 Found 97 hits with Last Name = 'vivas' and Initial = 'l'
Found 97 hits with Last Name = 'vivas' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23232
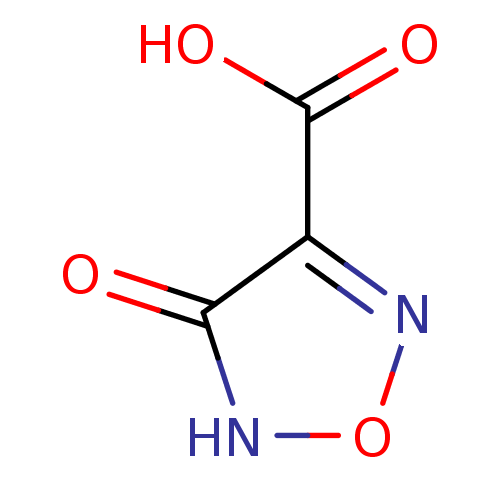
(1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...)Show InChI InChI=1S/C3H2N2O4/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23251
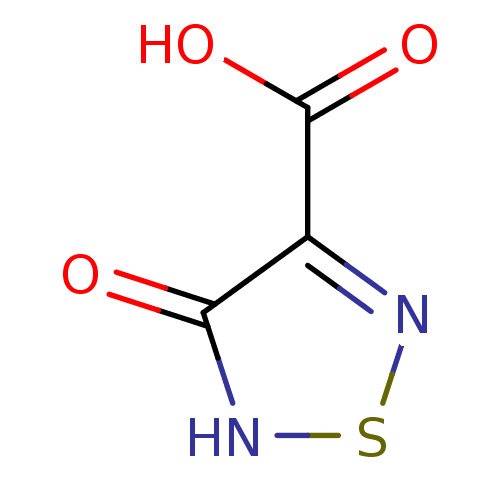
(1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...)Show InChI InChI=1S/C3H2N2O3S/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23242
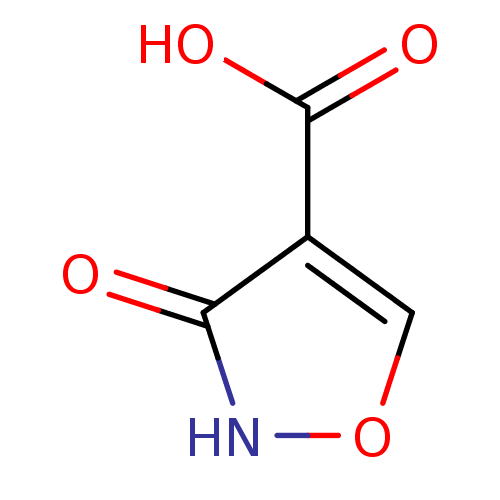
(1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...)Show InChI InChI=1S/C4H3NO4/c6-3-2(4(7)8)1-9-5-3/h1H,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM308131
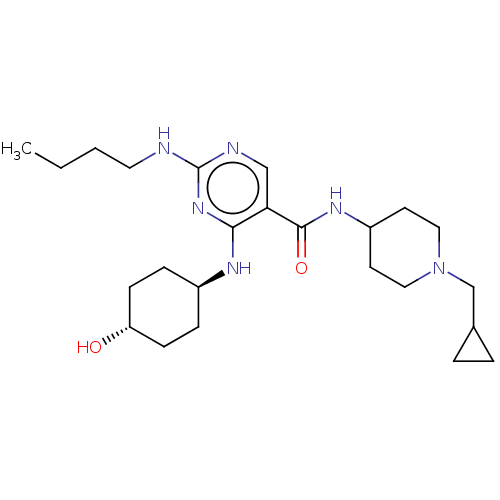
(US9649309, Compound UNC2876A)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CC3CC3)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.25,wD:27.29,(-10.11,-3.85,;-8.77,-3.08,;-7.44,-3.85,;-6.1,-3.08,;-4.77,-3.85,;-3.44,-3.08,;-2.1,-3.85,;-.77,-3.08,;-.77,-1.54,;.56,-.77,;.56,.77,;1.9,-1.54,;3.23,-.77,;4.56,-1.54,;5.9,-.77,;5.9,.77,;7.23,1.54,;8.57,.77,;9.34,-.56,;10.11,.77,;4.56,1.54,;3.23,.77,;-2.1,-.77,;-2.1,.77,;-3.44,1.54,;-4.77,.77,;-6.1,1.54,;-6.1,3.08,;-7.44,3.85,;-4.77,3.85,;-3.44,3.08,;-3.44,-1.54,)| Show InChI InChI=1S/C24H40N6O2/c1-2-3-12-25-24-26-15-21(22(29-24)27-18-6-8-20(31)9-7-18)23(32)28-19-10-13-30(14-11-19)16-17-4-5-17/h15,17-20,31H,2-14,16H2,1H3,(H,28,32)(H2,25,26,27,29)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50602486
