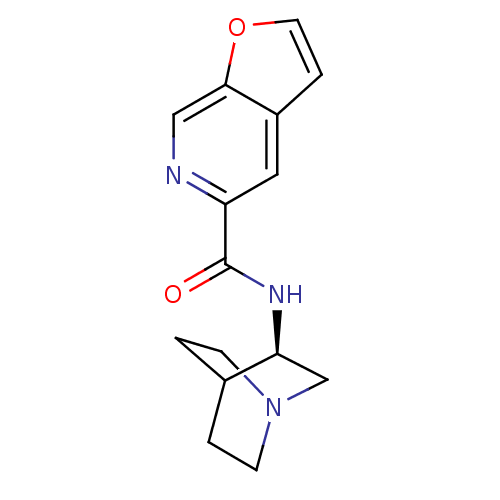
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 1.50E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM50161764
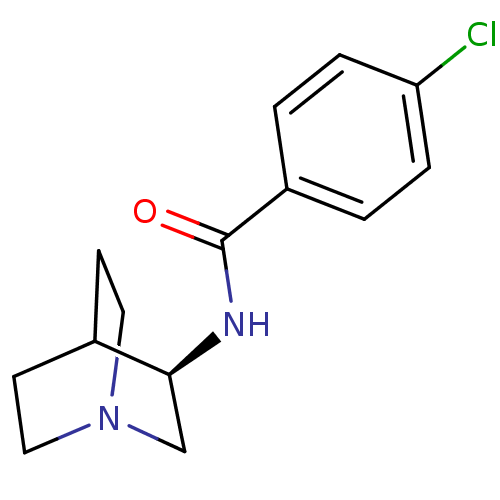
((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 3.30E+4 | -25.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM91595
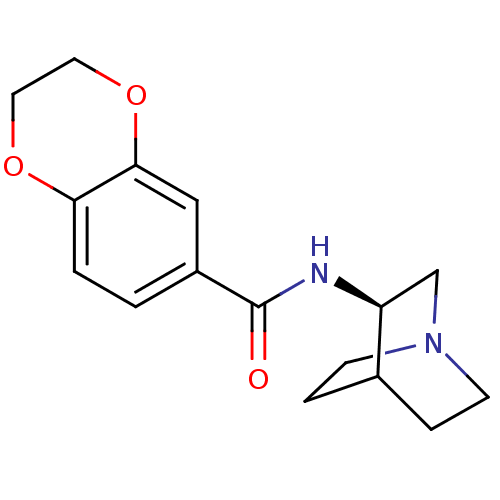
(PHA568487)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2OCCOc2c1 |wU:3.2,THB:2:3:6.7:9.10,(5.33,-4.62,;5.33,-3.08,;6.67,-2.31,;8,-3.08,;9.22,-2.29,;9.13,-3.83,;10.33,-5.04,;9.09,-5.75,;7.93,-4.59,;6.35,-5.14,;7.23,-4.3,;4,-2.31,;2.67,-3.08,;1.33,-2.31,;1.33,-.77,;;,1.54,;1.33,2.31,;2.67,1.54,;2.67,,;4,-.77,)| Show InChI InChI=1S/C16H20N2O3/c19-16(17-13-10-18-5-3-11(13)4-6-18)12-1-2-14-15(9-12)21-8-7-20-14/h1-2,9,11,13H,3-8,10H2,(H,17,19)/t13-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 3.30E+4 | -25.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego
| Assay Description
Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... |
Taylor Research Group (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM350321
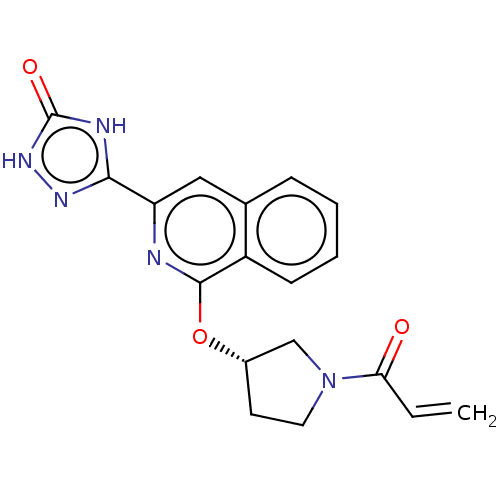
((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EGFR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ITK |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Jak3 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50097393
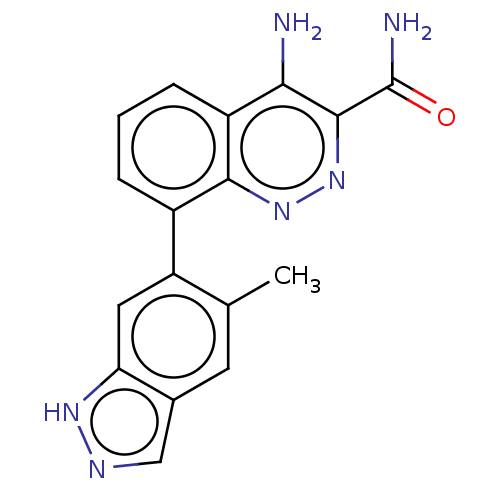
(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Btk |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK2 by LanthaScreen Binding assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580180

