

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15613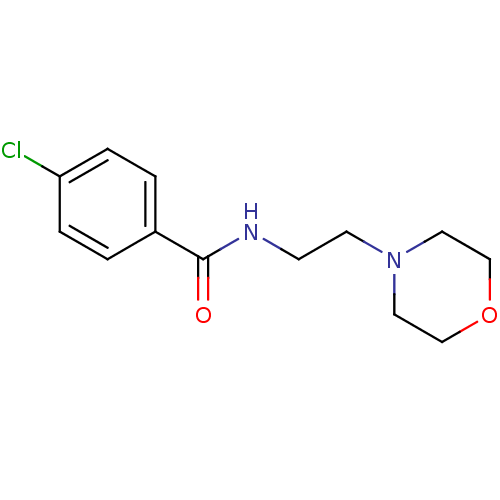 (4-chloro-N-(2-morpholin-4-ylethyl)benzamide | 4-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579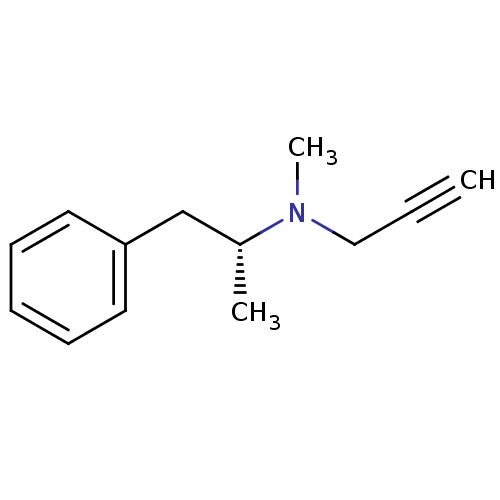 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50420209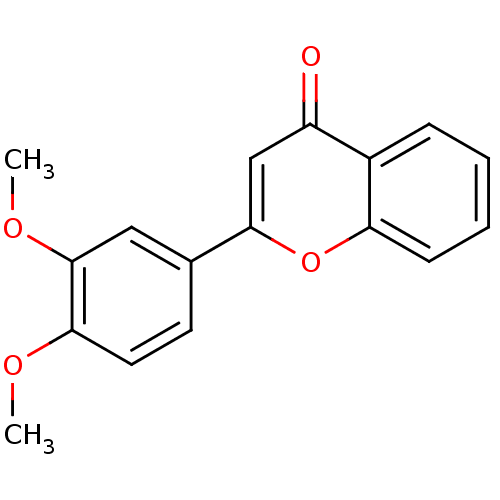 (CHEMBL91153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50011421 (2-(4-Nitro-phenyl)-chromen-4-one | CHEMBL64780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150763 (2-(Naphthalen-1-yl)-4H-chromen-4-one (3o)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150758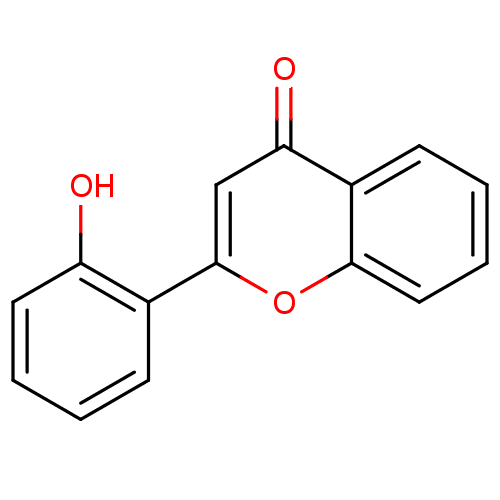 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150762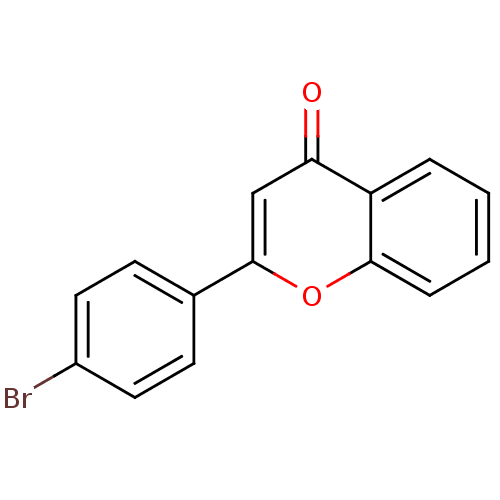 (2-(4-Bromophenyl)-4H-chromen-4-one (3n)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150757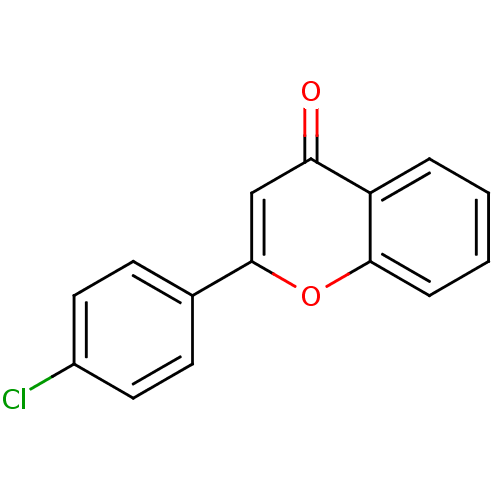 (2-(4-Chlorophenyl)-4H-chromen-4-one (3c)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150763 (2-(Naphthalen-1-yl)-4H-chromen-4-one (3o)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50431165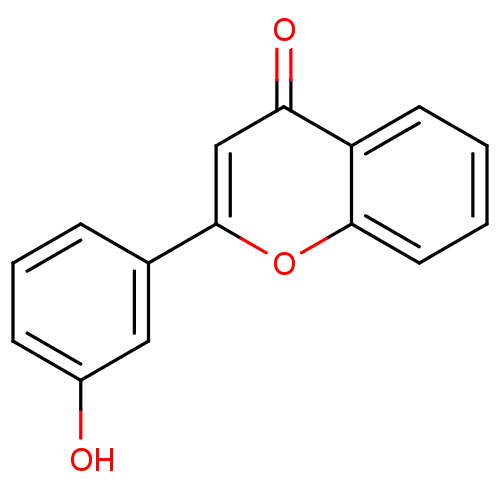 (2-(3-hydroxyphenyl)-4H-chromen-4-one (2) | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50011421 (2-(4-Nitro-phenyl)-chromen-4-one | CHEMBL64780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150760 (2-(3-Methoxyphenyl)-4H-chromen-4-one (3h)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150761 (4-(4-Oxo-4H-chromen-2-yl)benzonitrile (3m)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50310190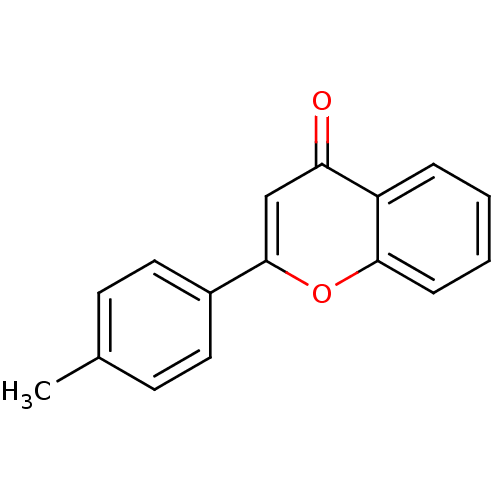 (2-p-Tolyl-chromen-4-one | 2-p-tolyl-4H-chromen-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50011446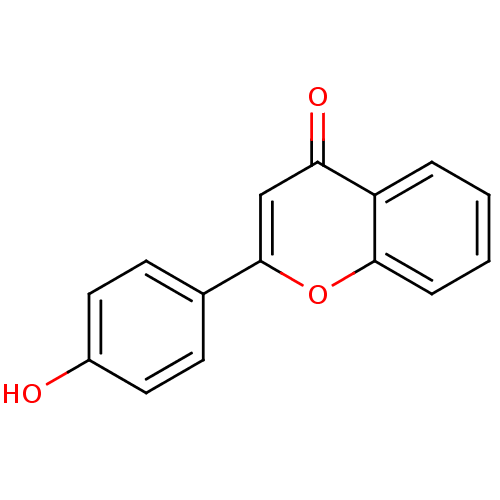 (2-(4-Hydroxy-phenyl)-chromen-4-one | 2-(4-hydroxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50028962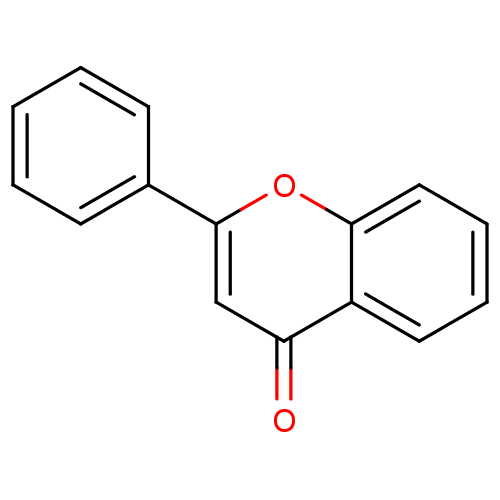 (CHEMBL275638 | flavone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150765 (2-(Anthracen-9-yl)-4H-chromen-4-one (3q)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150756 (2-(2-Chlorophenyl)-4H-1-Benzopyran-4-one (3b)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150764 (2-(Naphthalen-2-yl)-4H-chromen-4-one (3p)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15613 (4-chloro-N-(2-morpholin-4-ylethyl)benzamide | 4-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150759 (2-(2-Methoxyphenyl)-4H-chromen-4-one (3g)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50310189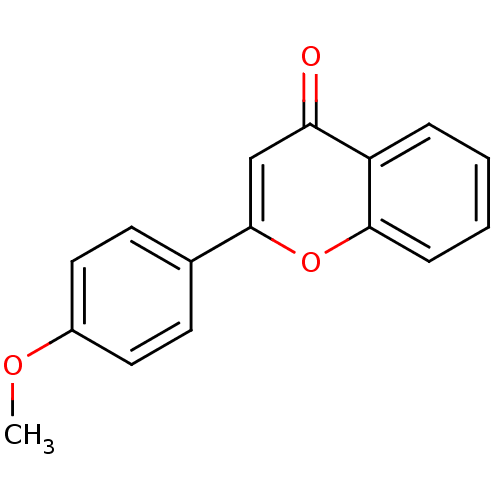 (2-(4-Methoxy-phenyl)-chromen-4-one | 2-(4-methoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150765 (2-(Anthracen-9-yl)-4H-chromen-4-one (3q)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150756 (2-(2-Chlorophenyl)-4H-1-Benzopyran-4-one (3b)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150767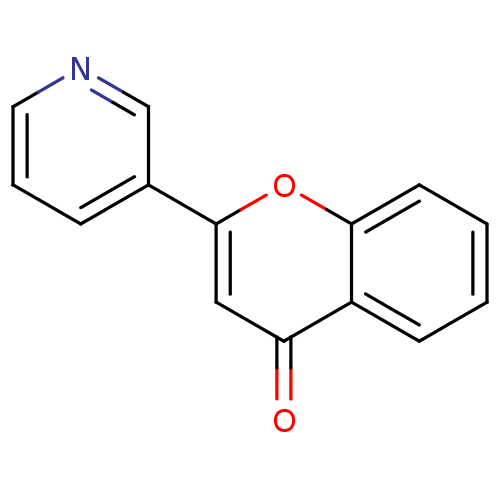 (2-(Pyridin-3-yl)-4H-chromen-4-one (3s)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150760 (2-(3-Methoxyphenyl)-4H-chromen-4-one (3h)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150762 (2-(4-Bromophenyl)-4H-chromen-4-one (3n)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50028962 (CHEMBL275638 | flavone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150764 (2-(Naphthalen-2-yl)-4H-chromen-4-one (3p)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50310189 (2-(4-Methoxy-phenyl)-chromen-4-one | 2-(4-methoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150766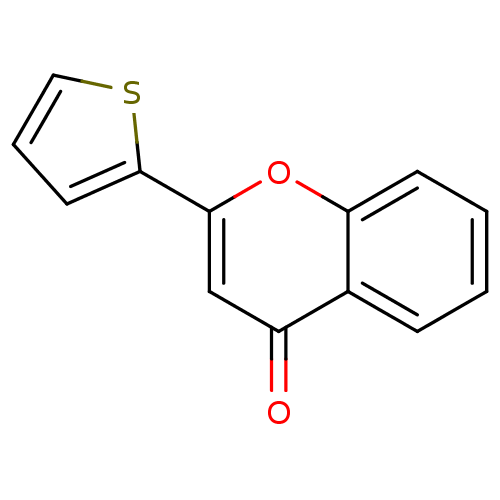 (2-(Thiophen-2-yl)-4H-chromen-4-one (3r)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50431165 (2-(3-hydroxyphenyl)-4H-chromen-4-one (2) | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150759 (2-(2-Methoxyphenyl)-4H-chromen-4-one (3g)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50310190 (2-p-Tolyl-chromen-4-one | 2-p-tolyl-4H-chromen-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150757 (2-(4-Chlorophenyl)-4H-chromen-4-one (3c)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50420209 (CHEMBL91153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50011446 (2-(4-Hydroxy-phenyl)-chromen-4-one | 2-(4-hydroxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150767 (2-(Pyridin-3-yl)-4H-chromen-4-one (3s)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150766 (2-(Thiophen-2-yl)-4H-chromen-4-one (3r)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 6.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM150761 (4-(4-Oxo-4H-chromen-2-yl)benzonitrile (3m)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 8.