 Found 87 hits with Last Name = 'viht' and Initial = 'k'
Found 87 hits with Last Name = 'viht' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599153
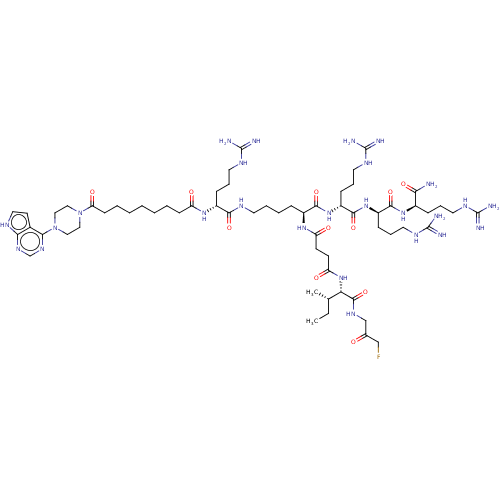
(CHEMBL5196521)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109209
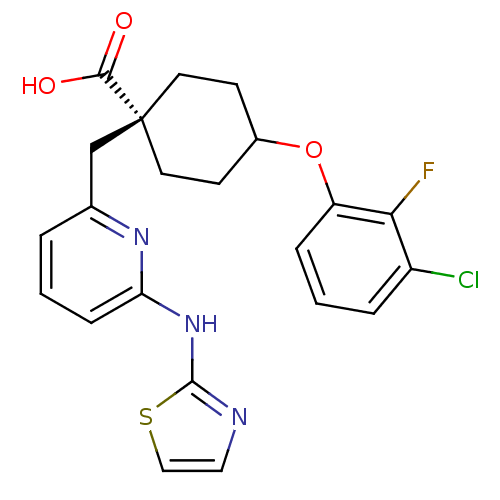
(VX-689)Show SMILES OC(=O)[C@]1(Cc2cccc(Nc3nccs3)n2)CCC(CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:3.3,(2.79,-10.07,;2.79,-8.53,;4.12,-7.76,;1.46,-7.76,;1.46,-9.3,;.12,-10.07,;.12,-11.61,;-1.21,-12.38,;-2.54,-11.61,;-2.54,-10.07,;-3.88,-9.3,;-3.88,-7.76,;-5.12,-6.85,;-4.65,-5.39,;-3.11,-5.39,;-2.63,-6.85,;-1.21,-9.3,;2.79,-6.99,;2.79,-5.45,;1.46,-4.68,;.12,-5.45,;.12,-6.99,;1.46,-3.14,;.12,-2.37,;-1.21,-3.14,;-2.54,-2.37,;-2.54,-.83,;-1.21,-.05,;-1.21,1.49,;.12,-.83,;1.46,-.06,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15?,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0155 | -62.7 | 3.88 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599152
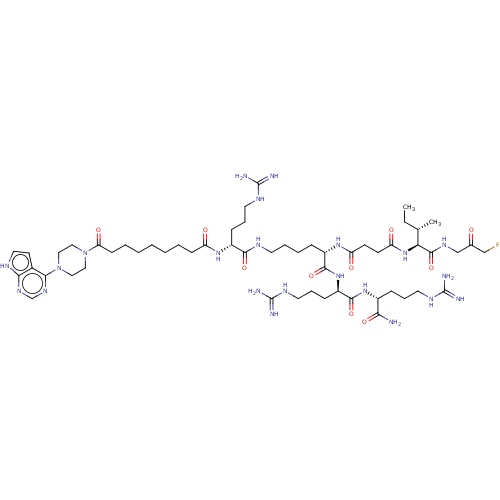
(CHEMBL5179794)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.136 | -57.3 | 34.2 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599149
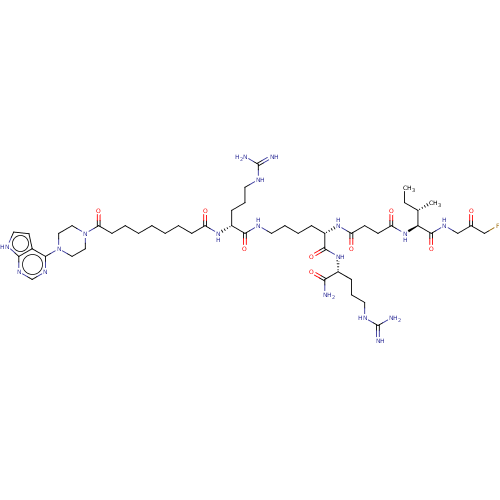
(CHEMBL5174535)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.86 | -50.7 | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM109209

(VX-689)Show SMILES OC(=O)[C@]1(Cc2cccc(Nc3nccs3)n2)CCC(CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:3.3,(2.79,-10.07,;2.79,-8.53,;4.12,-7.76,;1.46,-7.76,;1.46,-9.3,;.12,-10.07,;.12,-11.61,;-1.21,-12.38,;-2.54,-11.61,;-2.54,-10.07,;-3.88,-9.3,;-3.88,-7.76,;-5.12,-6.85,;-4.65,-5.39,;-3.11,-5.39,;-2.63,-6.85,;-1.21,-9.3,;2.79,-6.99,;2.79,-5.45,;1.46,-4.68,;.12,-5.45,;.12,-6.99,;1.46,-3.14,;.12,-2.37,;-1.21,-3.14,;-2.54,-2.37,;-2.54,-.83,;-1.21,-.05,;-1.21,1.49,;.12,-.83,;1.46,-.06,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15?,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.17 | -50.3 | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599151
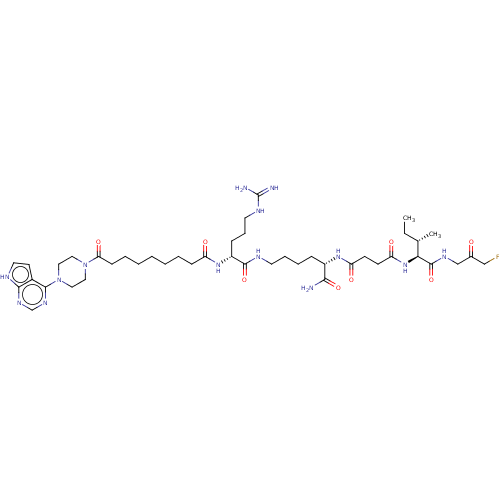
(CHEMBL5172486)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of 5-TAMRA-labeled ARC-1530 from CK2alpha (unknown origin) (1 to 335 residues) after 15 to 60 mins by luminescence assay |
Bioorg Med Chem 26: 5062-5068 (2018)
Article DOI: 10.1016/j.bmc.2018.09.003
BindingDB Entry DOI: 10.7270/Q26112V7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109209

(VX-689)Show SMILES OC(=O)[C@]1(Cc2cccc(Nc3nccs3)n2)CCC(CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:3.3,(2.79,-10.07,;2.79,-8.53,;4.12,-7.76,;1.46,-7.76,;1.46,-9.3,;.12,-10.07,;.12,-11.61,;-1.21,-12.38,;-2.54,-11.61,;-2.54,-10.07,;-3.88,-9.3,;-3.88,-7.76,;-5.12,-6.85,;-4.65,-5.39,;-3.11,-5.39,;-2.63,-6.85,;-1.21,-9.3,;2.79,-6.99,;2.79,-5.45,;1.46,-4.68,;.12,-5.45,;.12,-6.99,;1.46,-3.14,;.12,-2.37,;-1.21,-3.14,;-2.54,-2.37,;-2.54,-.83,;-1.21,-.05,;-1.21,1.49,;.12,-.83,;1.46,-.06,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15?,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM). Subseque... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM). Subseque... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50010942
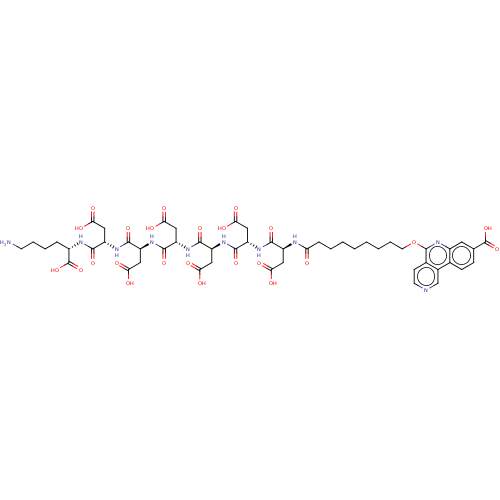
(CHEMBL4077554)Show SMILES NCCCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCCCCCCCOc1nc2cc(ccc2c2cnccc12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H40N4O5S/c1-3-31(4-2)16-17-32(25(35)13-19-37-18-12-21-8-6-5-7-9-21)15-14-29-20-24(34)22-10-11-23(33)26-27(22)38-28(36)30-26/h5-11,24,29,33-34H,3-4,12-20H2,1-2H3,(H,30,36)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of CK2alpha (unknown origin) (1 to 335 residues) using 5-TAMRA-RADDSDDDDD as substrate after 30 mins by fluorescence imaging method |
Bioorg Med Chem 25: 2277-2284 (2017)
Article DOI: 10.1016/j.bmc.2017.02.055
BindingDB Entry DOI: 10.7270/Q21G0PRW |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50199310

(2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)NC(=O)CCCCCNCCNS(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C69H116N34O14S/c70-54-50-55(93-37-92-54)103(38-94-50)63-52(107)51(106)53(117-63)62(114)85-26-6-2-4-22-48(104)96-41(15-8-27-86-64(71)72)56(108)97-42(16-9-28-87-65(73)74)57(109)98-43(17-10-29-88-66(75)76)58(110)99-44(18-11-30-89-67(77)78)59(111)100-45(19-12-31-90-68(79)80)60(112)101-46(20-13-32-91-69(81)82)61(113)102-49(105)23-3-1-5-25-83-34-35-95-118(115,116)47-21-7-14-39-36-84-33-24-40(39)47/h7,14,21,24,33,36-38,41-46,51-53,63,83,95,106-107H,1-6,8-13,15-20,22-23,25-32,34-35H2,(H,85,114)(H,96,104)(H,97,108)(H,98,109)(H,99,110)(H,100,111)(H,101,112)(H2,70,92,93)(H4,71,72,86)(H4,73,74,87)(H4,75,76,88)(H4,77,78,89)(H4,79,80,90)(H4,81,82,91)(H,102,105,113)/t41-,42-,43-,44-,45-,46-,51+,52-,53+,63-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50199310

(2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)NC(=O)CCCCCNCCNS(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C69H116N34O14S/c70-54-50-55(93-37-92-54)103(38-94-50)63-52(107)51(106)53(117-63)62(114)85-26-6-2-4-22-48(104)96-41(15-8-27-86-64(71)72)56(108)97-42(16-9-28-87-65(73)74)57(109)98-43(17-10-29-88-66(75)76)58(110)99-44(18-11-30-89-67(77)78)59(111)100-45(19-12-31-90-68(79)80)60(112)101-46(20-13-32-91-69(81)82)61(113)102-49(105)23-3-1-5-25-83-34-35-95-118(115,116)47-21-7-14-39-36-84-33-24-40(39)47/h7,14,21,24,33,36-38,41-46,51-53,63,83,95,106-107H,1-6,8-13,15-20,22-23,25-32,34-35H2,(H,85,114)(H,96,104)(H,97,108)(H,98,109)(H,99,110)(H,100,111)(H,101,112)(H2,70,92,93)(H4,71,72,86)(H4,73,74,87)(H4,75,76,88)(H4,77,78,89)(H4,79,80,90)(H4,81,82,91)(H,102,105,113)/t41-,42-,43-,44-,45-,46-,51+,52-,53+,63-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599148
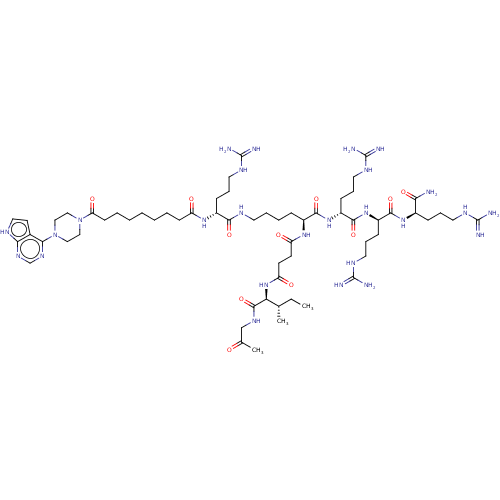
(CHEMBL5188777)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(C)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM27248
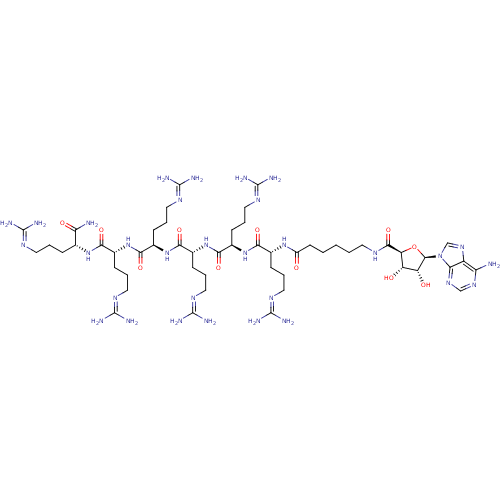
(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM27248

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of CK2alpha (unknown origin) (1 to 335 residues) using 5-TAMRA-RADDSDDDDD as substrate after 30 mins by fluorescence imaging method |
Bioorg Med Chem 25: 2277-2284 (2017)
Article DOI: 10.1016/j.bmc.2017.02.055
BindingDB Entry DOI: 10.7270/Q21G0PRW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599147

(CHEMBL5201435)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(C)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50156669
(4,5,6,7-TETRABROMO-BENZIMIDAZOLE | 4,5,6,7-tetrabr...)Show InChI InChI=1S/C7H2Br4N2/c8-2-3(9)5(11)7-6(4(2)10)12-1-13-7/h1H,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of 5-TAMRA-labeled ARC-1530 from CK2alpha (unknown origin) (1 to 335 residues) after 15 to 60 mins by luminescence assay |
Bioorg Med Chem 26: 5062-5068 (2018)
Article DOI: 10.1016/j.bmc.2018.09.003
BindingDB Entry DOI: 10.7270/Q26112V7 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50010974

(CHEMBL4067866)Show SMILES OC(=O)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCCCCCn1c2cc(ccc2c2cnccc2c1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H39ClN4O4S/c1-33(24-7-8-24)15-3-16-34(26(36)12-19-38-18-11-21-4-2-5-23(30)20-21)17-14-31-13-10-22-6-9-25(35)27-28(22)39-29(37)32-27/h2,4-6,9,20,24,31,35H,3,7-8,10-19H2,1H3,(H,32,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of CK2alpha (unknown origin) (1 to 335 residues) using 5-TAMRA-RADDSDDDDD as substrate after 30 mins by fluorescence imaging method |
Bioorg Med Chem 25: 2277-2284 (2017)
Article DOI: 10.1016/j.bmc.2017.02.055
BindingDB Entry DOI: 10.7270/Q21G0PRW |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599150
(CHEMBL5174274)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599150

(CHEMBL5174274)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599149

(CHEMBL5174535)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599146
(CHEMBL5189362)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(C)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599149

(CHEMBL5174535)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599149

(CHEMBL5174535)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599150

(CHEMBL5174274)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599145

(CHEMBL5179913)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O)C(=O)NCC(C)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109206
(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-[(4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)c2ccc(Nc3ncc4CN=C(c5cc(Cl)ccc5-c4n3)c3c(F)cccc3OC)cc2OC)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,c:9,54| Show InChI InChI=1S/C65H69ClFN9O11S2/c1-8-75-51-29-25-42(88(80,81)82)35-47(51)64(3,4)55(75)20-11-10-12-21-56-65(5,48-36-43(89(83,84)85)26-30-52(48)76(56)9-2)31-16-22-57(77)69-32-14-13-18-50(61(68)78)73-62(79)45-28-24-41(34-54(45)87-7)72-63-71-38-39-37-70-60(58-49(67)17-15-19-53(58)86-6)46-33-40(66)23-27-44(46)59(39)74-63/h10-12,15,17,19-21,23-30,33-36,38,50H,8-9,13-14,16,18,22,31-32,37H2,1-7H3,(H6-,68,69,71,72,73,74,77,78,79,80,81,82,83,84,85)/t50-,65?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.273 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109207
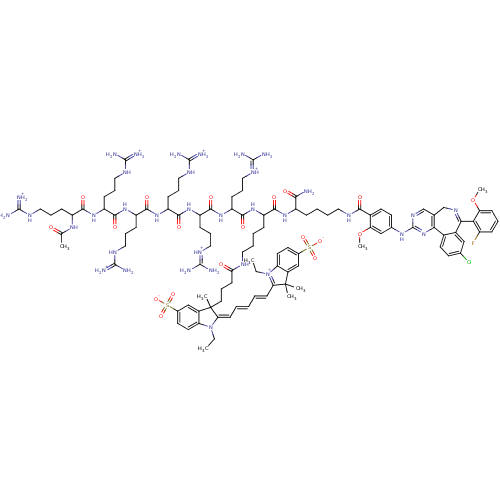
(Compound II: Ac-D-Arg6-D-Lys(PromoFluor 647)-D-Lys...)Show SMILES [#6]-[#6]-[#7]-1\[#6](=[#6]\[#6]=[#6]\[#6]=[#6]\[#6]2=[#7+](-[#6]-[#6])-c3ccc(cc3C2([#6])[#6])S([#8-])(=O)=O)C([#6])([#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7+]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c2ccc(-[#7]-c3ncc4-[#6]-[#7]=[#6](-c5cc(Cl)ccc5-c4n3)-c3c(F)cccc3-[#8]-[#6])cc2-[#8]-[#6])-[#6](-[#7])=O)c2cc(ccc-12)S([#8-])(=O)=O |c:9,132| Show InChI InChI=1S/C109H155ClFN35O19S2/c1-9-145-82-45-41-66(166(158,159)160)58-71(82)108(4,5)86(145)36-12-11-13-37-87-109(6,72-59-67(167(161,162)163)42-46-83(72)146(87)10-2)47-19-38-88(148)125-48-16-15-28-76(95(152)137-74(92(112)149)27-14-17-49-126-93(150)69-44-40-65(57-85(69)165-8)136-107-134-61-63-60-133-91(89-73(111)26-18-35-84(89)164-7)70-56-64(110)39-43-68(70)90(63)144-107)138-96(153)78(31-22-52-129-103(117)118)140-98(155)80(33-24-54-131-105(121)122)142-100(157)81(34-25-55-132-106(123)124)143-99(156)79(32-23-53-130-104(119)120)141-97(154)77(30-21-51-128-102(115)116)139-94(151)75(135-62(3)147)29-20-50-127-101(113)114/h11-13,18,26,35-37,39-46,56-59,61,74-81H,9-10,14-17,19-25,27-34,38,47-55,60H2,1-8H3,(H38-,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,134,135,136,137,138,139,140,141,142,143,144,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163)/p+5 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109208
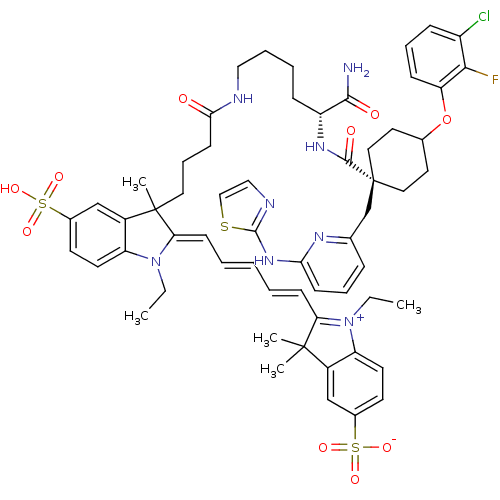
(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-{[4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@]2(Cc3cccc(Nc4nccs4)n3)CCC(CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:42.43,38.40,wD:42.44,c:9,(-16.27,-52.94,;-14.86,-52.31,;-14.7,-50.78,;-15.85,-49.75,;-17.35,-50.07,;-18.44,-48.98,;-19.93,-49.38,;-21.02,-48.29,;-22.51,-48.69,;-23.6,-47.6,;-25.12,-47.84,;-25.82,-49.22,;-27.36,-49.22,;-25.82,-46.47,;-27.3,-46.07,;-27.7,-44.59,;-26.61,-43.5,;-25.13,-43.89,;-24.73,-45.38,;-23.36,-46.08,;-22.27,-44.99,;-21.87,-46.48,;-27.01,-42.01,;-27.41,-40.52,;-25.52,-41.61,;-28.5,-42.41,;-15.22,-48.34,;-15.22,-46.8,;-16.56,-47.57,;-16.56,-46.03,;-17.89,-45.26,;-17.89,-43.72,;-16.56,-42.95,;-19.22,-42.95,;-19.22,-41.41,;-20.56,-40.64,;-20.56,-39.1,;-21.89,-38.33,;-21.89,-36.79,;-23.22,-36.02,;-23.22,-34.48,;-21.89,-33.71,;-24.56,-33.71,;-24.56,-35.25,;-25.89,-36.02,;-25.89,-37.56,;-27.23,-38.34,;-28.56,-37.57,;-28.56,-36.03,;-29.89,-35.26,;-29.89,-33.72,;-31.14,-32.81,;-30.66,-31.35,;-29.12,-31.35,;-28.65,-32.81,;-27.22,-35.26,;-23.22,-32.94,;-23.22,-31.4,;-24.56,-30.63,;-25.89,-31.4,;-25.89,-32.94,;-24.56,-29.09,;-25.89,-28.32,;-27.22,-29.09,;-28.56,-28.32,;-28.56,-26.78,;-27.22,-26.01,;-27.22,-24.47,;-25.89,-26.78,;-24.56,-26.01,;-20.56,-36.02,;-19.22,-36.79,;-20.56,-34.48,;-13.69,-48.51,;-12.55,-47.48,;-11.08,-47.95,;-10.76,-49.46,;-11.91,-50.49,;-13.37,-50.01,;-9.94,-46.92,;-8.85,-45.83,;-11.03,-45.83,;-8.85,-48.01,)| Show InChI InChI=1S/C60H70ClFN8O10S3/c1-6-69-47-26-24-41(82(74,75)76)36-43(47)58(3,4)50(69)20-9-8-10-21-51-59(5,44-37-42(83(77,78)79)25-27-48(44)70(51)7-2)30-15-23-53(71)64-33-12-11-18-46(55(63)72)67-56(73)60(38-39-16-13-22-52(66-39)68-57-65-34-35-81-57)31-28-40(29-32-60)80-49-19-14-17-45(61)54(49)62/h8-10,13-14,16-17,19-22,24-27,34-37,40,46H,6-7,11-12,15,18,23,28-33,38H2,1-5H3,(H6-,63,64,65,66,67,68,71,72,73,74,75,76,77,78,79)/t40?,46-,59?,60-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM109206

(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-[(4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)c2ccc(Nc3ncc4CN=C(c5cc(Cl)ccc5-c4n3)c3c(F)cccc3OC)cc2OC)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,c:9,54| Show InChI InChI=1S/C65H69ClFN9O11S2/c1-8-75-51-29-25-42(88(80,81)82)35-47(51)64(3,4)55(75)20-11-10-12-21-56-65(5,48-36-43(89(83,84)85)26-30-52(48)76(56)9-2)31-16-22-57(77)69-32-14-13-18-50(61(68)78)73-62(79)45-28-24-41(34-54(45)87-7)72-63-71-38-39-37-70-60(58-49(67)17-15-19-53(58)86-6)46-33-40(66)23-27-44(46)59(39)74-63/h10-12,15,17,19-21,23-30,33-36,38,50H,8-9,13-14,16,18,22,31-32,37H2,1-7H3,(H6-,68,69,71,72,73,74,77,78,79,80,81,82,83,84,85)/t50-,65?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.527 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM109207

(Compound II: Ac-D-Arg6-D-Lys(PromoFluor 647)-D-Lys...)Show SMILES [#6]-[#6]-[#7]-1\[#6](=[#6]\[#6]=[#6]\[#6]=[#6]\[#6]2=[#7+](-[#6]-[#6])-c3ccc(cc3C2([#6])[#6])S([#8-])(=O)=O)C([#6])([#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7+]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c2ccc(-[#7]-c3ncc4-[#6]-[#7]=[#6](-c5cc(Cl)ccc5-c4n3)-c3c(F)cccc3-[#8]-[#6])cc2-[#8]-[#6])-[#6](-[#7])=O)c2cc(ccc-12)S([#8-])(=O)=O |c:9,132| Show InChI InChI=1S/C109H155ClFN35O19S2/c1-9-145-82-45-41-66(166(158,159)160)58-71(82)108(4,5)86(145)36-12-11-13-37-87-109(6,72-59-67(167(161,162)163)42-46-83(72)146(87)10-2)47-19-38-88(148)125-48-16-15-28-76(95(152)137-74(92(112)149)27-14-17-49-126-93(150)69-44-40-65(57-85(69)165-8)136-107-134-61-63-60-133-91(89-73(111)26-18-35-84(89)164-7)70-56-64(110)39-43-68(70)90(63)144-107)138-96(153)78(31-22-52-129-103(117)118)140-98(155)80(33-24-54-131-105(121)122)142-100(157)81(34-25-55-132-106(123)124)143-99(156)79(32-23-53-130-104(119)120)141-97(154)77(30-21-51-128-102(115)116)139-94(151)75(135-62(3)147)29-20-50-127-101(113)114/h11-13,18,26,35-37,39-46,56-59,61,74-81H,9-10,14-17,19-25,27-34,38,47-55,60H2,1-8H3,(H38-,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,134,135,136,137,138,139,140,141,142,143,144,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163)/p+5 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.34 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM109208

(2-[(1E,3E)-5-[(2E)-3-(3-{[(5R)-5-carbamoyl-5-{[4- ...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCCC[C@@H](NC(=O)[C@]2(Cc3cccc(Nc4nccs4)n3)CCC(CC2)Oc2cccc(Cl)c2F)C(N)=O)c2cc(ccc12)S(O)(=O)=O |r,wU:42.43,38.40,wD:42.44,c:9,(-16.27,-52.94,;-14.86,-52.31,;-14.7,-50.78,;-15.85,-49.75,;-17.35,-50.07,;-18.44,-48.98,;-19.93,-49.38,;-21.02,-48.29,;-22.51,-48.69,;-23.6,-47.6,;-25.12,-47.84,;-25.82,-49.22,;-27.36,-49.22,;-25.82,-46.47,;-27.3,-46.07,;-27.7,-44.59,;-26.61,-43.5,;-25.13,-43.89,;-24.73,-45.38,;-23.36,-46.08,;-22.27,-44.99,;-21.87,-46.48,;-27.01,-42.01,;-27.41,-40.52,;-25.52,-41.61,;-28.5,-42.41,;-15.22,-48.34,;-15.22,-46.8,;-16.56,-47.57,;-16.56,-46.03,;-17.89,-45.26,;-17.89,-43.72,;-16.56,-42.95,;-19.22,-42.95,;-19.22,-41.41,;-20.56,-40.64,;-20.56,-39.1,;-21.89,-38.33,;-21.89,-36.79,;-23.22,-36.02,;-23.22,-34.48,;-21.89,-33.71,;-24.56,-33.71,;-24.56,-35.25,;-25.89,-36.02,;-25.89,-37.56,;-27.23,-38.34,;-28.56,-37.57,;-28.56,-36.03,;-29.89,-35.26,;-29.89,-33.72,;-31.14,-32.81,;-30.66,-31.35,;-29.12,-31.35,;-28.65,-32.81,;-27.22,-35.26,;-23.22,-32.94,;-23.22,-31.4,;-24.56,-30.63,;-25.89,-31.4,;-25.89,-32.94,;-24.56,-29.09,;-25.89,-28.32,;-27.22,-29.09,;-28.56,-28.32,;-28.56,-26.78,;-27.22,-26.01,;-27.22,-24.47,;-25.89,-26.78,;-24.56,-26.01,;-20.56,-36.02,;-19.22,-36.79,;-20.56,-34.48,;-13.69,-48.51,;-12.55,-47.48,;-11.08,-47.95,;-10.76,-49.46,;-11.91,-50.49,;-13.37,-50.01,;-9.94,-46.92,;-8.85,-45.83,;-11.03,-45.83,;-8.85,-48.01,)| Show InChI InChI=1S/C60H70ClFN8O10S3/c1-6-69-47-26-24-41(82(74,75)76)36-43(47)58(3,4)50(69)20-9-8-10-21-51-59(5,44-37-42(83(77,78)79)25-27-48(44)70(51)7-2)30-15-23-53(71)64-33-12-11-18-46(55(63)72)67-56(73)60(38-39-16-13-22-52(66-39)68-57-65-34-35-81-57)31-28-40(29-32-60)80-49-19-14-17-45(61)54(49)62/h8-10,13-14,16-17,19-22,24-27,34-37,40,46H,6-7,11-12,15,18,23,28-33,38H2,1-5H3,(H6-,63,64,65,66,67,68,71,72,73,74,75,76,77,78,79)/t40?,46-,59?,60-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.544 | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
A stock solution was prepared containing complex of Aurora A and Compound I (final total concentrations of 6nM and 5nM, respectively). Separately, se... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224008
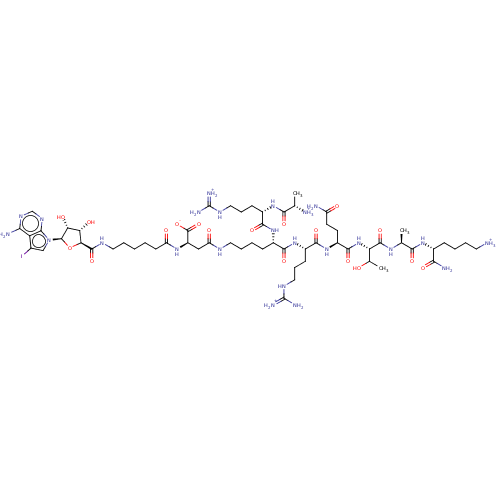
(ARC-3429)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCC[NH3+])C(N)=O |r| Show InChI InChI=1S/C60H101IN24O18/c1-28(63)49(93)80-34(15-11-23-73-59(67)68)52(96)81-33(14-7-10-21-71-40(89)25-37(58(101)102)78-39(88)17-5-4-9-22-72-56(100)45-43(90)44(91)57(103-45)85-26-31(61)41-46(65)75-27-76-48(41)85)51(95)82-35(16-12-24-74-60(69)70)53(97)83-36(18-19-38(64)87)54(98)84-42(30(3)86)55(99)77-29(2)50(94)79-32(47(66)92)13-6-8-20-62/h26-30,32-37,42-45,57,86,90-91H,4-25,62-63H2,1-3H3,(H2,64,87)(H2,66,92)(H,71,89)(H,72,100)(H,77,99)(H,78,88)(H,79,94)(H,80,93)(H,81,96)(H,82,95)(H,83,97)(H,84,98)(H,101,102)(H2,65,75,76)(H4,67,68,73)(H4,69,70,74)/p+3/t28-,29-,30?,32+,33-,34-,35-,36-,37+,42-,43-,44+,45-,57+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224008

(ARC-3429)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCC[NH3+])C(N)=O |r| Show InChI InChI=1S/C60H101IN24O18/c1-28(63)49(93)80-34(15-11-23-73-59(67)68)52(96)81-33(14-7-10-21-71-40(89)25-37(58(101)102)78-39(88)17-5-4-9-22-72-56(100)45-43(90)44(91)57(103-45)85-26-31(61)41-46(65)75-27-76-48(41)85)51(95)82-35(16-12-24-74-60(69)70)53(97)83-36(18-19-38(64)87)54(98)84-42(30(3)86)55(99)77-29(2)50(94)79-32(47(66)92)13-6-8-20-62/h26-30,32-37,42-45,57,86,90-91H,4-25,62-63H2,1-3H3,(H2,64,87)(H2,66,92)(H,71,89)(H,72,100)(H,77,99)(H,78,88)(H,79,94)(H,80,93)(H,81,96)(H,82,95)(H,83,97)(H,84,98)(H,101,102)(H2,65,75,76)(H4,67,68,73)(H4,69,70,74)/p+3/t28-,29-,30?,32+,33-,34-,35-,36-,37+,42-,43-,44+,45-,57+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0190 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224009
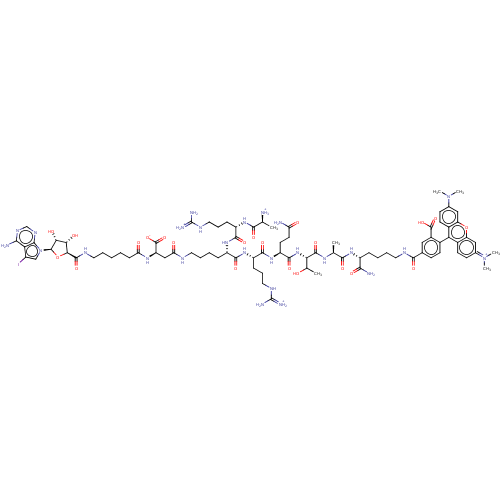
(ARC-3430)Show SMILES C[C@H]([NH3+])C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:6.5,62.64,82.84,38.37,40.45,wD:17.16,1.1,73.75,89.92,94.96,26.26,41.41,43.44,(11.91,-18.78,;11.91,-17.24,;10.58,-16.47,;13.25,-16.47,;13.25,-14.93,;14.58,-17.24,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;17.25,-17.24,;17.25,-18.78,;18.58,-16.47,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;12.28,-31.1,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;21.25,-16.47,;21.25,-14.93,;22.58,-17.24,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;25.25,-17.24,;25.25,-18.78,;26.59,-16.47,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;29.25,-16.47,;29.25,-14.93,;30.59,-17.24,;31.92,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H121IN26O22/c1-41(87)72(120)105-54(19-15-33-98-84(91)92)76(124)106-53(18-11-13-30-95-62(116)38-57(83(131)132)103-61(115)21-9-8-12-31-97-80(128)68-66(117)67(118)81(134-68)112-39-51(86)64-69(89)100-40-101-71(64)112)75(123)107-55(20-16-34-99-85(93)94)77(125)108-56(28-29-60(88)114)78(126)109-65(43(3)113)79(127)102-42(2)73(121)104-52(70(90)119)17-10-14-32-96-74(122)44-22-25-47(50(35-44)82(129)130)63-48-26-23-45(110(4)5)36-58(48)133-59-37-46(111(6)7)24-27-49(59)63/h22-27,35-37,39-43,52-57,65-68,81,113,117-118H,8-21,28-34,38,87H2,1-7H3,(H26-,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,114,115,116,119,120,121,122,123,124,125,126,127,128,129,130,131,132)/p+3/t41-,42-,43?,52+,53-,54-,55-,56-,57+,65-,66-,67+,68-,81+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224009

(ARC-3430)Show SMILES C[C@H]([NH3+])C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:6.5,62.64,82.84,38.37,40.45,wD:17.16,1.1,73.75,89.92,94.96,26.26,41.41,43.44,(11.91,-18.78,;11.91,-17.24,;10.58,-16.47,;13.25,-16.47,;13.25,-14.93,;14.58,-17.24,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;17.25,-17.24,;17.25,-18.78,;18.58,-16.47,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;12.28,-31.1,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;21.25,-16.47,;21.25,-14.93,;22.58,-17.24,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;25.25,-17.24,;25.25,-18.78,;26.59,-16.47,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;29.25,-16.47,;29.25,-14.93,;30.59,-17.24,;31.92,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H121IN26O22/c1-41(87)72(120)105-54(19-15-33-98-84(91)92)76(124)106-53(18-11-13-30-95-62(116)38-57(83(131)132)103-61(115)21-9-8-12-31-97-80(128)68-66(117)67(118)81(134-68)112-39-51(86)64-69(89)100-40-101-71(64)112)75(123)107-55(20-16-34-99-85(93)94)77(125)108-56(28-29-60(88)114)78(126)109-65(43(3)113)79(127)102-42(2)73(121)104-52(70(90)119)17-10-14-32-96-74(122)44-22-25-47(50(35-44)82(129)130)63-48-26-23-45(110(4)5)36-58(48)133-59-37-46(111(6)7)24-27-49(59)63/h22-27,35-37,39-43,52-57,65-68,81,113,117-118H,8-21,28-34,38,87H2,1-7H3,(H26-,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,114,115,116,119,120,121,122,123,124,125,126,127,128,129,130,131,132)/p+3/t41-,42-,43?,52+,53-,54-,55-,56-,57+,65-,66-,67+,68-,81+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224010
(ARC-3384)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:71.81,16.23,3.3,48.47,50.55,wD:27.70,82.86,7.12,88.91,93.95,36.36,51.51,53.54,(30.59,-14.16,;31.92,-14.93,;33.25,-14.16,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H122N26O22/c1-42(86)72(119)104-55(19-15-34-97-84(90)91)76(123)105-54(18-11-13-31-94-63(115)40-58(83(130)131)102-62(114)21-9-8-12-32-96-80(127)68-66(116)67(117)81(133-68)111-36-30-51-69(88)99-41-100-71(51)111)75(122)106-56(20-16-35-98-85(92)93)77(124)107-57(28-29-61(87)113)78(125)108-65(44(3)112)79(126)101-43(2)73(120)103-53(70(89)118)17-10-14-33-95-74(121)45-22-25-48(52(37-45)82(128)129)64-49-26-23-46(109(4)5)38-59(49)132-60-39-47(110(6)7)24-27-50(60)64/h22-27,30,36-39,41-44,53-58,65-68,81,112,116-117H,8-21,28-29,31-35,40,86H2,1-7H3,(H26-,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,113,114,115,118,119,120,121,122,123,124,125,126,127,128,129,130,131)/p+3/t42-,43-,44?,53+,54-,55-,56-,57-,58+,65-,66-,67+,68-,81+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224011
(ARC-3380)Show SMILES CCCCCCCCCCCCCC(=O)NCCCC[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(C)O)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:97.107,42.49,29.29,74.73,76.81,117.119,wD:53.96,108.112,33.38,24.24,20.20,62.62,77.77,79.80,(21.25,-5.69,;22.58,-4.92,;23.92,-5.69,;25.25,-4.92,;26.59,-5.69,;27.92,-4.92,;29.25,-5.69,;30.59,-4.92,;31.92,-5.69,;33.25,-4.92,;34.59,-5.69,;34.59,-7.23,;35.92,-8,;35.92,-9.54,;34.59,-10.31,;37.25,-10.31,;37.25,-11.85,;38.59,-12.62,;38.59,-14.16,;39.92,-14.93,;39.92,-16.47,;38.59,-17.24,;37.25,-16.47,;37.25,-14.93,;35.92,-17.24,;35.92,-18.78,;34.59,-16.47,;33.25,-17.24,;33.25,-18.78,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;41.26,-17.24,;41.26,-18.78,;42.59,-16.47,;43.92,-17.24,;43.92,-18.78,;42.59,-19.55,;42.59,-21.09,;41.26,-21.86,;41.26,-23.4,;39.92,-24.17,;38.59,-23.4,;39.92,-25.71,;38.59,-26.48,;38.59,-28.02,;39.92,-28.79,;41.26,-28.02,;41.26,-26.48,;42.74,-28.42,;43.14,-29.91,;43.83,-27.33,;39.92,-30.33,;38.59,-31.1,;37.25,-30.33,;35.92,-31.1,;35.92,-32.64,;37.25,-33.41,;38.59,-32.64,;39.92,-33.41,;41.26,-32.64,;42.59,-33.41,;43.92,-32.64,;43.92,-31.1,;42.59,-30.33,;41.26,-31.1,;45.26,-33.41,;46.59,-32.64,;45.26,-34.95,;34.59,-33.41,;33.25,-32.64,;34.59,-34.95,;45.26,-16.47,;46.59,-17.24,;45.26,-14.93,)| Show InChI InChI=1S/C105H160N28O24/c1-9-10-11-12-13-14-15-16-17-18-20-36-80(136)114-47-26-23-32-71(94(145)124-70(89(109)141)31-22-28-50-116-93(144)62-38-41-65(69(54-62)102(152)153)83-66-42-39-63(131(5)6)55-77(66)156-78-56-64(132(7)8)40-43-67(78)83)126-92(143)60(3)122-99(150)84(61(4)134)130-98(149)75(44-45-79(107)135)129-97(148)74(35-30-52-119-105(112)113)128-95(146)72(127-96(147)73(125-91(142)59(2)106)34-29-51-118-104(110)111)33-24-27-48-115-82(138)57-76(103(154)155)123-81(137)37-21-19-25-49-117-100(151)87-85(139)86(140)101(157-87)133-53-46-68-88(108)120-58-121-90(68)133/h38-43,46,53-56,58-61,70-76,84-87,101,134,139-140H,9-37,44-45,47-52,57,106H2,1-8H3,(H28-,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,135,136,137,138,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155)/p+3/t59-,60-,61?,70+,71+,72-,73-,74-,75-,76+,84-,85-,86+,87-,101+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM224008

(ARC-3429)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCC[NH3+])C(N)=O |r| Show InChI InChI=1S/C60H101IN24O18/c1-28(63)49(93)80-34(15-11-23-73-59(67)68)52(96)81-33(14-7-10-21-71-40(89)25-37(58(101)102)78-39(88)17-5-4-9-22-72-56(100)45-43(90)44(91)57(103-45)85-26-31(61)41-46(65)75-27-76-48(41)85)51(95)82-35(16-12-24-74-60(69)70)53(97)83-36(18-19-38(64)87)54(98)84-42(30(3)86)55(99)77-29(2)50(94)79-32(47(66)92)13-6-8-20-62/h26-30,32-37,42-45,57,86,90-91H,4-25,62-63H2,1-3H3,(H2,64,87)(H2,66,92)(H,71,89)(H,72,100)(H,77,99)(H,78,88)(H,79,94)(H,80,93)(H,81,96)(H,82,95)(H,83,97)(H,84,98)(H,101,102)(H2,65,75,76)(H4,67,68,73)(H4,69,70,74)/p+3/t28-,29-,30?,32+,33-,34-,35-,36-,37+,42-,43-,44+,45-,57+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 94 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM224009

(ARC-3430)Show SMILES C[C@H]([NH3+])C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:6.5,62.64,82.84,38.37,40.45,wD:17.16,1.1,73.75,89.92,94.96,26.26,41.41,43.44,(11.91,-18.78,;11.91,-17.24,;10.58,-16.47,;13.25,-16.47,;13.25,-14.93,;14.58,-17.24,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;17.25,-17.24,;17.25,-18.78,;18.58,-16.47,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;12.28,-31.1,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;21.25,-16.47,;21.25,-14.93,;22.58,-17.24,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;25.25,-17.24,;25.25,-18.78,;26.59,-16.47,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;29.25,-16.47,;29.25,-14.93,;30.59,-17.24,;31.92,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H121IN26O22/c1-41(87)72(120)105-54(19-15-33-98-84(91)92)76(124)106-53(18-11-13-30-95-62(116)38-57(83(131)132)103-61(115)21-9-8-12-31-97-80(128)68-66(117)67(118)81(134-68)112-39-51(86)64-69(89)100-40-101-71(64)112)75(123)107-55(20-16-34-99-85(93)94)77(125)108-56(28-29-60(88)114)78(126)109-65(43(3)113)79(127)102-42(2)73(121)104-52(70(90)119)17-10-14-32-96-74(122)44-22-25-47(50(35-44)82(129)130)63-48-26-23-45(110(4)5)36-58(48)133-59-37-46(111(6)7)24-27-49(59)63/h22-27,35-37,39-43,52-57,65-68,81,113,117-118H,8-21,28-34,38,87H2,1-7H3,(H26-,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,114,115,116,119,120,121,122,123,124,125,126,127,128,129,130,131,132)/p+3/t41-,42-,43?,52+,53-,54-,55-,56-,57+,65-,66-,67+,68-,81+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM224010

(ARC-3384)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:71.81,16.23,3.3,48.47,50.55,wD:27.70,82.86,7.12,88.91,93.95,36.36,51.51,53.54,(30.59,-14.16,;31.92,-14.93,;33.25,-14.16,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H122N26O22/c1-42(86)72(119)104-55(19-15-34-97-84(90)91)76(123)105-54(18-11-13-31-94-63(115)40-58(83(130)131)102-62(114)21-9-8-12-32-96-80(127)68-66(116)67(117)81(133-68)111-36-30-51-69(88)99-41-100-71(51)111)75(122)106-56(20-16-35-98-85(92)93)77(124)107-57(28-29-61(87)113)78(125)108-65(44(3)112)79(126)101-43(2)73(120)103-53(70(89)118)17-10-14-33-95-74(121)45-22-25-48(52(37-45)82(128)129)64-49-26-23-46(109(4)5)38-59(49)132-60-39-47(110(6)7)24-27-50(60)64/h22-27,30,36-39,41-44,53-58,65-68,81,112,116-117H,8-21,28-29,31-35,40,86H2,1-7H3,(H26-,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,113,114,115,118,119,120,121,122,123,124,125,126,127,128,129,130,131)/p+3/t42-,43-,44?,53+,54-,55-,56-,57-,58+,65-,66-,67+,68-,81+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM224011

(ARC-3380)Show SMILES CCCCCCCCCCCCCC(=O)NCCCC[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(C)O)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:97.107,42.49,29.29,74.73,76.81,117.119,wD:53.96,108.112,33.38,24.24,20.20,62.62,77.77,79.80,(21.25,-5.69,;22.58,-4.92,;23.92,-5.69,;25.25,-4.92,;26.59,-5.69,;27.92,-4.92,;29.25,-5.69,;30.59,-4.92,;31.92,-5.69,;33.25,-4.92,;34.59,-5.69,;34.59,-7.23,;35.92,-8,;35.92,-9.54,;34.59,-10.31,;37.25,-10.31,;37.25,-11.85,;38.59,-12.62,;38.59,-14.16,;39.92,-14.93,;39.92,-16.47,;38.59,-17.24,;37.25,-16.47,;37.25,-14.93,;35.92,-17.24,;35.92,-18.78,;34.59,-16.47,;33.25,-17.24,;33.25,-18.78,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;41.26,-17.24,;41.26,-18.78,;42.59,-16.47,;43.92,-17.24,;43.92,-18.78,;42.59,-19.55,;42.59,-21.09,;41.26,-21.86,;41.26,-23.4,;39.92,-24.17,;38.59,-23.4,;39.92,-25.71,;38.59,-26.48,;38.59,-28.02,;39.92,-28.79,;41.26,-28.02,;41.26,-26.48,;42.74,-28.42,;43.14,-29.91,;43.83,-27.33,;39.92,-30.33,;38.59,-31.1,;37.25,-30.33,;35.92,-31.1,;35.92,-32.64,;37.25,-33.41,;38.59,-32.64,;39.92,-33.41,;41.26,-32.64,;42.59,-33.41,;43.92,-32.64,;43.92,-31.1,;42.59,-30.33,;41.26,-31.1,;45.26,-33.41,;46.59,-32.64,;45.26,-34.95,;34.59,-33.41,;33.25,-32.64,;34.59,-34.95,;45.26,-16.47,;46.59,-17.24,;45.26,-14.93,)| Show InChI InChI=1S/C105H160N28O24/c1-9-10-11-12-13-14-15-16-17-18-20-36-80(136)114-47-26-23-32-71(94(145)124-70(89(109)141)31-22-28-50-116-93(144)62-38-41-65(69(54-62)102(152)153)83-66-42-39-63(131(5)6)55-77(66)156-78-56-64(132(7)8)40-43-67(78)83)126-92(143)60(3)122-99(150)84(61(4)134)130-98(149)75(44-45-79(107)135)129-97(148)74(35-30-52-119-105(112)113)128-95(146)72(127-96(147)73(125-91(142)59(2)106)34-29-51-118-104(110)111)33-24-27-48-115-82(138)57-76(103(154)155)123-81(137)37-21-19-25-49-117-100(151)87-85(139)86(140)101(157-87)133-53-46-68-88(108)120-58-121-90(68)133/h38-43,46,53-56,58-61,70-76,84-87,101,134,139-140H,9-37,44-45,47-52,57,106H2,1-8H3,(H28-,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,135,136,137,138,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155)/p+3/t59-,60-,61?,70+,71+,72-,73-,74-,75-,76+,84-,85-,86+,87-,101+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
All measurements were performed in HEPES (50 mm, pH 7.5) containing NaCl (150 mm), Tween-20 (0.005%) and DTT (5 mm) according to a previously publish... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224009

(ARC-3430)Show SMILES C[C@H]([NH3+])C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12)C([O-])=O)C(=O)N[C@@H](CCCNC(N)=[NH2+])C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:6.5,62.64,82.84,38.37,40.45,wD:17.16,1.1,73.75,89.92,94.96,26.26,41.41,43.44,(11.91,-18.78,;11.91,-17.24,;10.58,-16.47,;13.25,-16.47,;13.25,-14.93,;14.58,-17.24,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;17.25,-17.24,;17.25,-18.78,;18.58,-16.47,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;12.28,-31.1,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;21.25,-16.47,;21.25,-14.93,;22.58,-17.24,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;25.25,-17.24,;25.25,-18.78,;26.59,-16.47,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;29.25,-16.47,;29.25,-14.93,;30.59,-17.24,;31.92,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H121IN26O22/c1-41(87)72(120)105-54(19-15-33-98-84(91)92)76(124)106-53(18-11-13-30-95-62(116)38-57(83(131)132)103-61(115)21-9-8-12-31-97-80(128)68-66(117)67(118)81(134-68)112-39-51(86)64-69(89)100-40-101-71(64)112)75(123)107-55(20-16-34-99-85(93)94)77(125)108-56(28-29-60(88)114)78(126)109-65(43(3)113)79(127)102-42(2)73(121)104-52(70(90)119)17-10-14-32-96-74(122)44-22-25-47(50(35-44)82(129)130)63-48-26-23-45(110(4)5)36-58(48)133-59-37-46(111(6)7)24-27-49(59)63/h22-27,35-37,39-43,52-57,65-68,81,113,117-118H,8-21,28-34,38,87H2,1-7H3,(H26-,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,114,115,116,119,120,121,122,123,124,125,126,127,128,129,130,131,132)/p+3/t41-,42-,43?,52+,53-,54-,55-,56-,57+,65-,66-,67+,68-,81+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
Association kinetics (Kon): All measurements were performed in HEPES (50 mM, pH 7.5) containing NaCl (150 mM), Tween-20 (0.005%) and DTT (5 mM) accor... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224010

(ARC-3384)Show SMILES CC(O)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:71.81,16.23,3.3,48.47,50.55,wD:27.70,82.86,7.12,88.91,93.95,36.36,51.51,53.54,(30.59,-14.16,;31.92,-14.93,;33.25,-14.16,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;33.25,-17.24,;33.25,-18.78,;34.59,-16.47,;35.92,-17.24,;35.92,-18.78,;37.25,-16.47,;37.25,-14.93,;38.59,-17.24,;39.92,-16.47,;39.92,-14.93,;38.59,-14.16,;38.59,-12.62,;37.25,-11.85,;37.25,-10.31,;35.92,-9.54,;34.59,-10.31,;35.92,-8,;34.59,-7.23,;34.59,-5.69,;35.92,-4.92,;37.25,-5.69,;37.25,-7.23,;38.74,-5.29,;39.14,-3.8,;39.83,-6.38,;35.92,-3.38,;34.59,-2.61,;33.25,-3.38,;31.92,-2.61,;31.92,-1.07,;33.25,-.3,;34.59,-1.07,;35.92,-.3,;37.25,-1.07,;38.59,-.3,;39.92,-1.07,;39.92,-2.61,;38.59,-3.38,;37.25,-2.61,;41.26,-.3,;41.26,1.24,;42.59,-1.07,;30.59,-.3,;30.59,1.24,;29.25,-1.07,;41.26,-17.24,;42.59,-16.47,;41.26,-18.78,)| Show InChI InChI=1S/C85H122N26O22/c1-42(86)72(119)104-55(19-15-34-97-84(90)91)76(123)105-54(18-11-13-31-94-63(115)40-58(83(130)131)102-62(114)21-9-8-12-32-96-80(127)68-66(116)67(117)81(133-68)111-36-30-51-69(88)99-41-100-71(51)111)75(122)106-56(20-16-35-98-85(92)93)77(124)107-57(28-29-61(87)113)78(125)108-65(44(3)112)79(126)101-43(2)73(120)103-53(70(89)118)17-10-14-33-95-74(121)45-22-25-48(52(37-45)82(128)129)64-49-26-23-46(109(4)5)38-59(49)132-60-39-47(110(6)7)24-27-50(60)64/h22-27,30,36-39,41-44,53-58,65-68,81,112,116-117H,8-21,28-29,31-35,40,86H2,1-7H3,(H26-,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,113,114,115,118,119,120,121,122,123,124,125,126,127,128,129,130,131)/p+3/t42-,43-,44?,53+,54-,55-,56-,57-,58+,65-,66-,67+,68-,81+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.84 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
Association kinetics (Kon): All measurements were performed in HEPES (50 mM, pH 7.5) containing NaCl (150 mM), Tween-20 (0.005%) and DTT (5 mM) accor... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin [470-798]
(Homo sapiens (Human)) | BDBM224011

(ARC-3380)Show SMILES CCCCCCCCCCCCCC(=O)NCCCC[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](CCCCNC(=O)C[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)C([O-])=O)NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@H](C)[NH3+])C(C)O)C(=O)N[C@H](CCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(N)=O |r,wU:97.107,42.49,29.29,74.73,76.81,117.119,wD:53.96,108.112,33.38,24.24,20.20,62.62,77.77,79.80,(21.25,-5.69,;22.58,-4.92,;23.92,-5.69,;25.25,-4.92,;26.59,-5.69,;27.92,-4.92,;29.25,-5.69,;30.59,-4.92,;31.92,-5.69,;33.25,-4.92,;34.59,-5.69,;34.59,-7.23,;35.92,-8,;35.92,-9.54,;34.59,-10.31,;37.25,-10.31,;37.25,-11.85,;38.59,-12.62,;38.59,-14.16,;39.92,-14.93,;39.92,-16.47,;38.59,-17.24,;37.25,-16.47,;37.25,-14.93,;35.92,-17.24,;35.92,-18.78,;34.59,-16.47,;33.25,-17.24,;33.25,-18.78,;31.92,-16.47,;30.59,-17.24,;29.25,-16.47,;29.25,-14.93,;27.92,-17.24,;27.92,-18.78,;26.59,-19.55,;26.59,-21.09,;27.92,-21.86,;25.25,-21.86,;26.59,-16.47,;25.25,-17.24,;25.25,-18.78,;23.92,-16.47,;23.92,-14.93,;22.58,-14.16,;22.58,-12.62,;21.25,-11.85,;21.25,-10.31,;22.58,-9.54,;19.92,-9.54,;22.58,-17.24,;21.25,-16.47,;21.25,-14.93,;19.92,-17.24,;19.92,-18.78,;18.58,-19.55,;18.58,-21.09,;17.25,-21.86,;17.25,-23.4,;15.92,-24.17,;14.58,-23.4,;15.92,-25.71,;14.58,-26.48,;13.25,-25.71,;11.91,-26.48,;11.91,-28.02,;10.58,-25.71,;9.25,-26.48,;7.91,-25.71,;6.58,-26.48,;5.25,-25.71,;3.91,-26.48,;3.91,-33.15,;2.58,-33.92,;5.25,-33.92,;6.54,-33.08,;7.73,-34.05,;7.18,-35.49,;8.02,-36.78,;5.64,-35.41,;4.68,-36.61,;9.22,-33.66,;9.77,-32.22,;11.31,-32.3,;11.71,-33.79,;13.08,-34.48,;14.37,-33.65,;13.16,-36.02,;11.87,-36.86,;10.5,-36.16,;10.42,-34.62,;14.58,-28.02,;15.92,-28.79,;13.25,-28.79,;18.58,-16.47,;17.25,-17.24,;17.25,-18.78,;15.92,-16.47,;15.92,-14.93,;14.58,-14.16,;14.58,-12.62,;13.25,-11.85,;13.25,-10.31,;14.58,-9.54,;11.91,-9.54,;14.58,-17.24,;13.25,-16.47,;13.25,-14.93,;11.91,-17.24,;11.91,-18.78,;10.58,-16.47,;31.92,-14.93,;30.59,-14.16,;33.25,-14.16,;41.26,-17.24,;41.26,-18.78,;42.59,-16.47,;43.92,-17.24,;43.92,-18.78,;42.59,-19.55,;42.59,-21.09,;41.26,-21.86,;41.26,-23.4,;39.92,-24.17,;38.59,-23.4,;39.92,-25.71,;38.59,-26.48,;38.59,-28.02,;39.92,-28.79,;41.26,-28.02,;41.26,-26.48,;42.74,-28.42,;43.14,-29.91,;43.83,-27.33,;39.92,-30.33,;38.59,-31.1,;37.25,-30.33,;35.92,-31.1,;35.92,-32.64,;37.25,-33.41,;38.59,-32.64,;39.92,-33.41,;41.26,-32.64,;42.59,-33.41,;43.92,-32.64,;43.92,-31.1,;42.59,-30.33,;41.26,-31.1,;45.26,-33.41,;46.59,-32.64,;45.26,-34.95,;34.59,-33.41,;33.25,-32.64,;34.59,-34.95,;45.26,-16.47,;46.59,-17.24,;45.26,-14.93,)| Show InChI InChI=1S/C105H160N28O24/c1-9-10-11-12-13-14-15-16-17-18-20-36-80(136)114-47-26-23-32-71(94(145)124-70(89(109)141)31-22-28-50-116-93(144)62-38-41-65(69(54-62)102(152)153)83-66-42-39-63(131(5)6)55-77(66)156-78-56-64(132(7)8)40-43-67(78)83)126-92(143)60(3)122-99(150)84(61(4)134)130-98(149)75(44-45-79(107)135)129-97(148)74(35-30-52-119-105(112)113)128-95(146)72(127-96(147)73(125-91(142)59(2)106)34-29-51-118-104(110)111)33-24-27-48-115-82(138)57-76(103(154)155)123-81(137)37-21-19-25-49-117-100(151)87-85(139)86(140)101(157-87)133-53-46-68-88(108)120-58-121-90(68)133/h38-43,46,53-56,58-61,70-76,84-87,101,134,139-140H,9-37,44-45,47-52,57,106H2,1-8H3,(H28-,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,135,136,137,138,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155)/p+3/t59-,60-,61?,70+,71+,72-,73-,74-,75-,76+,84-,85-,86+,87-,101+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 14.9 | n/a | n/a | n/a | 7.5 | n/a |
University of Tartu
| Assay Description
Association kinetics (Kon): All measurements were performed in HEPES (50 mM, pH 7.5) containing NaCl (150 mM), Tween-20 (0.005%) and DTT (5 mM) accor... |
Chembiochem 18: 790-798 (2017)
Article DOI: 10.1002/cbic.201600697
BindingDB Entry DOI: 10.7270/Q28914QN |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50010979
(CHEMBL4072540)Show SMILES CCN1c2cc(ccc2\C(=C/C=C/C=C/C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C1(C)CCCC(=O)NCCCC[C@H](NC(=O)CCCCCCCCn1c2cc(ccc2c2cnccc2c1=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O)S(O)(=O)=O |r,c:16| Show InChI InChI=1S/C29H42N4O5S/c1-4-32(5-2)17-18-33(26(35)13-20-38-19-12-22-7-6-8-24(21-22)37-3)16-15-30-14-11-23-9-10-25(34)27-28(23)39-29(36)31-27/h6-10,21,30,34H,4-5,11-20H2,1-3H3,(H,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of ARC-1504 binding to CK2alpha (unknown origin) (1 to 335 residues) measured after 15 mins by fluorescence anisotropic method |
Bioorg Med Chem 25: 2277-2284 (2017)
Article DOI: 10.1016/j.bmc.2017.02.055
BindingDB Entry DOI: 10.7270/Q21G0PRW |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50010963
(CHEMBL4076226)Show SMILES CCN1c2cc(ccc2\C(=C/C=C/C=C/C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C1(C)CCCC(=O)NCCCC[C@H](NC(=O)CCCCCCCCOc1nc2cc(ccc2c2cnccc12)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O)S(O)(=O)=O |r,c:16| Show InChI InChI=1S/C25H34N4O4S/c26-12-4-15-29(22(31)11-18-33-17-10-19-5-2-1-3-6-19)16-14-27-13-9-20-7-8-21(30)23-24(20)34-25(32)28-23/h1-3,5-8,27,30H,4,9-18,26H2,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of ARC-1504 binding to CK2alpha (unknown origin) (1 to 335 residues) measured after 15 mins by fluorescence anisotropic method |
Bioorg Med Chem 25: 2277-2284 (2017)
Article DOI: 10.1016/j.bmc.2017.02.055
BindingDB Entry DOI: 10.7270/Q21G0PRW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data