(CHEMBL5073653) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TNK2 by LanthaScreen Binding assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK1 by LanthaScreen Binding assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580179
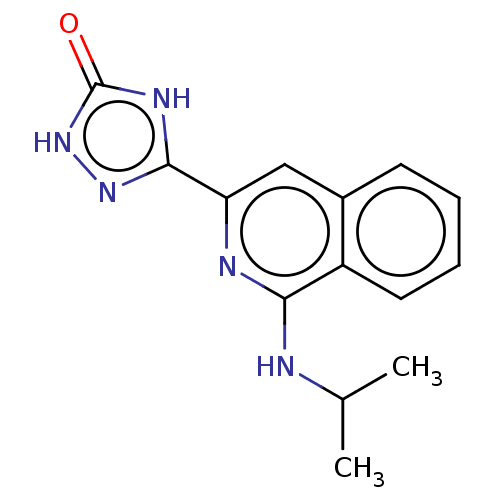
(CHEMBL5072186) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580181

(CHEMBL5083068) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580182

(CHEMBL5083885)Show SMILES CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM350318

((R)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Blk |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Bmx |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase FRK
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Frk |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Fgr |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580177
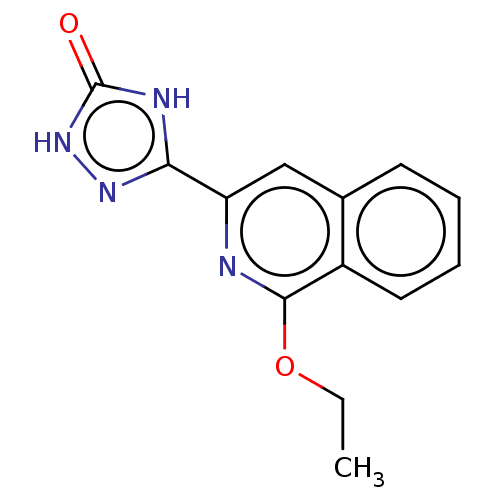
(CHEMBL5073944) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LynB |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tec |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LynA |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Hck |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580178
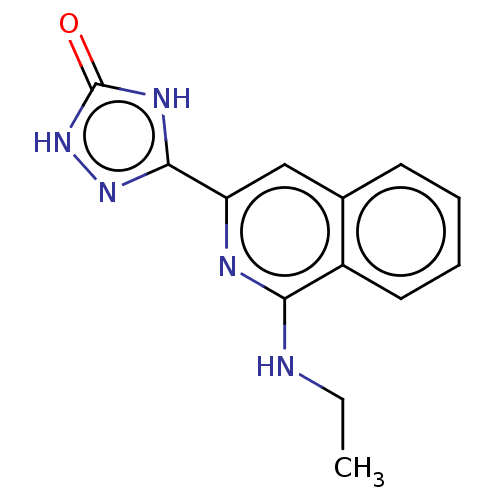
(CHEMBL5077105) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580173

(CHEMBL5075973) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Itk |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580175

(CHEMBL5094686) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Src (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Lck (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50097394
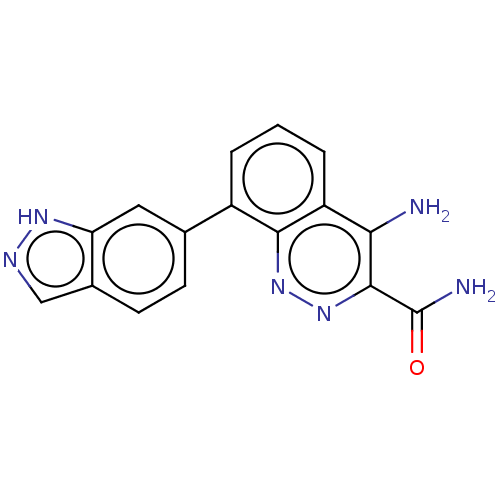
(CHEMBL3586446)Show InChI InChI=1S/C16H12N6O/c17-13-11-3-1-2-10(14(11)21-22-15(13)16(18)23)8-4-5-9-7-19-20-12(9)6-8/h1-7H,(H2,17,21)(H2,18,23)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580172
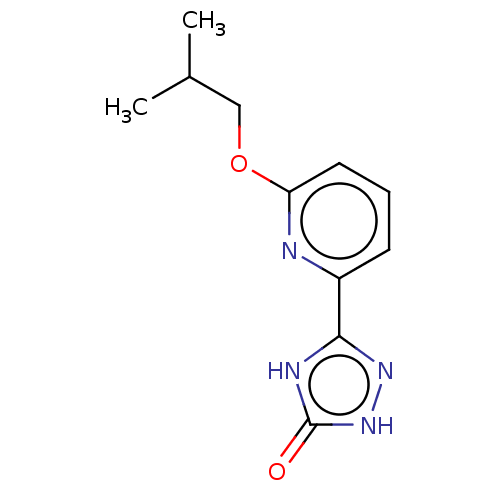
(CHEMBL5089713) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580176

(CHEMBL5074849) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50097398
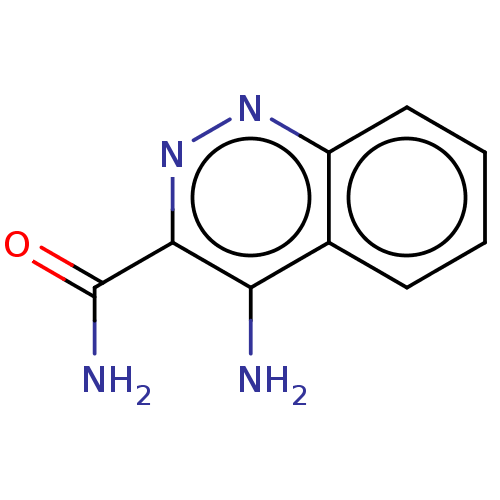
(CHEMBL449216 | HTS-9026)Show InChI InChI=1S/C9H8N4O/c10-7-5-3-1-2-4-6(5)12-13-8(7)9(11)14/h1-4H,(H2,10,12)(H2,11,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50097398

(CHEMBL449216 | HTS-9026)Show InChI InChI=1S/C9H8N4O/c10-7-5-3-1-2-4-6(5)12-13-8(7)9(11)14/h1-4H,(H2,10,12)(H2,11,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human Raji cells after 30 mins by HTRF assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50097393

(CHEMBL3586447)Show SMILES Cc1cc2cn[nH]c2cc1-c1cccc2c(N)c(nnc12)C(N)=O Show InChI InChI=1S/C17H14N6O/c1-8-5-9-7-20-21-13(9)6-12(8)10-3-2-4-11-14(18)16(17(19)24)23-22-15(10)11/h2-7H,1H3,(H2,18,22)(H2,19,24)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human Raji cells after 30 mins by HTRF assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580174

(CHEMBL5089990) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50097396
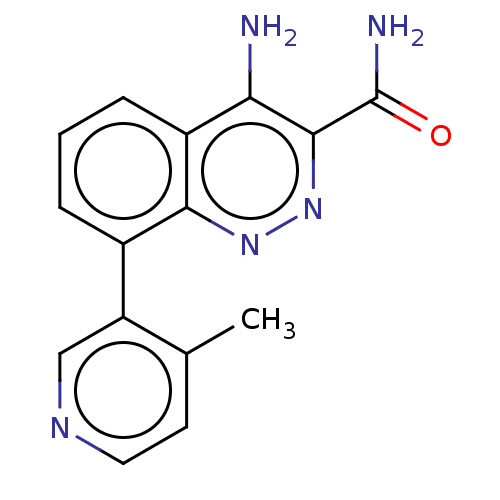
(CHEMBL3586444)Show InChI InChI=1S/C15H13N5O/c1-8-5-6-18-7-11(8)9-3-2-4-10-12(16)14(15(17)21)20-19-13(9)10/h2-7H,1H3,(H2,16,19)(H2,17,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580170

(CHEMBL5080191) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50097395
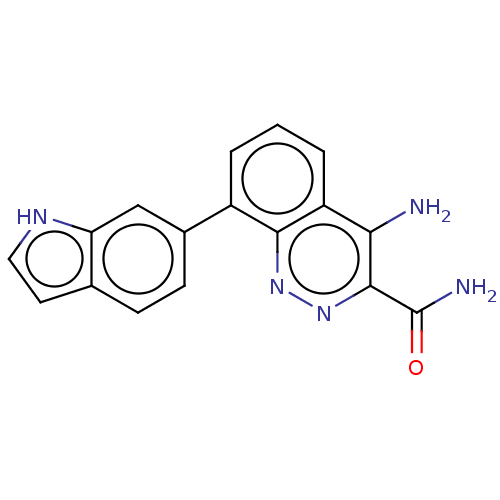
(CHEMBL3586445)Show InChI InChI=1S/C17H13N5O/c18-14-12-3-1-2-11(15(12)21-22-16(14)17(19)23)10-5-4-9-6-7-20-13(9)8-10/h1-8,20H,(H2,18,21)(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50580164
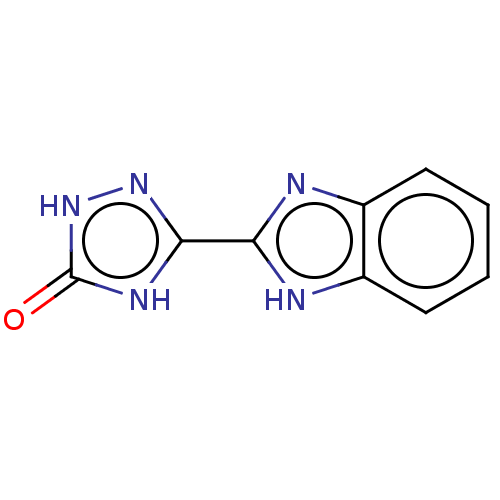
(CHEMBL5079178) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Btk (unknown origin) assessed as inhibition of phosphorylation of FAM-labelled peptide substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50097394

(CHEMBL3586446)Show InChI InChI=1S/C16H12N6O/c17-13-11-3-1-2-10(14(11)21-22-15(13)16(18)23)8-4-5-9-7-19-20-12(9)6-8/h1-7H,(H2,17,21)(H2,18,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human Raji cells after 30 mins by HTRF assay |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50097397

(CHEMBL3586443)Show InChI InChI=1S/C13H12N6O/c1-19-6-7(5-16-19)8-3-2-4-9-10(14)12(13(15)20)18-17-11(8)9/h2-6H,1H3,(H2,14,17)(H2,15,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
J Med Chem 58: 5437-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00734
BindingDB Entry DOI: 10.7270/Q25B047D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data