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152653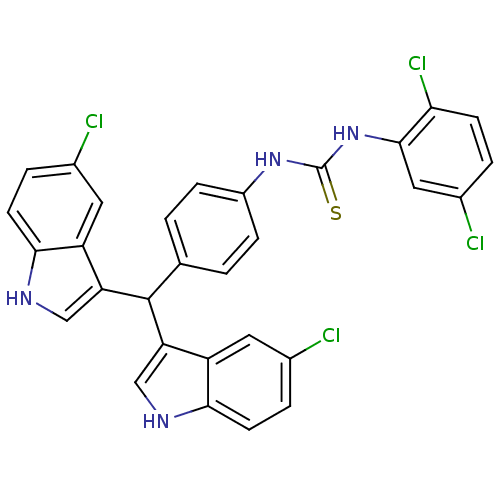 (4-(1,3-Dioxoisoindolin-2-yl)-N-(4-ethylphenyl)benz...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.82E+4 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152640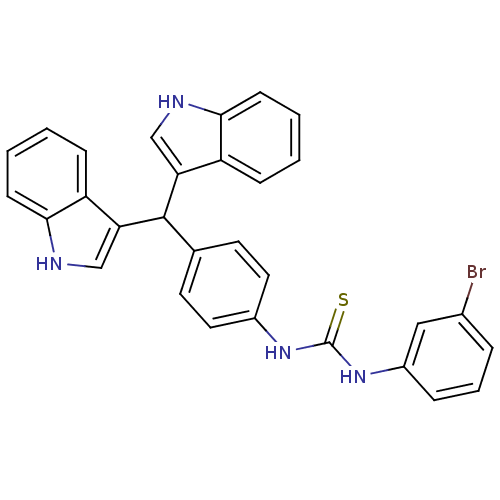 (4-(1,3-Dioxoisoindolin-2-yl)-N,N-diethyl benzenesu...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152640 (4-(1,3-Dioxoisoindolin-2-yl)-N,N-diethyl benzenesu...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152642 (2-(4-(Piperidin-1-ylsulphonyl)phenyl)isoindoline-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152643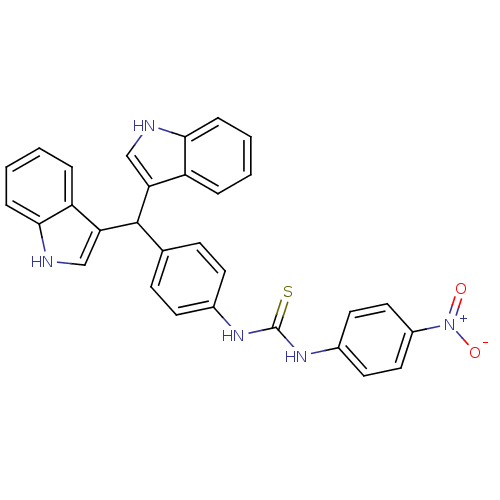 (2-(4-(4-Methylpiperazin-1-ylsulphonyl)phenyl)isoin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152644 (4-(1,3-Dioxoisoindolin-2-yl)-N-phenylbenzenesulpho...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152645 (4-(1,3-Dioxoisoindolin-2-yl)-N-(2-hydroxyphenyl)be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2 (Virus)) | BDBM152646 (4-(1,3-Dioxoisoindolin-2-yl)-N-(3-methoxyphenyl)be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.5 | n/a |
Birla Institute of Technology | Assay Description To measure the inhibitory activities of the compounds, the DENV2 NS2B-NS3 protease activities were measured in the presence of the compounds using Bz... | Bioorg Chem 62: 74-82 (2015) Article DOI: 10.1016/j.bioorg.2015.07.005 BindingDB Entry DOI: 10.7270/Q2VT1QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |