

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50018796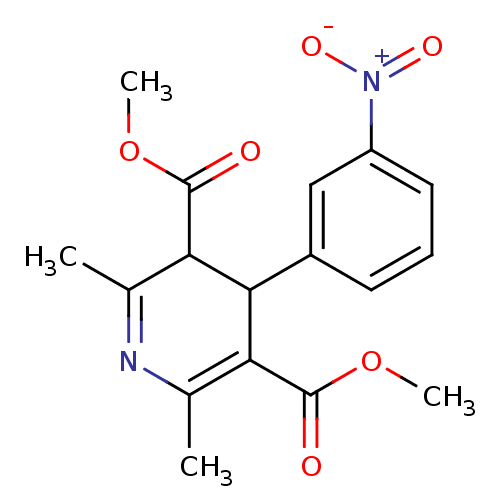 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072809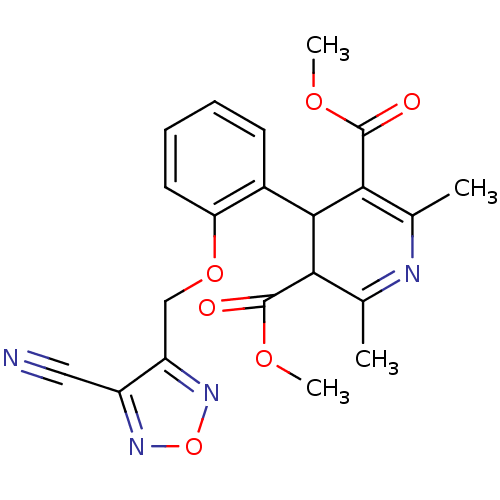 (4-[2-(4-Cyano-furazan-3-ylmethoxy)-phenyl]-2,6-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072811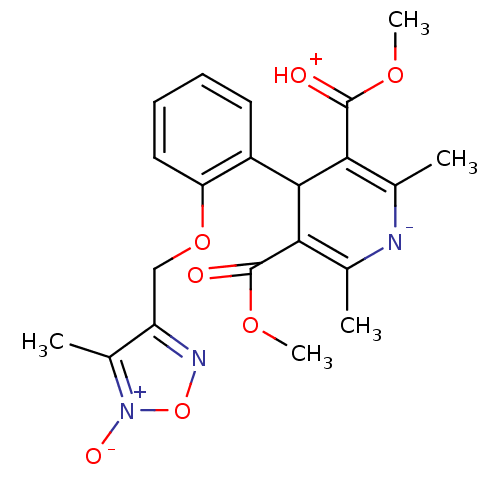 (2,6-Dimethyl-4-[2-(4-methyl-5-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072818 (4-[2-(4-Cyano-5-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 1 (Homo sapiens (Human)) | BDBM26335 (3-[2-({5-[(4-ethylphenyl)methyl]-1-[(4-methoxyphen...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072803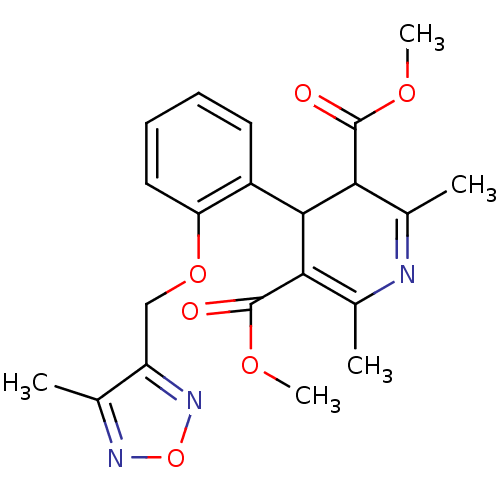 (2,6-Dimethyl-4-[2-(4-methyl-furazan-3-ylmethoxy)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072814 (4-[2-(4-Cyano-2-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072810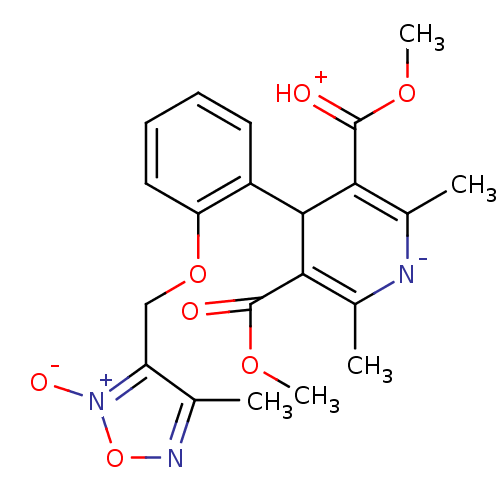 (2,6-Dimethyl-4-[2-(4-methyl-2-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072816 (2,6-Dimethyl-4-[2-(2-nitrooxy-ethoxy)-phenyl]-1,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072804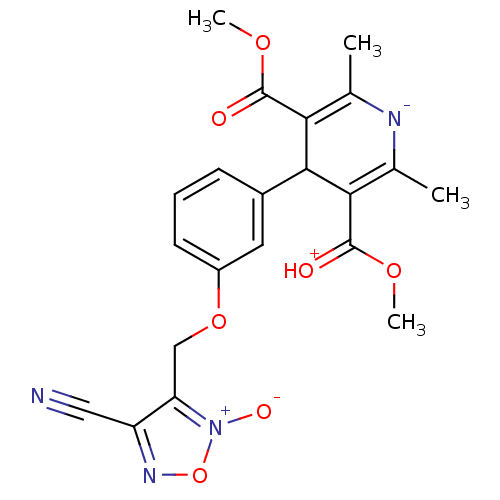 (4-[3-(4-Cyano-2-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072817 (4-[3-(4-Cyano-5-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072806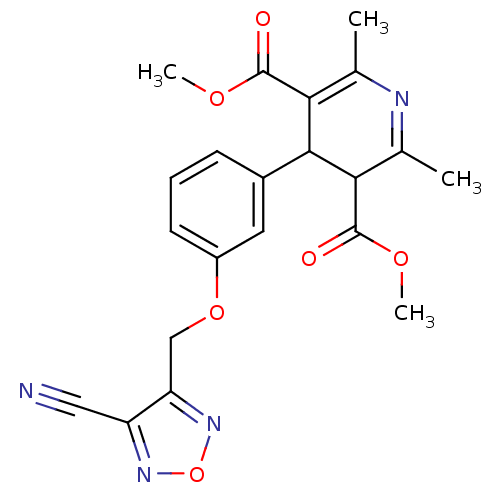 (4-[3-(4-Cyano-furazan-3-ylmethoxy)-phenyl]-2,6-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072813 (2,6-Dimethyl-4-[3-(4-methyl-2-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072812 (2,6-Dimethyl-4-[3-(4-methyl-furazan-3-ylmethoxy)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072807 (4-[2-(4-Carbamoyl-2-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072800 (2,6-Dimethyl-4-[3-(4-methyl-5-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072801 (4-[2-(4-Carbamoyl-furazan-3-ylmethoxy)-phenyl]-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072802 (2,6-Dimethyl-4-[3-(2-nitrooxy-ethoxy)-phenyl]-1,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 1 (Homo sapiens (Human)) | BDBM26336 (6-[(2-aminoethyl)amino]-3-[(4-ethylphenyl)methyl]-...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 440 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072815 (4-[2-(4-Carbamoyl-5-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072808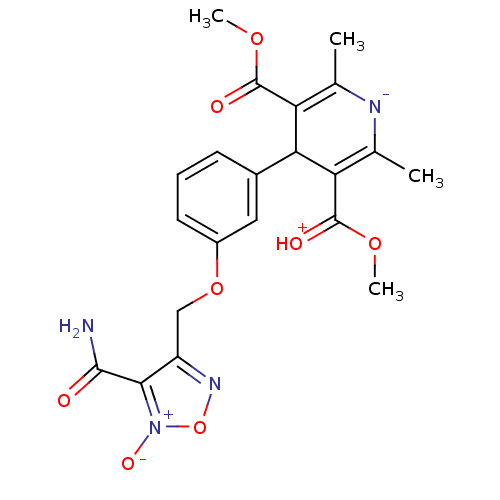 (4-[3-(4-Carbamoyl-5-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072819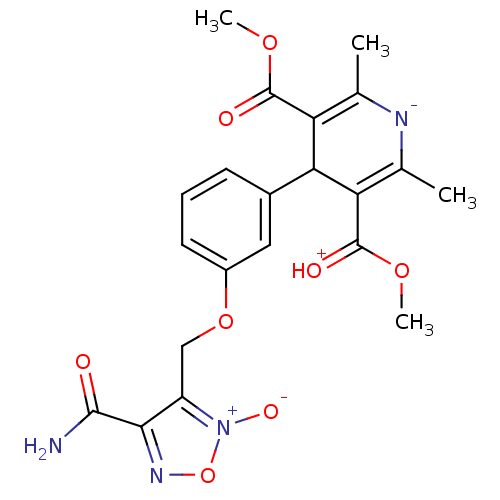 (4-[3-(4-Carbamoyl-2-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Homo sapiens (Human)) | BDBM26335 (3-[2-({5-[(4-ethylphenyl)methyl]-1-[(4-methoxyphen...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.61E+3 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072805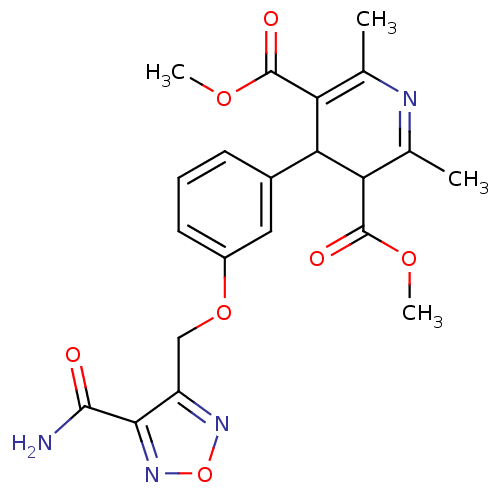 (4-[3-(4-Carbamoyl-furazan-3-ylmethoxy)-phenyl]-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 1 (Homo sapiens (Human)) | BDBM26337 (6-{[2-(4,5-dihydro-1H-imidazol-2-ylamino)ethyl]ami...