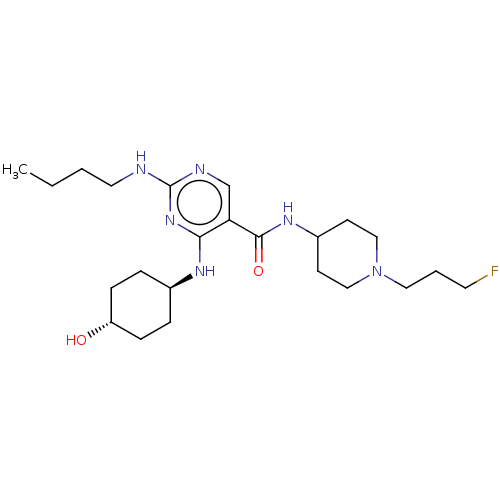
(CHEMBL5196154)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.24,wD:27.28,(26.44,-12.6,;27.77,-11.83,;29.1,-12.6,;30.44,-11.83,;31.77,-12.6,;33.11,-11.83,;34.44,-12.6,;35.78,-11.83,;35.77,-10.28,;37.1,-9.51,;37.1,-7.97,;38.44,-10.27,;39.77,-9.5,;41.1,-10.27,;42.43,-9.5,;42.43,-7.96,;43.77,-7.19,;45.1,-7.96,;46.43,-7.19,;47.77,-7.96,;41.1,-7.19,;39.76,-7.96,;34.44,-9.52,;34.43,-7.98,;33.1,-7.21,;31.77,-7.98,;30.43,-7.21,;30.43,-5.67,;29.1,-4.9,;31.77,-4.9,;33.1,-5.67,;33.11,-10.29,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50602485
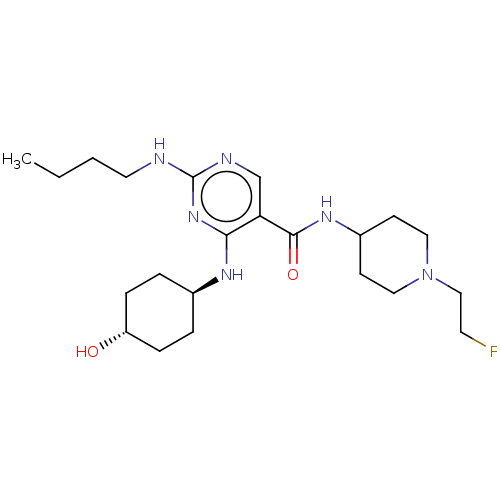
(CHEMBL5171919)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:23.23,wD:26.27,(2.87,-11.2,;4.2,-10.43,;5.54,-11.2,;6.87,-10.43,;8.2,-11.2,;9.54,-10.43,;10.87,-11.2,;12.21,-10.43,;12.2,-8.88,;13.54,-8.11,;13.53,-6.57,;14.87,-8.87,;16.2,-8.1,;17.54,-8.87,;18.86,-8.1,;18.87,-6.56,;20.2,-5.79,;21.53,-6.56,;22.87,-5.79,;17.53,-5.79,;16.19,-6.56,;10.87,-8.12,;10.86,-6.58,;9.53,-5.81,;8.2,-6.58,;6.87,-5.81,;6.86,-4.27,;5.53,-3.5,;8.2,-3.5,;9.53,-4.27,;9.54,-8.89,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8 |
J Nat Prod 76: 1064-70 (2013)
Article DOI: 10.1021/np400083k
BindingDB Entry DOI: 10.7270/Q2S75K77 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM308131

(US9649309, Compound UNC2876A)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CC3CC3)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.25,wD:27.29,(-10.11,-3.85,;-8.77,-3.08,;-7.44,-3.85,;-6.1,-3.08,;-4.77,-3.85,;-3.44,-3.08,;-2.1,-3.85,;-.77,-3.08,;-.77,-1.54,;.56,-.77,;.56,.77,;1.9,-1.54,;3.23,-.77,;4.56,-1.54,;5.9,-.77,;5.9,.77,;7.23,1.54,;8.57,.77,;9.34,-.56,;10.11,.77,;4.56,1.54,;3.23,.77,;-2.1,-.77,;-2.1,.77,;-3.44,1.54,;-4.77,.77,;-6.1,1.54,;-6.1,3.08,;-7.44,3.85,;-4.77,3.85,;-3.44,3.08,;-3.44,-1.54,)| Show InChI InChI=1S/C24H40N6O2/c1-2-3-12-25-24-26-15-21(22(29-24)27-18-6-8-20(31)9-7-18)23(32)28-19-10-13-30(14-11-19)16-17-4-5-17/h15,17-20,31H,2-14,16H2,1H3,(H,28,32)(H2,25,26,27,29)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50602486

(CHEMBL5196154)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.24,wD:27.28,(26.44,-12.6,;27.77,-11.83,;29.1,-12.6,;30.44,-11.83,;31.77,-12.6,;33.11,-11.83,;34.44,-12.6,;35.78,-11.83,;35.77,-10.28,;37.1,-9.51,;37.1,-7.97,;38.44,-10.27,;39.77,-9.5,;41.1,-10.27,;42.43,-9.5,;42.43,-7.96,;43.77,-7.19,;45.1,-7.96,;46.43,-7.19,;47.77,-7.96,;41.1,-7.19,;39.76,-7.96,;34.44,-9.52,;34.43,-7.98,;33.1,-7.21,;31.77,-7.98,;30.43,-7.21,;30.43,-5.67,;29.1,-4.9,;31.77,-4.9,;33.1,-5.67,;33.11,-10.29,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50602485

(CHEMBL5171919)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:23.23,wD:26.27,(2.87,-11.2,;4.2,-10.43,;5.54,-11.2,;6.87,-10.43,;8.2,-11.2,;9.54,-10.43,;10.87,-11.2,;12.21,-10.43,;12.2,-8.88,;13.54,-8.11,;13.53,-6.57,;14.87,-8.87,;16.2,-8.1,;17.54,-8.87,;18.86,-8.1,;18.87,-6.56,;20.2,-5.79,;21.53,-6.56,;22.87,-5.79,;17.53,-5.79,;16.19,-6.56,;10.87,-8.12,;10.86,-6.58,;9.53,-5.81,;8.2,-6.58,;6.87,-5.81,;6.86,-4.27,;5.53,-3.5,;8.2,-3.5,;9.53,-4.27,;9.54,-8.89,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM308131

(US9649309, Compound UNC2876A)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CC3CC3)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.25,wD:27.29,(-10.11,-3.85,;-8.77,-3.08,;-7.44,-3.85,;-6.1,-3.08,;-4.77,-3.85,;-3.44,-3.08,;-2.1,-3.85,;-.77,-3.08,;-.77,-1.54,;.56,-.77,;.56,.77,;1.9,-1.54,;3.23,-.77,;4.56,-1.54,;5.9,-.77,;5.9,.77,;7.23,1.54,;8.57,.77,;9.34,-.56,;10.11,.77,;4.56,1.54,;3.23,.77,;-2.1,-.77,;-2.1,.77,;-3.44,1.54,;-4.77,.77,;-6.1,1.54,;-6.1,3.08,;-7.44,3.85,;-4.77,3.85,;-3.44,3.08,;-3.44,-1.54,)| Show InChI InChI=1S/C24H40N6O2/c1-2-3-12-25-24-26-15-21(22(29-24)27-18-6-8-20(31)9-7-18)23(32)28-19-10-13-30(14-11-19)16-17-4-5-17/h15,17-20,31H,2-14,16H2,1H3,(H,28,32)(H2,25,26,27,29)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50602486

(CHEMBL5196154)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.24,wD:27.28,(26.44,-12.6,;27.77,-11.83,;29.1,-12.6,;30.44,-11.83,;31.77,-12.6,;33.11,-11.83,;34.44,-12.6,;35.78,-11.83,;35.77,-10.28,;37.1,-9.51,;37.1,-7.97,;38.44,-10.27,;39.77,-9.5,;41.1,-10.27,;42.43,-9.5,;42.43,-7.96,;43.77,-7.19,;45.1,-7.96,;46.43,-7.19,;47.77,-7.96,;41.1,-7.19,;39.76,-7.96,;34.44,-9.52,;34.43,-7.98,;33.1,-7.21,;31.77,-7.98,;30.43,-7.21,;30.43,-5.67,;29.1,-4.9,;31.77,-4.9,;33.1,-5.67,;33.11,-10.29,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50602485

(CHEMBL5171919)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:23.23,wD:26.27,(2.87,-11.2,;4.2,-10.43,;5.54,-11.2,;6.87,-10.43,;8.2,-11.2,;9.54,-10.43,;10.87,-11.2,;12.21,-10.43,;12.2,-8.88,;13.54,-8.11,;13.53,-6.57,;14.87,-8.87,;16.2,-8.1,;17.54,-8.87,;18.86,-8.1,;18.87,-6.56,;20.2,-5.79,;21.53,-6.56,;22.87,-5.79,;17.53,-5.79,;16.19,-6.56,;10.87,-8.12,;10.86,-6.58,;9.53,-5.81,;8.2,-6.58,;6.87,-5.81,;6.86,-4.27,;5.53,-3.5,;8.2,-3.5,;9.53,-4.27,;9.54,-8.89,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM308131

(US9649309, Compound UNC2876A)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CC3CC3)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.25,wD:27.29,(-10.11,-3.85,;-8.77,-3.08,;-7.44,-3.85,;-6.1,-3.08,;-4.77,-3.85,;-3.44,-3.08,;-2.1,-3.85,;-.77,-3.08,;-.77,-1.54,;.56,-.77,;.56,.77,;1.9,-1.54,;3.23,-.77,;4.56,-1.54,;5.9,-.77,;5.9,.77,;7.23,1.54,;8.57,.77,;9.34,-.56,;10.11,.77,;4.56,1.54,;3.23,.77,;-2.1,-.77,;-2.1,.77,;-3.44,1.54,;-4.77,.77,;-6.1,1.54,;-6.1,3.08,;-7.44,3.85,;-4.77,3.85,;-3.44,3.08,;-3.44,-1.54,)| Show InChI InChI=1S/C24H40N6O2/c1-2-3-12-25-24-26-15-21(22(29-24)27-18-6-8-20(31)9-7-18)23(32)28-19-10-13-30(14-11-19)16-17-4-5-17/h15,17-20,31H,2-14,16H2,1H3,(H,28,32)(H2,25,26,27,29)/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50247629
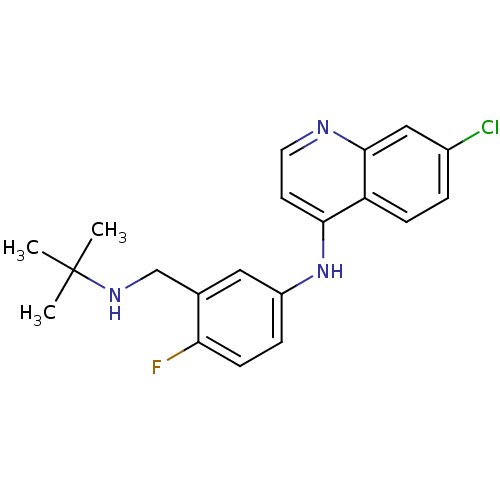
(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50602485

(CHEMBL5171919)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:23.23,wD:26.27,(2.87,-11.2,;4.2,-10.43,;5.54,-11.2,;6.87,-10.43,;8.2,-11.2,;9.54,-10.43,;10.87,-11.2,;12.21,-10.43,;12.2,-8.88,;13.54,-8.11,;13.53,-6.57,;14.87,-8.87,;16.2,-8.1,;17.54,-8.87,;18.86,-8.1,;18.87,-6.56,;20.2,-5.79,;21.53,-6.56,;22.87,-5.79,;17.53,-5.79,;16.19,-6.56,;10.87,-8.12,;10.86,-6.58,;9.53,-5.81,;8.2,-6.58,;6.87,-5.81,;6.86,-4.27,;5.53,-3.5,;8.2,-3.5,;9.53,-4.27,;9.54,-8.89,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50602486

(CHEMBL5196154)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CCCF)CC2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:24.24,wD:27.28,(26.44,-12.6,;27.77,-11.83,;29.1,-12.6,;30.44,-11.83,;31.77,-12.6,;33.11,-11.83,;34.44,-12.6,;35.78,-11.83,;35.77,-10.28,;37.1,-9.51,;37.1,-7.97,;38.44,-10.27,;39.77,-9.5,;41.1,-10.27,;42.43,-9.5,;42.43,-7.96,;43.77,-7.19,;45.1,-7.96,;46.43,-7.19,;47.77,-7.96,;41.1,-7.19,;39.76,-7.96,;34.44,-9.52,;34.43,-7.98,;33.1,-7.21,;31.77,-7.98,;30.43,-7.21,;30.43,-5.67,;29.1,-4.9,;31.77,-4.9,;33.1,-5.67,;33.11,-10.29,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113822
BindingDB Entry DOI: 10.7270/Q2TX3KFC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056190
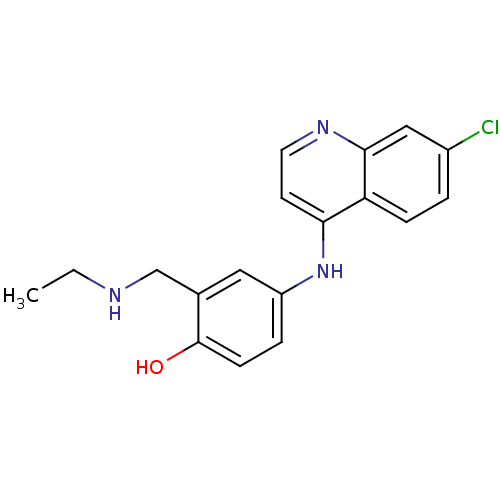
(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134931
(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM23251

(1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...)Show InChI InChI=1S/C3H2N2O3S/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23243
(1,2(1,5)-Isoxazole, IOA2 | 4-hydroxy-1,2-oxazole-3...)Show InChI InChI=1S/C4H3NO4/c6-2-1-9-5-3(2)4(7)8/h1,6H,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using DEF substrate |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using DEF substrate |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM50056923
((+)-usnic acid | (9bR)-2,6-diacetyl-3,7,9-trihydro...)Show SMILES CC(=O)C1C(=O)C=C2Oc3c(c(O)c(C)c(O)c3C(C)=O)[C@@]2(C)C1=O |r,t:6| Show InChI InChI=1S/C18H16O7/c1-6-14(22)12(8(3)20)16-13(15(6)23)18(4)10(25-16)5-9(21)11(7(2)19)17(18)24/h5,11,22-23H,1-4H3/t11?,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8 |
J Nat Prod 76: 1064-70 (2013)
Article DOI: 10.1021/np400083k
BindingDB Entry DOI: 10.7270/Q2S75K77 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM23242

(1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...)Show InChI InChI=1S/C4H3NO4/c6-3-2(4(7)8)1-9-5-3/h1H,(H,5,6)(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM23232

(1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...)Show InChI InChI=1S/C3H2N2O4/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 7.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23250
(1,2(1,5)-Isoxazole, IOA9 | 4-hydroxy-1,2-oxazole-3...)Show InChI InChI=1S/C5H3NO6/c7-2-1(4(8)9)6-12-3(2)5(10)11/h7H,(H,8,9)(H,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM50478424
(Vulpinic Acid)Show SMILES COC(=O)C(=C1\OC(=O)C(=C1O)c1ccccc1)\c1ccccc1 |c:9| Show InChI InChI=1S/C19H14O5/c1-23-18(21)15(13-10-6-3-7-11-13)17-16(20)14(19(22)24-17)12-8-4-2-5-9-12/h2-11,20H,1H3/b17-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8 |
J Nat Prod 76: 1064-70 (2013)
Article DOI: 10.1021/np400083k
BindingDB Entry DOI: 10.7270/Q2S75K77 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM50491952

(EVERNIC ACID)Show SMILES COc1cc(C)c(C(=O)Oc2cc(C)c(C(O)=O)c(O)c2)c(O)c1 Show InChI InChI=1S/C17H16O7/c1-8-5-11(7-12(18)14(8)16(20)21)24-17(22)15-9(2)4-10(23-3)6-13(15)19/h4-7,18-19H,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli FabI using 2-dodecenoyl-CoA as substrate at pH 8 |
J Nat Prod 76: 1064-70 (2013)
Article DOI: 10.1021/np400083k
BindingDB Entry DOI: 10.7270/Q2S75K77 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23258
(1H-1,2,3-triazole-5-carboxylic acid | Triazole, TR...)Show InChI InChI=1S/C3H3N3O2/c7-3(8)2-1-4-6-5-2/h1H,(H,7,8)(H,4,5,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23244
(1,2(1,5)-Isoxazole, IOA3 | 3-hydroxy-5-methyl-1,2-...)Show InChI InChI=1S/C5H5NO4/c1-2-3(5(8)9)4(7)6-10-2/h1H3,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23245
(1,2(1,5)-Isoxazole, IOA4 | 2-methyl-3-oxo-2,3-dihy...)Show InChI InChI=1S/C5H5NO4/c1-6-4(7)3(2-10-6)5(8)9/h2H,1H3,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23246
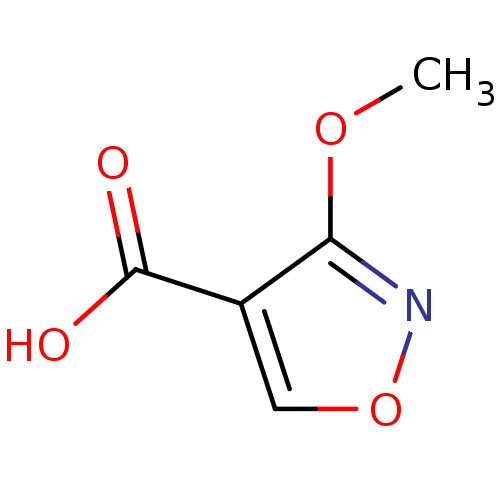
(1,2(1,5)-Isoxazole, IOA5 | 3-methoxy-1,2-oxazole-4...)Show InChI InChI=1S/C5H5NO4/c1-9-4-3(5(7)8)2-10-6-4/h2H,1H3,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data