

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50384537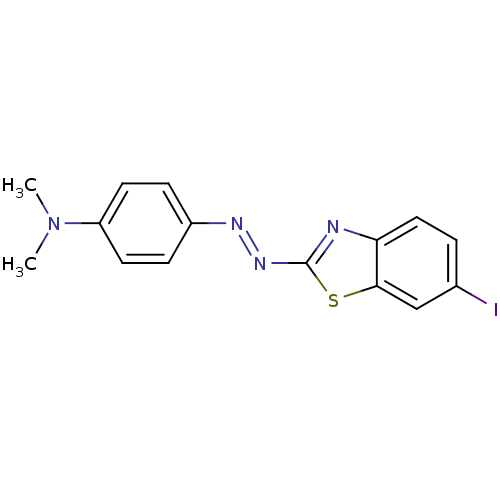 (CHEMBL2036430) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay | ACS Med Chem Lett 3: 58-62 (2012) Article DOI: 10.1021/ml200230e BindingDB Entry DOI: 10.7270/Q2PR7X1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50384538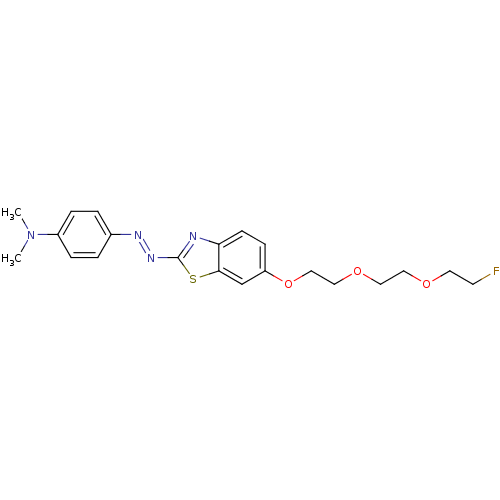 (CHEMBL2036419 | CHEMBL2036420) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay | ACS Med Chem Lett 3: 58-62 (2012) Article DOI: 10.1021/ml200230e BindingDB Entry DOI: 10.7270/Q2PR7X1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50103634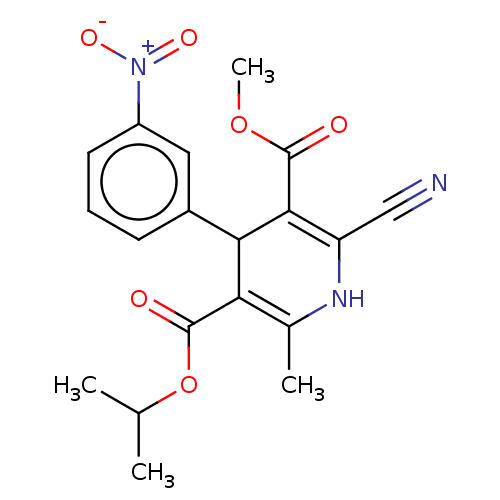 (CL-287389 | FK-235 | Nilvadipine | Nivadipine | SK...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576972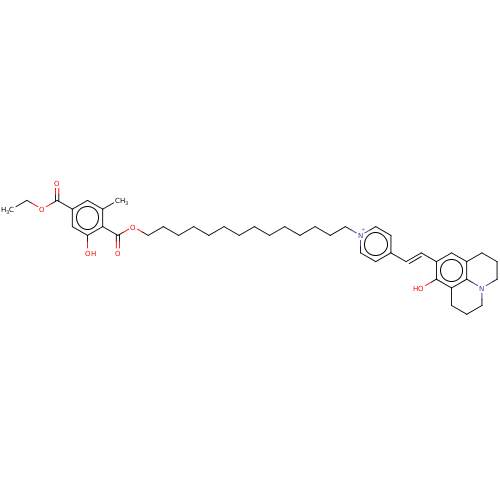 (CHEMBL4879019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227967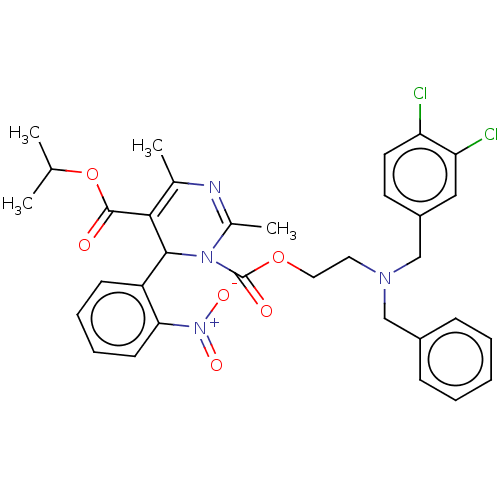 (CHEMBL78973) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101817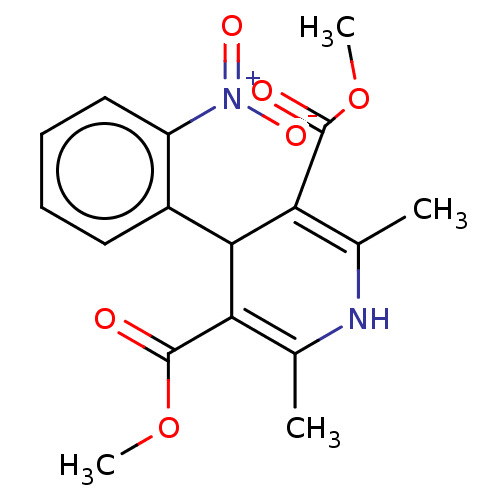 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101815 (CHEBI:7550 | Nicardipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227968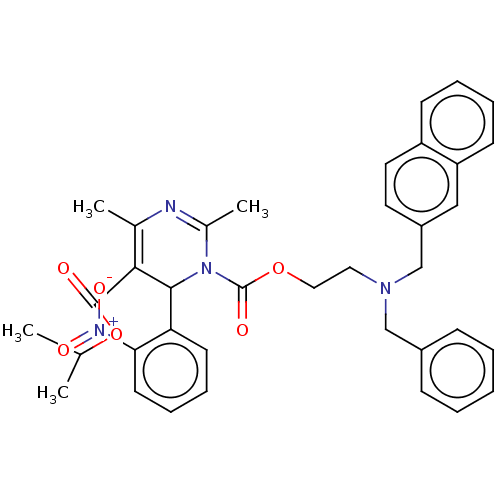 (CHEMBL310953) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227969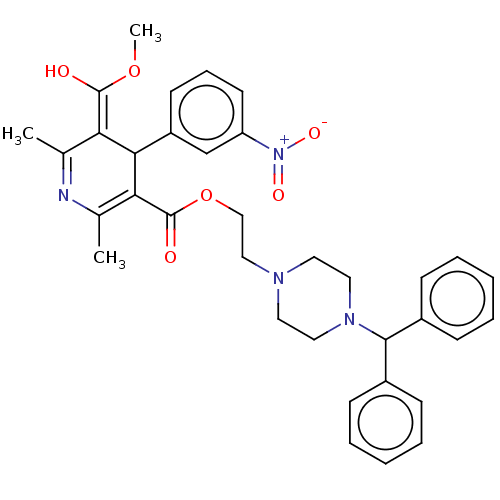 (CHEMBL312176 | CV-4093) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011938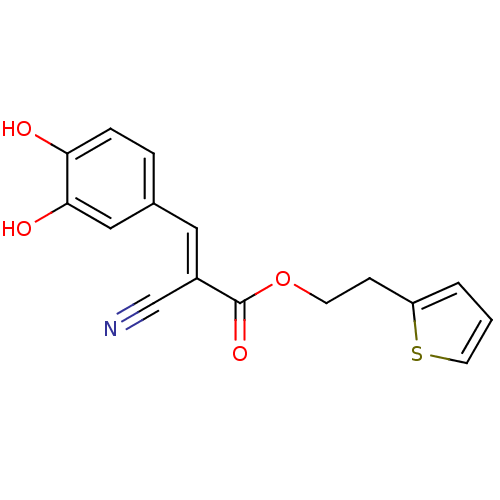 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 2-th...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011932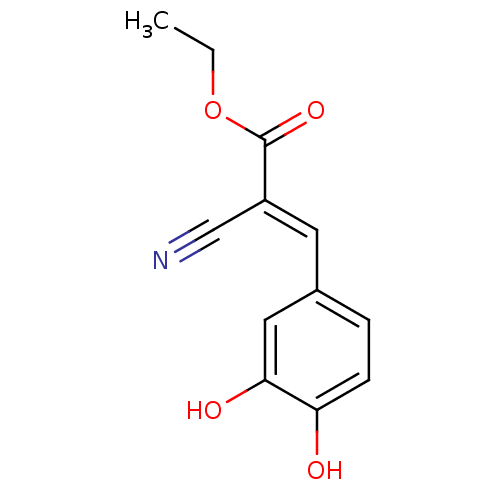 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576974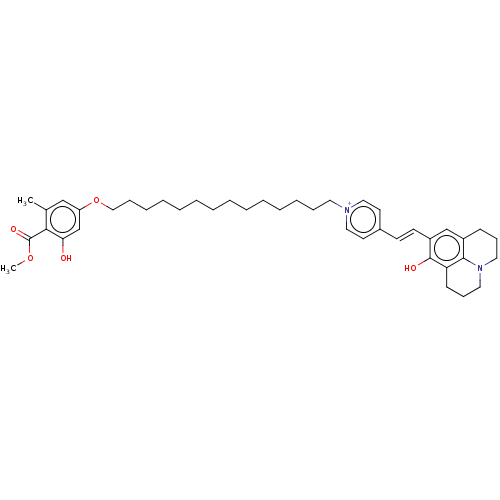 (CHEMBL4872515) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011934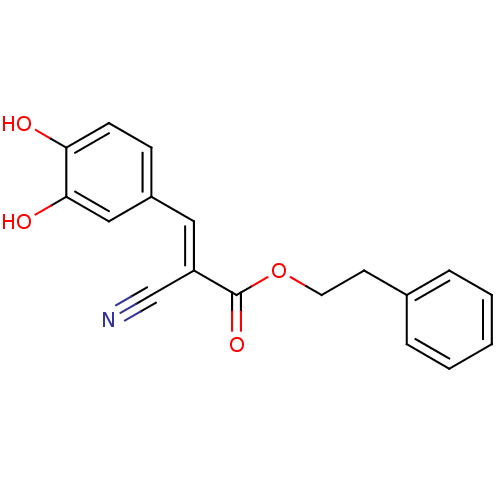 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011930 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 3-ph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011931 ((2E)‐3‐phenylprop‐2‐enR...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011935 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 2-(2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50011938 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 2-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50011935 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 2-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50576973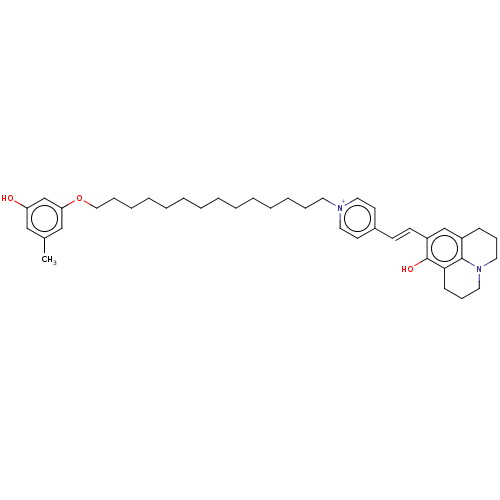 (CHEMBL4857072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Trypanosoma brucei brucei alternative oxidase using ubiquinol as substrate preincubated for 2 mins followed by substrate ad... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113470 BindingDB Entry DOI: 10.7270/Q2N87FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50011930 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 3-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50011934 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50011937 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 8-(3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50011933 (2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 4-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50384538 (CHEMBL2036419 | CHEMBL2036420) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay | ACS Med Chem Lett 3: 58-62 (2012) Article DOI: 10.1021/ml200230e BindingDB Entry DOI: 10.7270/Q2PR7X1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15B (Rattus norvegicus) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytes | J Med Chem 34: 1503-5 (1991) BindingDB Entry DOI: 10.7270/Q22Z14G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491199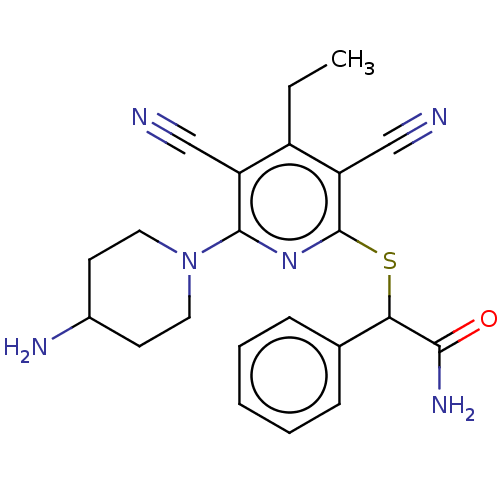 (US10975056, Example 143) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491200 (US10975056, Example 144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491201 (2-((6-((2-Amino-2-oxoethyl)(methyl)amino)-3,5-dicy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491202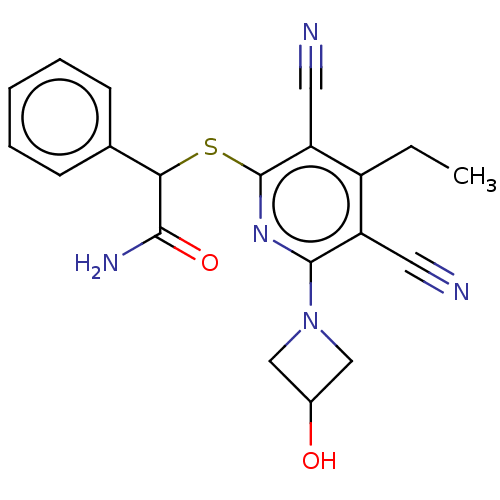 (US10975056, Example 146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491479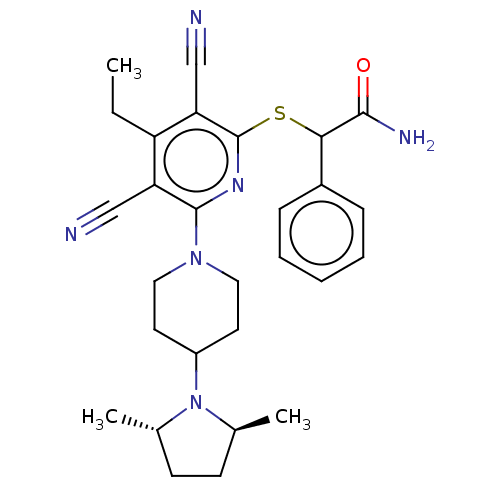 (US10975056, Example 462) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491480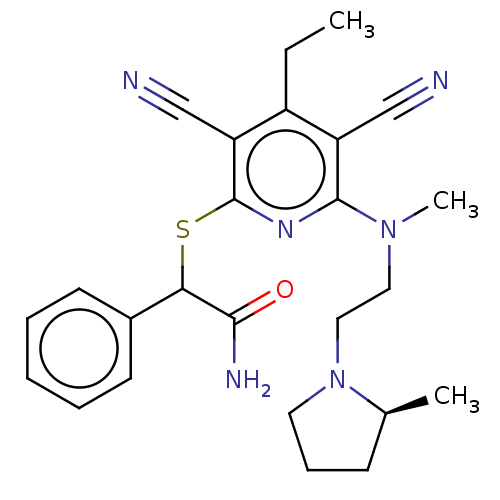 (US10975056, Example 463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491481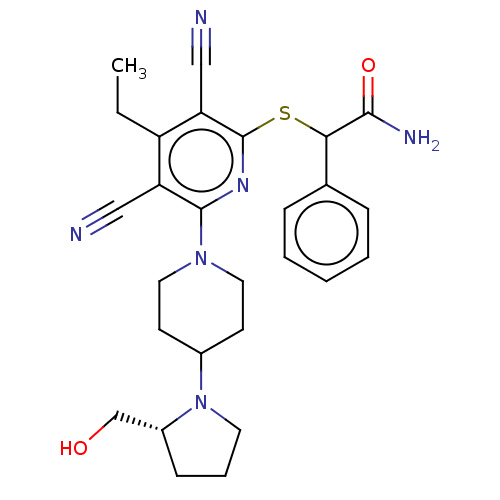 (US10975056, Example 464) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491482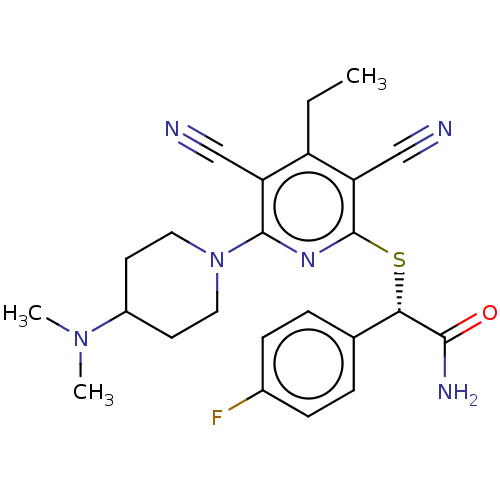 (US10975056, Example 465) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491483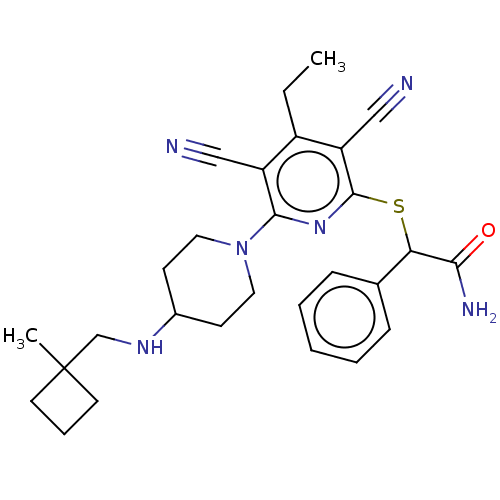 (US10975056, Example 466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491484 (US10975056, Example 467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491485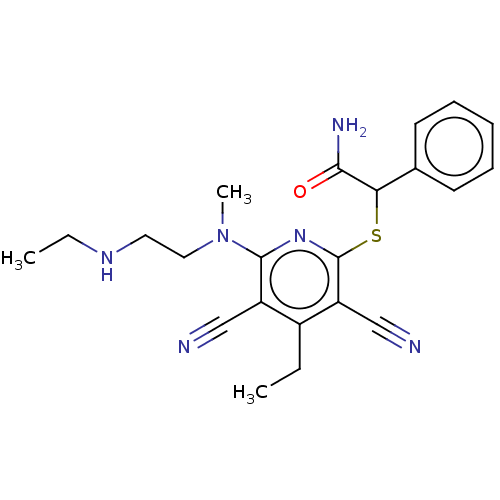 (US10975056, Example 468) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491486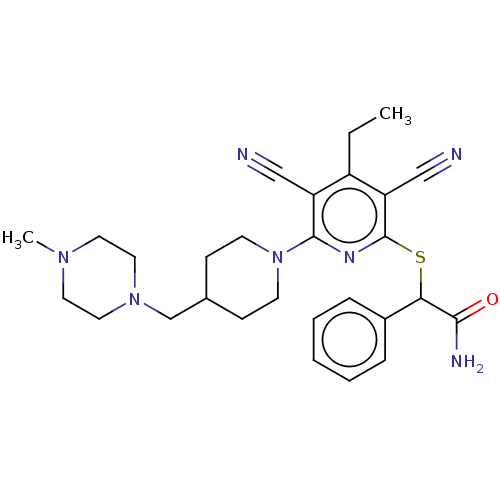 (US10975056, Example 469) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491487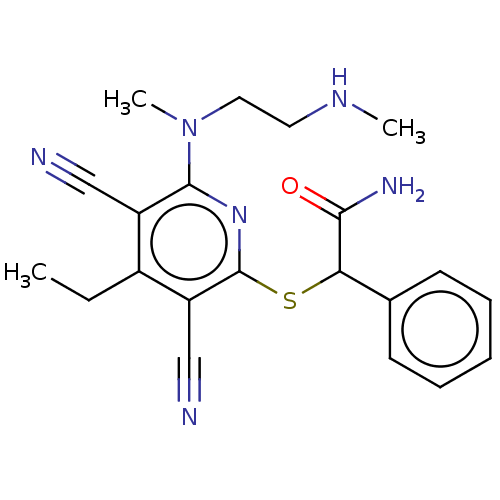 (US10975056, Example 470) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491488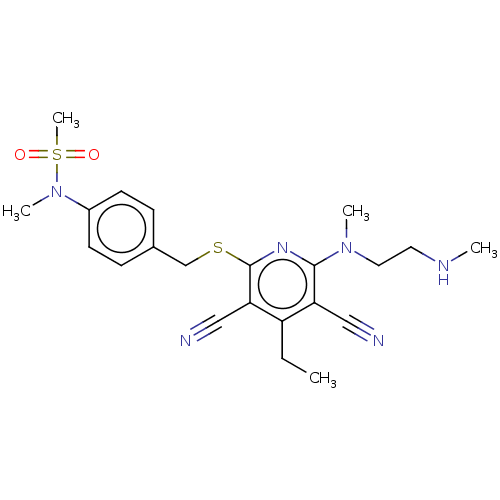 (US10975056, Example 471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491489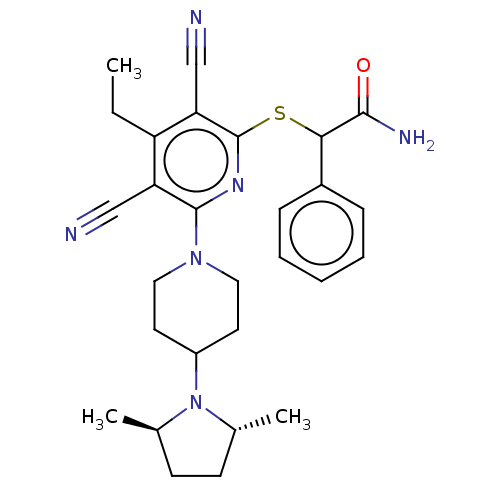 (US10975056, Example 472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491490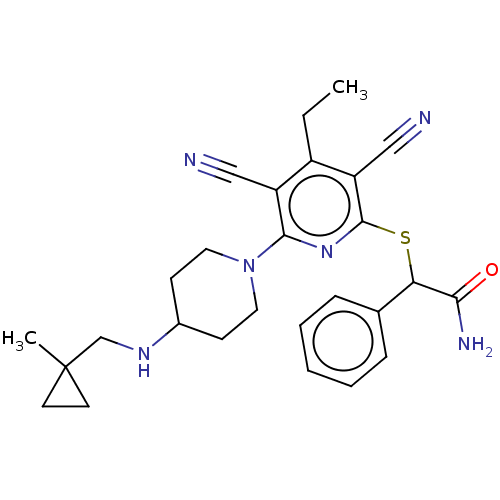 (US10975056, Example 473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491491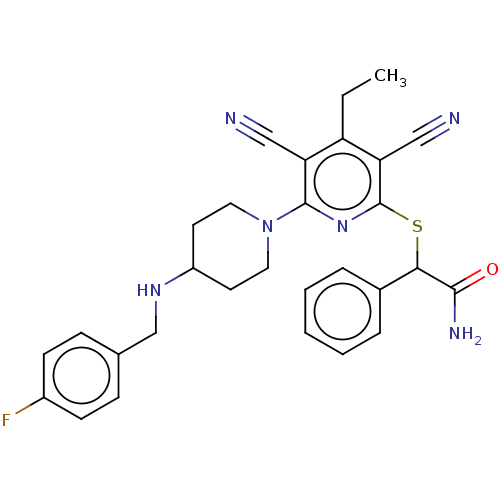 (US10975056, Example 474) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491492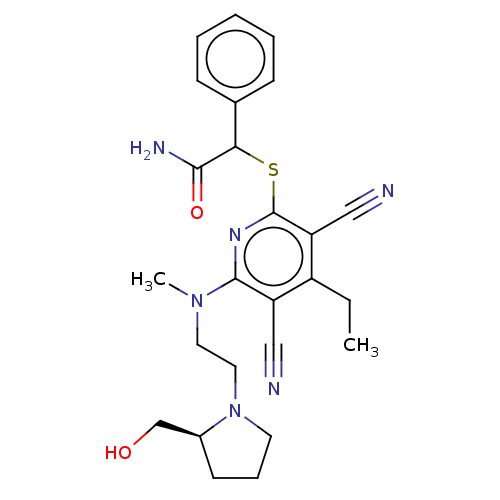 (US10975056, Example 475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491493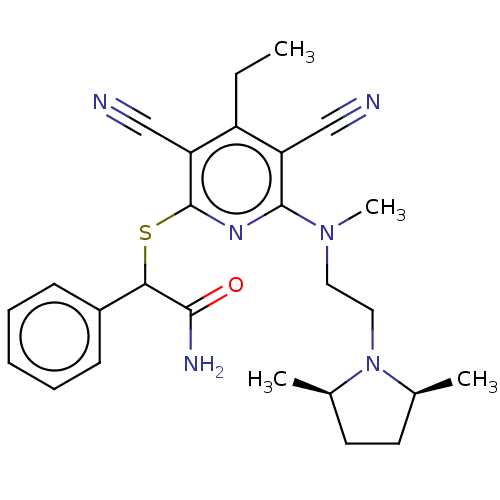 (US10975056, Example 476) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491494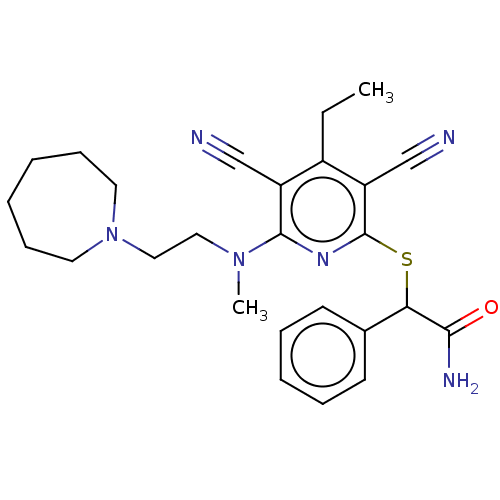 (US10975056, Example 477) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491495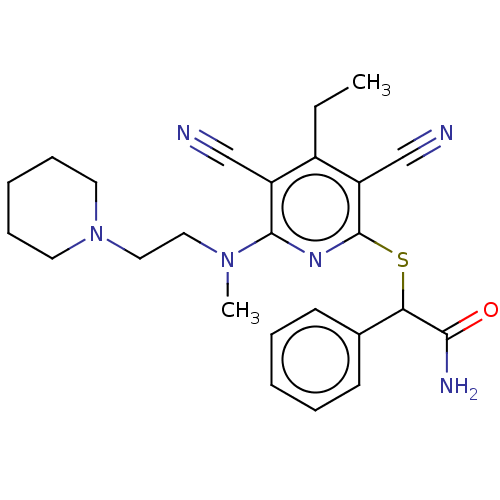 (US10975056, Example 478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491496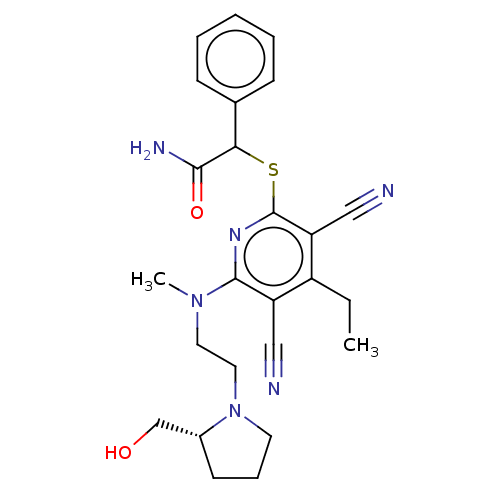 (US10975056, Example 479) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491497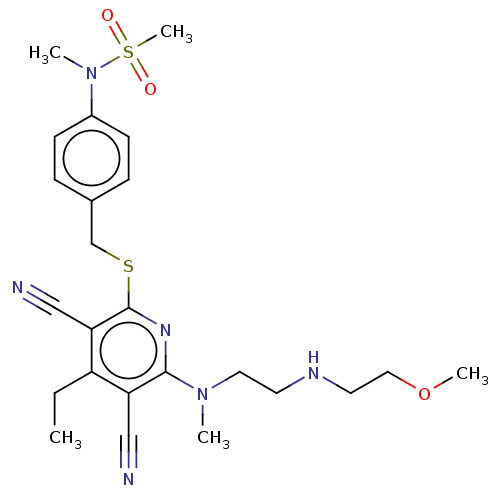 (US10975056, Example 480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491498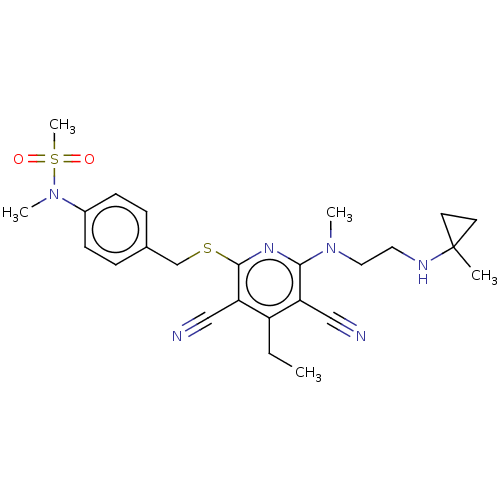 (US10975056, Example 481) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 776 total ) | Next | Last >> |