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.72E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Homo sapiens (Human)) | BDBM26336 (6-[(2-aminoethyl)amino]-3-[(4-ethylphenyl)methyl]-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.34E+4 | -27.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Homo sapiens (Human)) | BDBM26337 (6-{[2-(4,5-dihydro-1H-imidazol-2-ylamino)ethyl]ami...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.12E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Ferrara | Assay Description Nonspecific binding was determined in the presence of 1 uM Bv8. Displacement curves were determined in triplicate. The inhibition constant (Ki) of th... | J Med Chem 51: 7635-9 (2008) Article DOI: 10.1021/jm800854e BindingDB Entry DOI: 10.7270/Q23J3B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50018796 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50005836 (4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50117930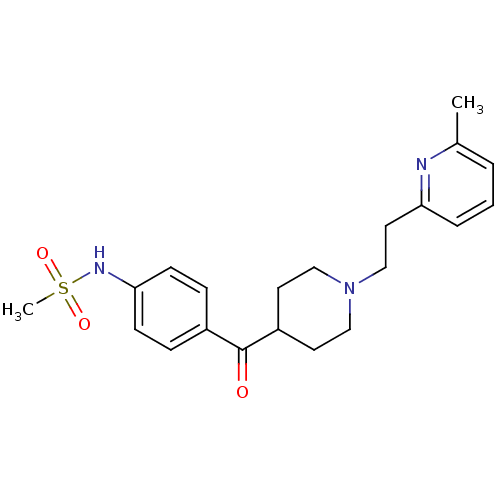 ((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072809 (4-[2-(4-Cyano-furazan-3-ylmethoxy)-phenyl]-2,6-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031720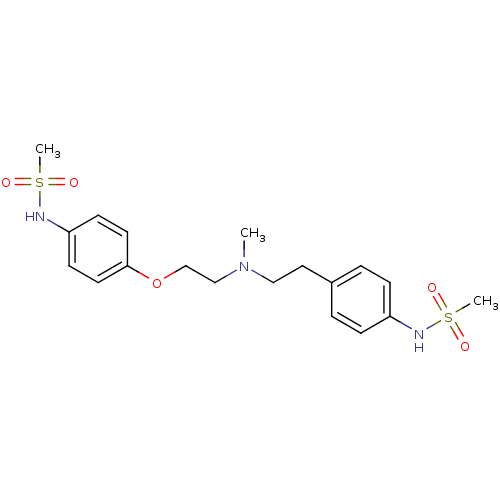 ((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50001786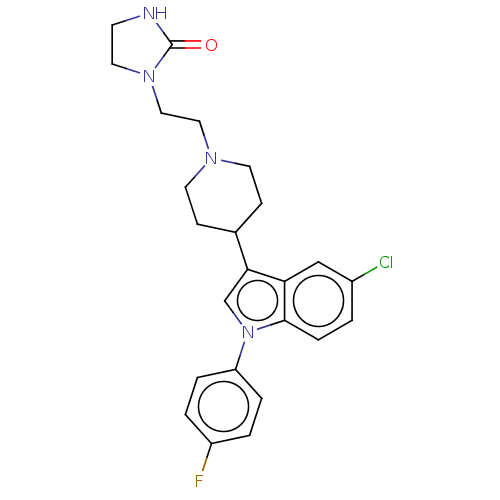 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50334150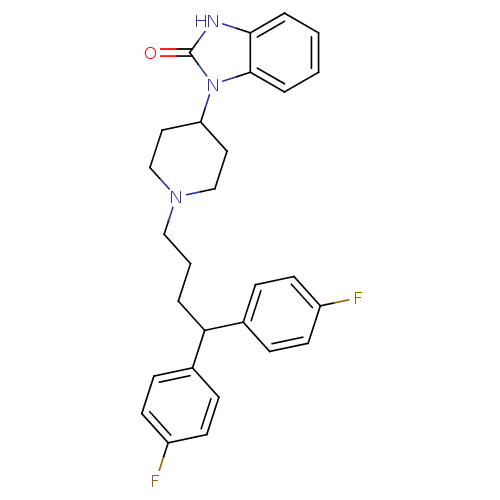 (1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072818 (4-[2-(4-Cyano-5-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50117925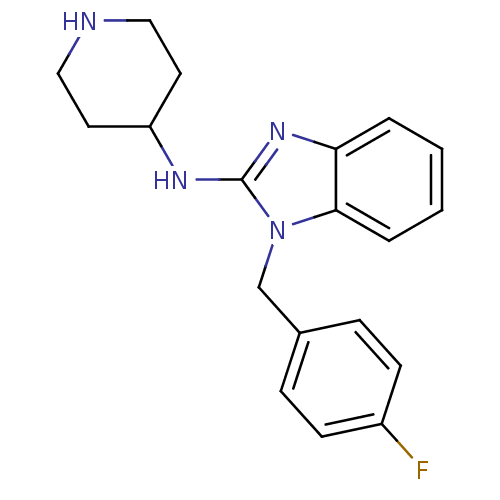 (1-(4-fluorobenzyl)-N-(piperidin-4-yl)-1H-benzo[d]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50017705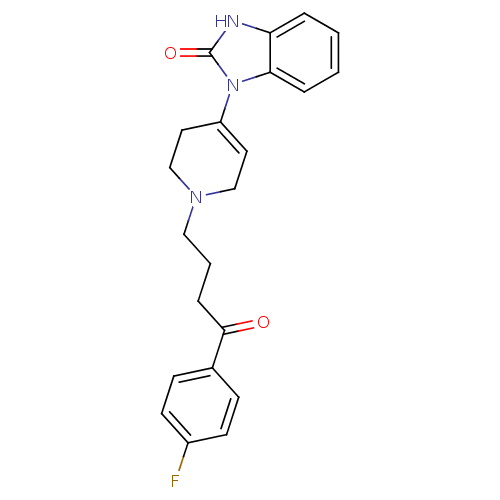 (1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50002338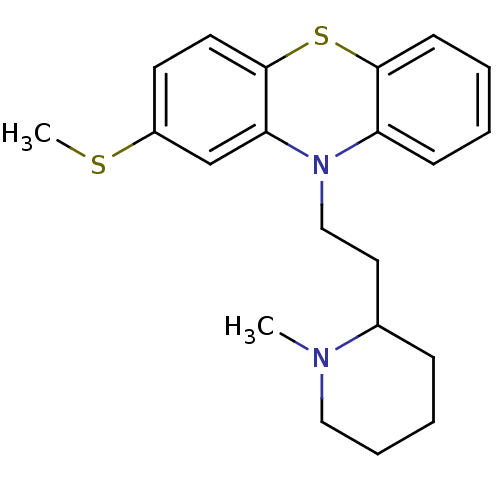 ((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 35.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072811 (2,6-Dimethyl-4-[2-(4-methyl-5-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072803 (2,6-Dimethyl-4-[2-(4-methyl-furazan-3-ylmethoxy)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072814 (4-[2-(4-Cyano-2-oxy-furazan-3-ylmethoxy)-phenyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072810 (2,6-Dimethyl-4-[2-(4-methyl-2-oxy-furazan-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072816 (2,6-Dimethyl-4-[2-(2-nitrooxy-ethoxy)-phenyl]-1,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50017376 ((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of human ERG in MCF7 cells | Eur J Med Chem 44: 1926-32 (2009) Article DOI: 10.1016/j.ejmech.2008.11.009 BindingDB Entry DOI: 10.7270/Q2TM7CCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |