 Found 111 hits with Last Name = 'tedesco' and Initial = 'd'
Found 111 hits with Last Name = 'tedesco' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monoglyceride lipase
(Rattus norvegicus (Rat)) | BDBM50160284
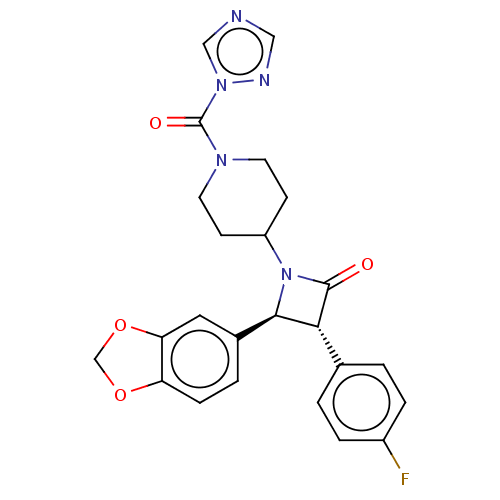
(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of rat MAGL |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160282
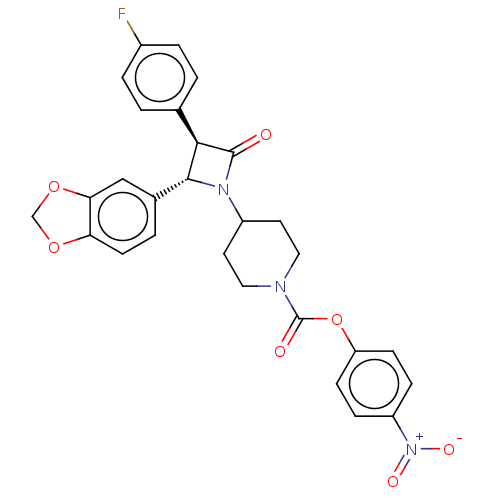
(CHEMBL3785760)Show SMILES [O-][N+](=O)c1ccc(OC(=O)N2CCC(CC2)N2[C@@H]([C@H](C2=O)c2ccc(F)cc2)c2ccc3OCOc3c2)cc1 |r| Show InChI InChI=1S/C28H24FN3O7/c29-19-4-1-17(2-5-19)25-26(18-3-10-23-24(15-18)38-16-37-23)31(27(25)33)20-11-13-30(14-12-20)28(34)39-22-8-6-21(7-9-22)32(35)36/h1-10,15,20,25-26H,11-14,16H2/t25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM22449
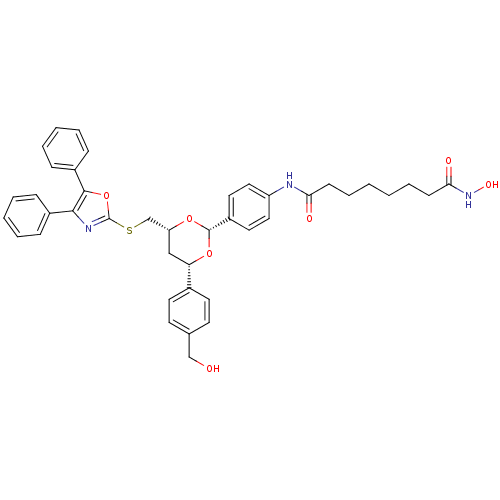
(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Rattus norvegicus (Rat)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in rat brain membranes preincubated for 20 mins followed by fluorophosphonate-rhodamine addition measured after 30 mins by competi... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
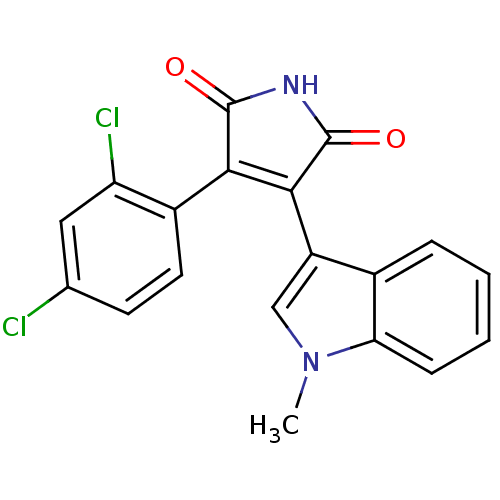
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) pre-incubated for 30 mins followed by ULight-GS (Ser641/pSer657) peptide substrate addition and measured afte... |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160280

(CHEMBL3787340)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C25H25N5O5/c1-33-19-5-2-16(3-6-19)22-23(17-4-7-20-21(12-17)35-15-34-20)30(24(22)31)18-8-10-28(11-9-18)25(32)29-14-26-13-27-29/h2-7,12-14,18,22-23H,8-11,15H2,1H3/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160277

(CHEMBL3785536)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](C(=O)N1C1CCN(CC1)C(=O)n1cncn1)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C25H27N5O4/c1-33-20-7-3-17(4-8-20)22-23(18-5-9-21(34-2)10-6-18)30(24(22)31)19-11-13-28(14-12-19)25(32)29-16-26-15-27-29/h3-10,15-16,19,22-23H,11-14H2,1-2H3/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409064

(CHEMBL5269954)Show InChI InChI=1S/C17H20N4O2/c22-15-10-13(11-16-14(15)2-9-23-16)12-20-5-7-21(8-6-20)17-18-3-1-4-19-17/h1-4,9,13H,5-8,10-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1B adrenoceptor in Wistar rat spleen assessed as phenylephrine induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160283
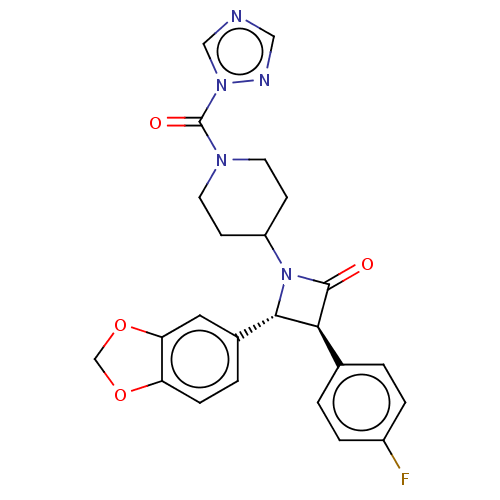
(CHEMBL3787346)Show SMILES Fc1ccc(cc1)[C@H]1[C@@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1D adrenoceptor in Wistar rat thoracic aorta assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409189

(CHEMBL5283402)Show InChI InChI=1S/C22H27N3/c1-2-23-16-15-20-17-24-22(25-20)14-13-21(18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,17,21,23H,2,13-16H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at alpha1A adrenoceptor in Wistar rat vas deferans assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409203
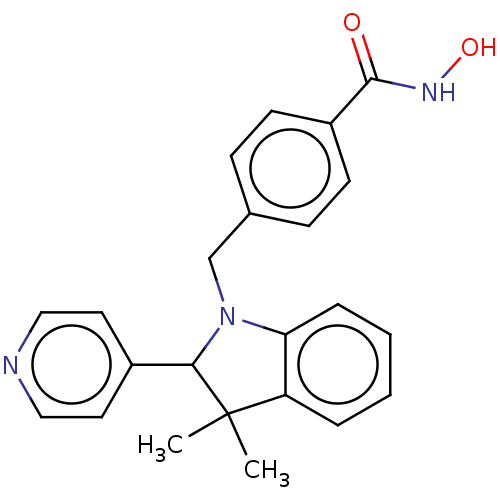
(CHEMBL5289038)Show InChI InChI=1S/C16H19ClN4S/c17-14-3-1-2-12(8-14)9-19-16(22)21-6-4-13(5-7-21)15-10-18-11-20-15/h1-3,8,10-11,13H,4-7,9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160279

(CHEMBL3787224)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C26H29N5O5/c1-34-20-7-4-17(5-8-20)23-24(18-6-9-21(35-2)22(14-18)36-3)31(25(23)32)19-10-12-29(13-11-19)26(33)30-16-27-15-28-30/h4-9,14-16,19,23-24H,10-13H2,1-3H3/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409199
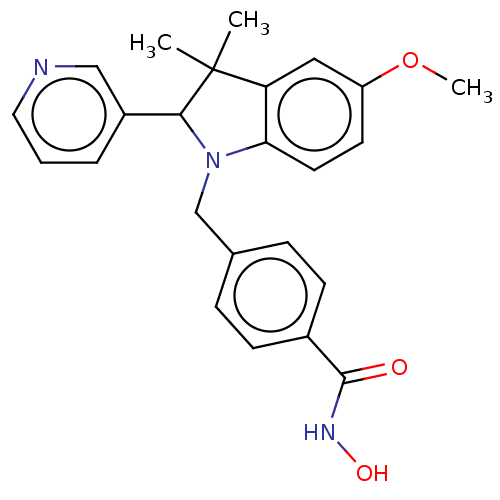
(CHEMBL5280583)Show InChI InChI=1S/C16H19IN4S/c17-14-4-2-1-3-13(14)9-19-16(22)21-7-5-12(6-8-21)15-10-18-11-20-15/h1-4,10-12H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409198
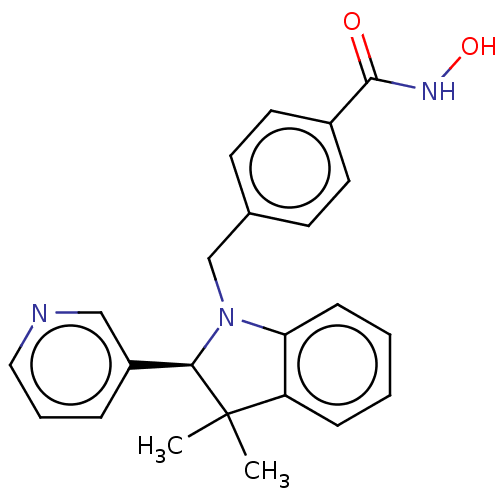
(CHEMBL5276531)Show InChI InChI=1S/C16H19IN4S/c17-14-3-1-12(2-4-14)9-19-16(22)21-7-5-13(6-8-21)15-10-18-11-20-15/h1-4,10-11,13H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409059
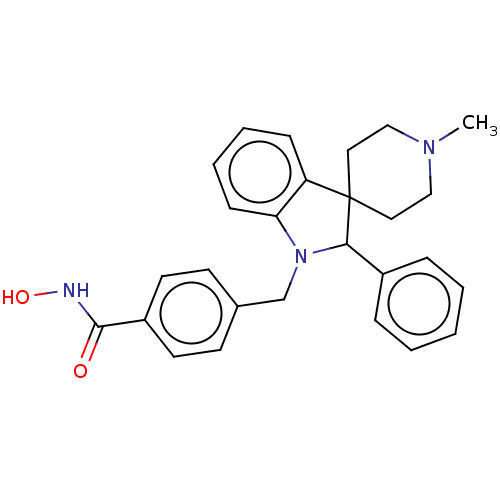
(CHEMBL5282958)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCN(CC2Cc3occc3C(=O)C2)CC1 Show InChI InChI=1S/C23H27FN2O3/c24-19-5-3-18(4-6-19)21(27)2-1-8-25-9-11-26(12-10-25)16-17-14-22(28)20-7-13-29-23(20)15-17/h3-7,13,17H,1-2,8-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1D adrenoceptor in Wistar rat thoracic aorta assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409190
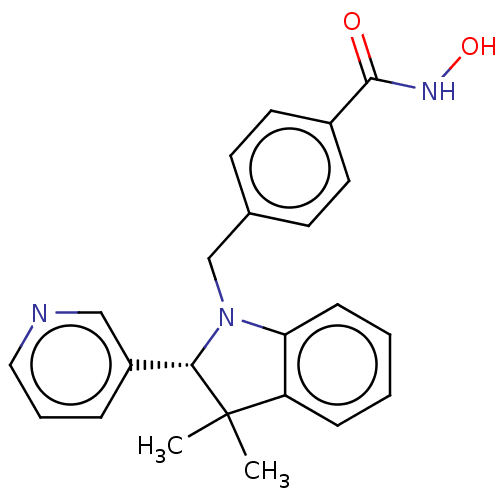
(CHEMBL5281527)Show SMILES C(Cc1ncc(CCN2CCCC2)[nH]1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H29N3/c1-3-9-20(10-4-1)23(21-11-5-2-6-12-21)13-14-24-25-19-22(26-24)15-18-27-16-7-8-17-27/h1-6,9-12,19,23H,7-8,13-18H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160281
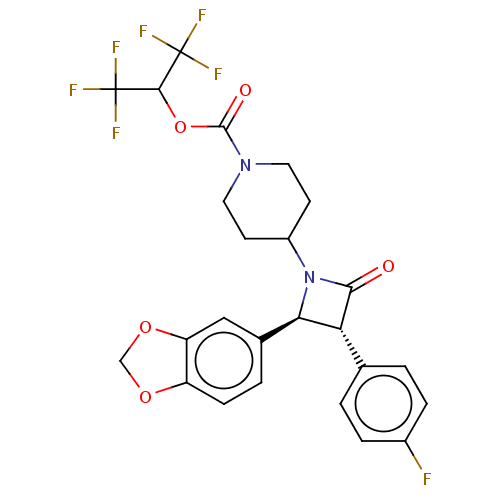
(CHEMBL3787592)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C25H21F7N2O5/c26-15-4-1-13(2-5-15)19-20(14-3-6-17-18(11-14)38-12-37-17)34(21(19)35)16-7-9-33(10-8-16)23(36)39-22(24(27,28)29)25(30,31)32/h1-6,11,16,19-20,22H,7-10,12H2/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human MAGL expressed in African green monkey COS cells using 2-arachidonoyl-[3H]-glycerol as substrate incubated for 20 mins by scintil... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409061

(CHEMBL5276825)Show InChI InChI=1S/C18H21N3O2/c22-16-11-14(12-17-15(16)4-10-23-17)13-20-6-8-21(9-7-20)18-3-1-2-5-19-18/h1-5,10,14H,6-9,11-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1B adrenoceptor in Wistar rat spleen assessed as phenylephrine induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409187

(CHEMBL5290574)Show InChI InChI=1S/C22H27N3/c23-16-15-20-17-24-22(25-20)14-8-7-13-21(18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-6,9-12,17,21H,7-8,13-16,23H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at alpha1A adrenoceptor in Wistar rat vas deferans assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409065
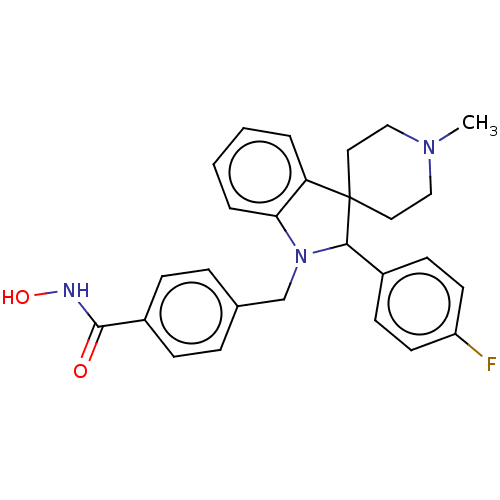
(CHEMBL5280477)Show SMILES Fc1ccc(cc1)C(=O)CCCCN1CCN(CC2Cc3occc3C(=O)C2)CC1 Show InChI InChI=1S/C24H29FN2O3/c25-20-6-4-19(5-7-20)22(28)3-1-2-9-26-10-12-27(13-11-26)17-18-15-23(29)21-8-14-30-24(21)16-18/h4-8,14,18H,1-3,9-13,15-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at alpha1A adrenoceptor in Wistar rat vas deferans assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409204

(CHEMBL5267454)Show InChI InChI=1S/C16H19FN4S/c17-14-4-2-1-3-13(14)9-19-16(22)21-7-5-12(6-8-21)15-10-18-11-20-15/h1-4,10-12H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409060
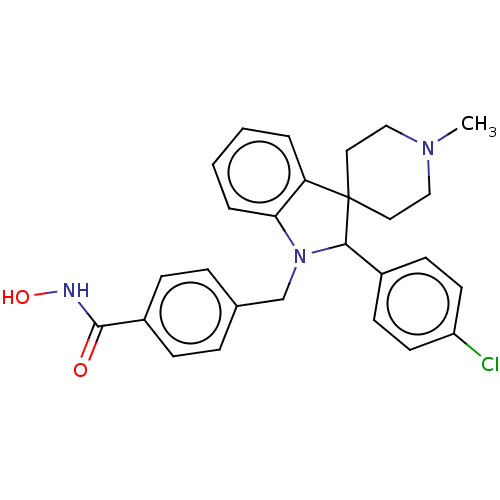
(CHEMBL5291414)Show InChI InChI=1S/C19H22N2O2/c22-18-12-15(13-19-17(18)6-11-23-19)14-20-7-9-21(10-8-20)16-4-2-1-3-5-16/h1-6,11,15H,7-10,12-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1D adrenoceptor in Wistar rat thoracic aorta assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409188

(CHEMBL5276718)Show SMILES C(Cc1cnc(CCC(c2ccccc2)c2ccccc2)[nH]1)NC1CC1 Show InChI InChI=1S/C23H27N3/c1-3-7-18(8-4-1)22(19-9-5-2-6-10-19)13-14-23-25-17-21(26-23)15-16-24-20-11-12-20/h1-10,17,20,22,24H,11-16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at Alpha-1D adrenoceptor in Wistar rat thoracic aorta assessed as noradrenaline induced contractions after 30 mins |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate preincubate... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate preincubate... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160278

(CHEMBL3786142)Show SMILES COc1ccc(cc1OC)[C@@H]1[C@H](C(=O)N1C1CCN(CC1)C(=O)n1cncn1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C27H31N5O6/c1-35-20-7-5-17(13-22(20)37-3)24-25(18-6-8-21(36-2)23(14-18)38-4)32(26(24)33)19-9-11-30(12-10-19)27(34)31-16-28-15-29-31/h5-8,13-16,19,24-25H,9-12H2,1-4H3/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM60622
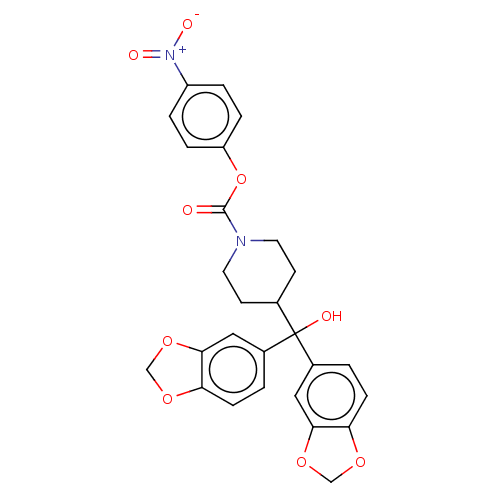
(BDBM50300355 | US11753371, Compound JZL-184 | US91...)Show SMILES OC(C1CCN(CC1)C(=O)Oc1ccc(cc1)[N+]([O-])=O)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C27H24N2O9/c30-26(38-21-5-3-20(4-6-21)29(32)33)28-11-9-17(10-12-28)27(31,18-1-7-22-24(13-18)36-15-34-22)19-2-8-23-25(14-19)37-16-35-23/h1-8,13-14,17,31H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160287

(CHEMBL3787453)Show SMILES Fc1ccc(cc1)[C@H]1CN([C@@H]1c1ccc2OCOc2c1)C1CCN(CC1)C(=O)n1cncn1 |r| Show InChI InChI=1S/C24H24FN5O3/c25-18-4-1-16(2-5-18)20-12-29(23(20)17-3-6-21-22(11-17)33-15-32-21)19-7-9-28(10-8-19)24(31)30-14-26-13-27-30/h1-6,11,13-14,19-20,23H,7-10,12,15H2/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
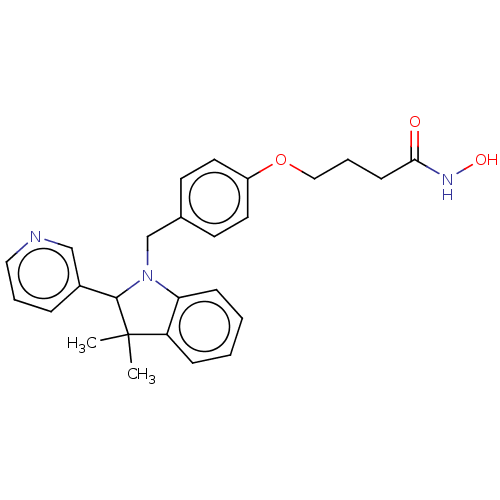
(Homo sapiens (Human)) | BDBM50409202
(CHEMBL5286210)Show InChI InChI=1S/C16H19FN4S/c17-14-3-1-12(2-4-14)9-19-16(22)21-7-5-13(6-8-21)15-10-18-11-20-15/h1-4,10-11,13H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 616 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7781
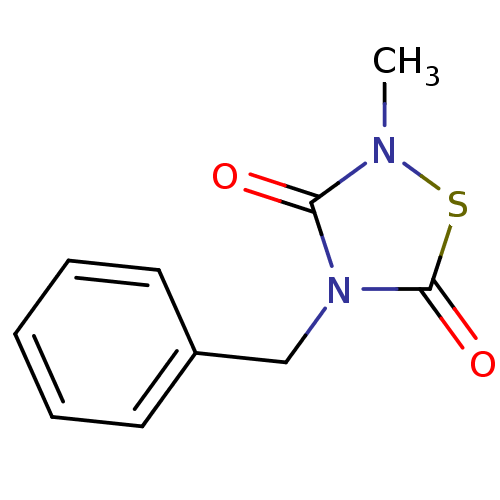
(4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...)Show InChI InChI=1S/C10H10N2O2S/c1-11-9(13)12(10(14)15-11)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM22449

(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50160279

(CHEMBL3787224)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C26H29N5O5/c1-34-20-7-4-17(5-8-20)23-24(18-6-9-21(35-2)22(14-18)36-3)31(25(23)32)19-10-12-29(13-11-19)26(33)30-16-27-15-28-30/h4-9,14-16,19,23-24H,10-13H2,1-3H3/t23-,24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in rat brain membranes using using N-arachidonoyl-[14C]-ethanolamine as substrate assessed as reduction in [14C]ethanolamine produ... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50160283

(CHEMBL3787346)Show SMILES Fc1ccc(cc1)[C@H]1[C@@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 729 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate preincubate... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50409201
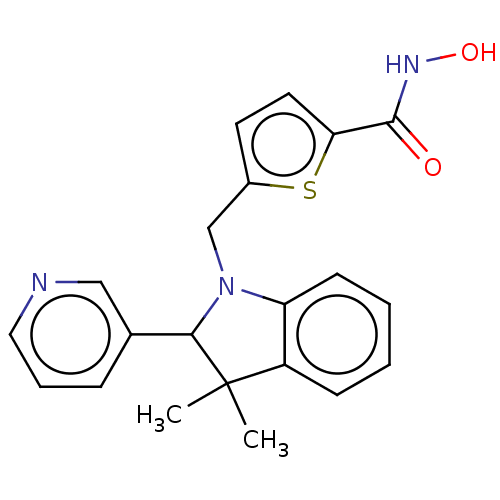
(CHEMBL5267435)Show InChI InChI=1S/C17H22N4S/c1-13-4-2-3-5-15(13)10-19-17(22)21-8-6-14(7-9-21)16-11-18-12-20-16/h2-5,11-12,14H,6-10H2,1H3,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 811 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50368627
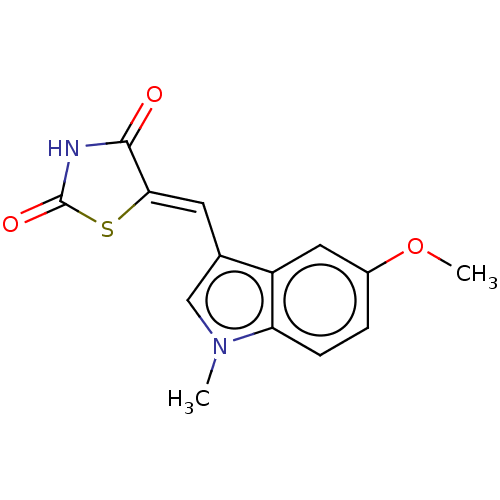
(CHEMBL4160653)Show InChI InChI=1S/C14H12N2O3S/c1-16-7-8(5-12-13(17)15-14(18)20-12)10-6-9(19-2)3-4-11(10)16/h3-7H,1-2H3,(H,15,17,18)/b12-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM22449

(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT3 receptor in guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of 2-methyl-5-HT-induced contrac... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50198886
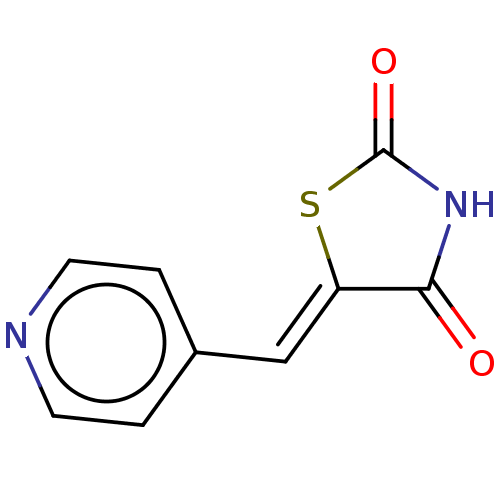
(CHEMBL3911800)Show InChI InChI=1S/C9H6N2O2S/c12-8-7(14-9(13)11-8)5-6-1-3-10-4-2-6/h1-5H,(H,11,12,13)/b7-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50409202

(CHEMBL5286210)Show InChI InChI=1S/C16H19FN4S/c17-14-3-1-12(2-4-14)9-19-16(22)21-7-5-13(6-8-21)15-10-18-11-20-15/h1-4,10-11,13H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT3 receptor in guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of 2-methyl-5-HT-induced contrac... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50409189

(CHEMBL5283402)Show InChI InChI=1S/C22H27N3/c1-2-23-16-15-20-17-24-22(25-20)14-13-21(18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,17,21,23H,2,13-16H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50160280

(CHEMBL3787340)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C25H25N5O5/c1-33-19-5-2-16(3-6-19)22-23(17-4-7-20-21(12-17)35-15-34-20)30(24(22)31)18-8-10-28(11-9-18)25(32)29-14-26-13-27-29/h2-7,12-14,18,22-23H,8-11,15H2,1H3/t22-,23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in rat brain membranes using using N-arachidonoyl-[14C]-ethanolamine as substrate assessed as reduction in [14C]ethanolamine produ... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50409198

(CHEMBL5276531)Show InChI InChI=1S/C16H19IN4S/c17-14-3-1-12(2-4-14)9-19-16(22)21-7-5-13(6-8-21)15-10-18-11-20-15/h1-4,10-11,13H,5-9H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT3 receptor in guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of 2-methyl-5-HT-induced contrac... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50409064

(CHEMBL5269954)Show InChI InChI=1S/C17H20N4O2/c22-15-10-13(11-16-14(15)2-9-23-16)12-20-5-7-21(8-6-20)17-18-3-1-4-19-17/h1-4,9,13H,5-8,10-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5-HT3 receptor in guinea pig ileum muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50368622
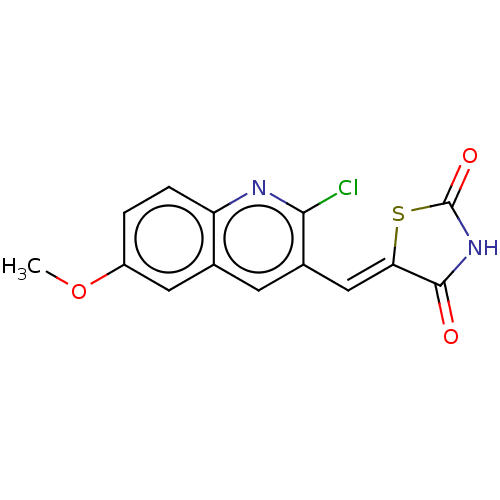
(CHEMBL4169102)Show InChI InChI=1S/C14H9ClN2O3S/c1-20-9-2-3-10-7(5-9)4-8(12(15)16-10)6-11-13(18)17-14(19)21-11/h2-6H,1H3,(H,17,18,19)/b11-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) using GS-2 peptide substrate incubated for 30 mins by kinase-Glo luminescent assay |
J Med Chem 61: 7640-7656 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00610
BindingDB Entry DOI: 10.7270/Q20Z75TR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50409060

(CHEMBL5291414)Show InChI InChI=1S/C19H22N2O2/c22-18-12-15(13-19-17(18)6-11-23-19)14-20-7-9-21(10-8-20)16-4-2-1-3-5-16/h1-6,11,15H,7-10,12-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT3 receptor in guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of 2-methyl-5-HT-induced contrac... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50160277

(CHEMBL3785536)Show SMILES COc1ccc(cc1)[C@@H]1[C@H](C(=O)N1C1CCN(CC1)C(=O)n1cncn1)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C25H27N5O4/c1-33-20-7-3-17(4-8-20)22-23(18-5-9-21(34-2)10-6-18)30(24(22)31)19-11-13-28(14-12-19)25(32)29-16-26-15-27-29/h3-10,15-16,19,22-23H,11-14H2,1-2H3/t22-,23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in rat brain membranes using using N-arachidonoyl-[14C]-ethanolamine as substrate assessed as reduction in [14C]ethanolamine produ... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50160284

(CHEMBL3785379)Show SMILES Fc1ccc(cc1)[C@@H]1[C@H](N(C2CCN(CC2)C(=O)n2cncn2)C1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H22FN5O4/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29/h1-6,11-13,18,21-22H,7-10,14H2/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His-tagged human FAAH expressed in sf21 cells using N-arachidonoyl-[14C]-ethanolamine as substrate assessed as r... |
J Med Chem 59: 2612-32 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01812
BindingDB Entry DOI: 10.7270/Q21R6SC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data