 Found 4850 hits with Last Name = 'wang' and Initial = 'k'
Found 4850 hits with Last Name = 'wang' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypsin
(Homo sapiens (Human)) | BDBM50125046
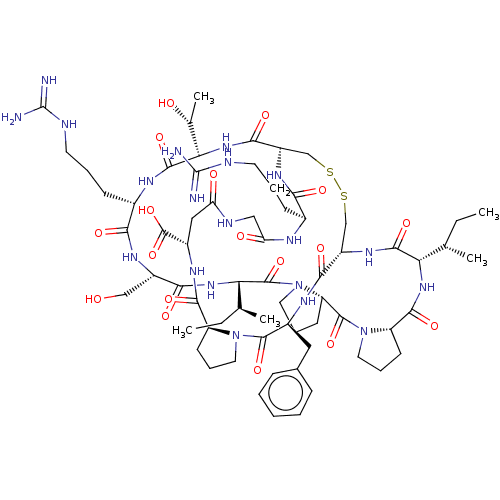
(CHEMBL3623792)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)C[C@H](NC2=O)C(O)=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-60(99)81-43-32-106-107-33-44(80-53(92)38(18-11-23-72-66(68)69)75-49(91)30-74-48(90)29-41(65(104)105)78-58(97)45-20-13-25-85(45)62(101)40(77-56(43)95)28-37-16-9-8-10-17-37)57(96)84-52(36(5)89)61(100)76-39(19-12-24-73-67(70)71)54(93)79-42(31-88)55(94)83-51(35(4)7-2)64(103)87-27-15-22-47(87)63(102)86-26-14-21-46(86)59(98)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,90)(H,75,91)(H,76,100)(H,77,95)(H,78,97)(H,79,93)(H,80,92)(H,81,99)(H,82,98)(H,83,94)(H,84,96)(H,104,105)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of beta-trypsin (unknown origin) using Bz-FVRpNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50124947
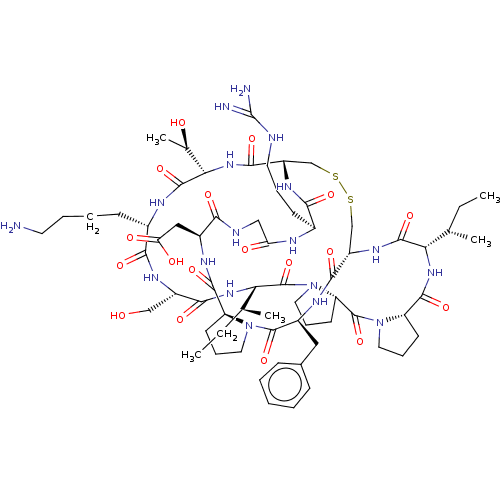
(CHEMBL453539)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of trypsin (unknown origin) |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50305263
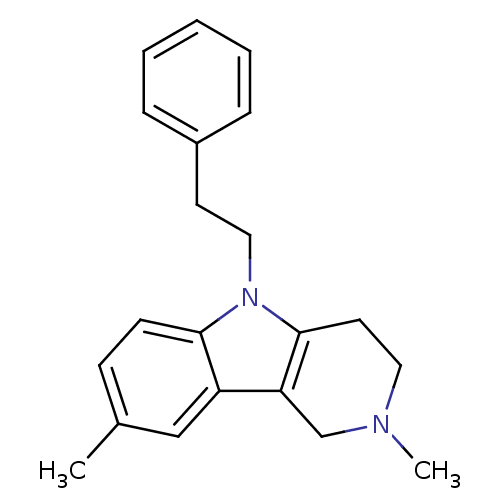
(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50125045
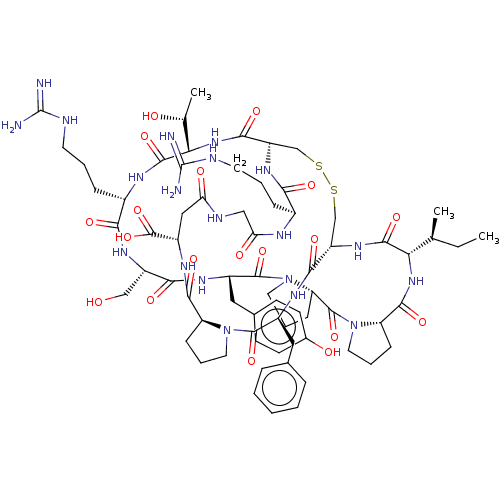
(CHEMBL3623793)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)C[C@H](NC2=O)C(O)=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C70H102N20O19S2/c1-4-36(2)54-63(103)85-47-34-110-111-35-48(84-56(96)41(15-8-24-75-69(71)72)78-53(95)32-77-52(94)31-45(68(108)109)82-61(101)49-17-10-26-88(49)65(105)43(81-59(47)99)29-38-13-6-5-7-14-38)60(100)87-55(37(3)92)64(104)79-42(16-9-25-76-70(73)74)57(97)83-46(33-91)58(98)80-44(30-39-20-22-40(93)23-21-39)66(106)90-28-12-19-51(90)67(107)89-27-11-18-50(89)62(102)86-54/h5-7,13-14,20-23,36-37,41-51,54-55,91-93H,4,8-12,15-19,24-35H2,1-3H3,(H,77,94)(H,78,95)(H,79,104)(H,80,98)(H,81,99)(H,82,101)(H,83,97)(H,84,96)(H,85,103)(H,86,102)(H,87,100)(H,108,109)(H4,71,72,75)(H4,73,74,76)/t36-,37+,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,54-,55-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of beta-trypsin (unknown origin) using Bz-FVRpNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50369383
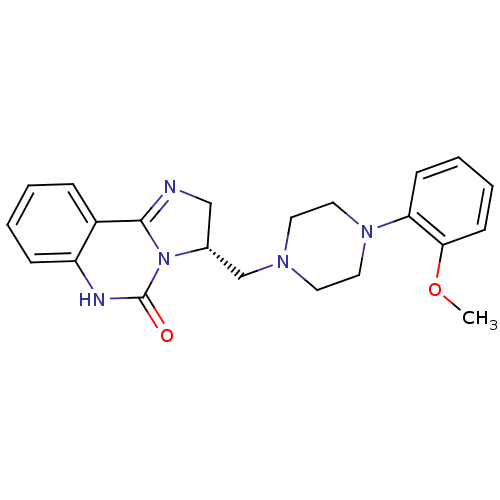
(CHEMBL1788222)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(=O)Nc2ccccc32)CC1 |r,c:16| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)15-16-14-23-21-17-6-2-3-7-18(17)24-22(28)27(16)21/h2-9,16H,10-15H2,1H3,(H,24,28)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50066109
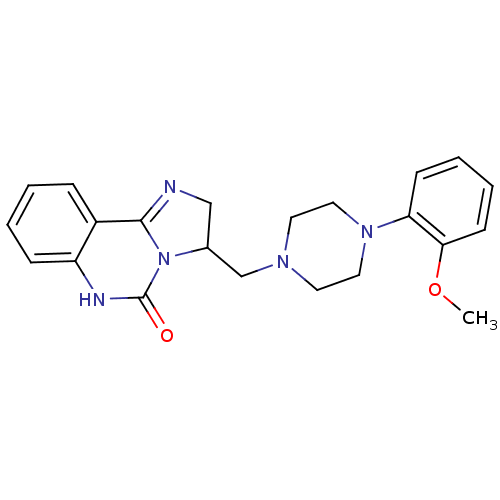
(3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN=C3N2C(=O)Nc2ccccc32)CC1 |c:16| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)15-16-14-23-21-17-6-2-3-7-18(17)24-22(28)27(16)21/h2-9,16H,10-15H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Kallikrein-14
(Homo sapiens (Human)) | BDBM50124906
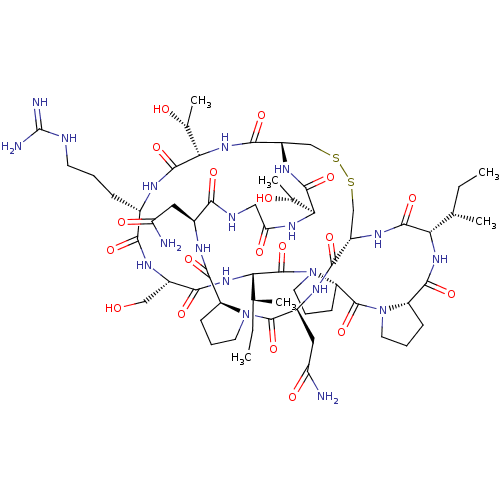
(CHEMBL3623776)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C60H97N19O19S2/c1-7-27(3)43-54(93)71-35-25-99-100-26-36(72-55(94)45(29(5)81)73-42(85)23-66-47(86)32(21-40(61)83)68-52(91)37-14-10-18-77(37)57(96)33(22-41(62)84)69-50(35)89)51(90)76-46(30(6)82)56(95)67-31(13-9-17-65-60(63)64)48(87)70-34(24-80)49(88)75-44(28(4)8-2)59(98)79-20-12-16-39(79)58(97)78-19-11-15-38(78)53(92)74-43/h27-39,43-46,80-82H,7-26H2,1-6H3,(H2,61,83)(H2,62,84)(H,66,86)(H,67,95)(H,68,91)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,88)(H,76,90)(H4,63,64,65)/t27-,28-,29+,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-,46-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK14 (unknown origin) expressed in Sf9 cells using Ac-YANR-pNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50369383

(CHEMBL1788222)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(=O)Nc2ccccc32)CC1 |r,c:16| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)15-16-14-23-21-17-6-2-3-7-18(17)24-22(28)27(16)21/h2-9,16H,10-15H2,1H3,(H,24,28)/t16-/m1/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50040253
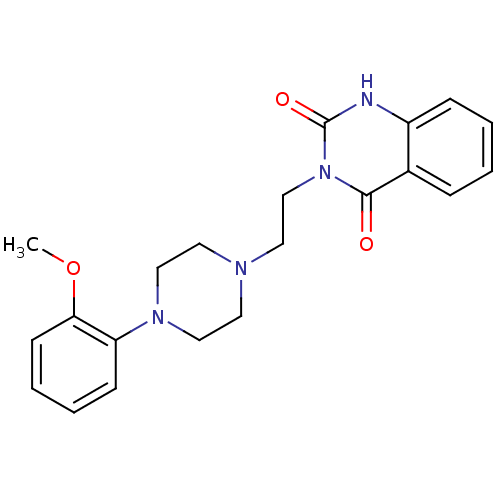
(3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...)Show SMILES COc1ccccc1N1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C21H24N4O3/c1-28-19-9-5-4-8-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-6-2-3-7-17(16)22-21(25)27/h2-9H,10-15H2,1H3,(H,22,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50040260
(2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2NC3=O)CC1 |c:17| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)14-16-15-27-21(23-16)17-6-2-3-7-18(17)24-22(27)28/h2-9,16H,10-15H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50066109

(3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN=C3N2C(=O)Nc2ccccc32)CC1 |c:16| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)15-16-14-23-21-17-6-2-3-7-18(17)24-22(28)27(16)21/h2-9,16H,10-15H2,1H3,(H,24,28) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50124906

(CHEMBL3623776)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C60H97N19O19S2/c1-7-27(3)43-54(93)71-35-25-99-100-26-36(72-55(94)45(29(5)81)73-42(85)23-66-47(86)32(21-40(61)83)68-52(91)37-14-10-18-77(37)57(96)33(22-41(62)84)69-50(35)89)51(90)76-46(30(6)82)56(95)67-31(13-9-17-65-60(63)64)48(87)70-34(24-80)49(88)75-44(28(4)8-2)59(98)79-20-12-16-39(79)58(97)78-19-11-15-38(78)53(92)74-43/h27-39,43-46,80-82H,7-26H2,1-6H3,(H2,61,83)(H2,62,84)(H,66,86)(H,67,95)(H,68,91)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,88)(H,76,90)(H4,63,64,65)/t27-,28-,29+,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of trypsin (unknown origin) |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Kallikrein-7
(Homo sapiens (Human)) | BDBM50125021
(CHEMBL3623779)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C58H92N20O20S2/c1-5-25(2)42-52(93)71-33-23-99-100-24-34(72-53(94)43(26(3)80)73-41(85)21-65-45(86)29(18-38(59)82)67-50(91)35-11-7-15-76(35)55(96)30(19-39(60)83)69-48(33)89)49(90)75-44(27(4)81)54(95)66-28(10-6-14-64-58(62)63)46(87)70-32(22-79)47(88)68-31(20-40(61)84)56(97)78-17-9-13-37(78)57(98)77-16-8-12-36(77)51(92)74-42/h25-37,42-44,79-81H,5-24H2,1-4H3,(H2,59,82)(H2,60,83)(H2,61,84)(H,65,86)(H,66,95)(H,67,91)(H,68,88)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,90)(H4,62,63,64)/t25-,26+,27+,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK7 (unknown origin) expressed in Pichia pastoris X33 using KHLY-pNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266979
(CHEMBL4061897)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C70H101N15O17S2/c1-7-10-24-43-59(91)74-44(31-41-20-13-11-14-21-41)60(92)77-47(35-86)61(93)82-56(39(5)9-3)70(102)85-30-19-27-52(85)69(101)84-29-18-26-51(84)65(97)81-55(38(4)8-2)66(98)78-49-37-104-103-36-48(62(94)73-43)79-67(99)57(40(6)87)80-54(89)34-72-58(90)45(33-53(71)88)75-64(96)50-25-17-28-83(50)68(100)46(76-63(49)95)32-42-22-15-12-16-23-42/h11-16,20-23,38-40,43-52,55-57,86-87H,7-10,17-19,24-37H2,1-6H3,(H2,71,88)(H,72,90)(H,73,94)(H,74,91)(H,75,96)(H,76,95)(H,77,92)(H,78,98)(H,79,99)(H,80,89)(H,81,97)(H,82,93)/t38-,39-,40+,43?,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM12623
(2,4-Diamino-5-ketopyrimidine 41 | 2-N-(1-methanesu...)Show SMILES COc1cc(F)c(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H20F3N5O4S/c1-30-12-7-11(19)14(20)15(21)13(12)16(27)10-8-23-18(25-17(10)22)24-9-3-5-26(6-4-9)31(2,28)29/h7-9H,3-6H2,1-2H3,(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266982

(CHEMBL4077874)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C69H97N15O19S2/c1-5-7-21-41-58(92)74-43(30-39-17-10-8-11-18-39)59(93)77-46(34-85)60(94)73-42(25-26-54(89)90)67(101)84-29-16-24-51(84)69(103)83-28-15-23-50(83)64(98)81-55(37(3)6-2)65(99)78-48-36-105-104-35-47(61(95)72-41)79-66(100)56(38(4)86)80-53(88)33-71-57(91)44(32-52(70)87)75-63(97)49-22-14-27-82(49)68(102)45(76-62(48)96)31-40-19-12-9-13-20-40/h8-13,17-20,37-38,41-51,55-56,85-86H,5-7,14-16,21-36H2,1-4H3,(H2,70,87)(H,71,91)(H,72,95)(H,73,94)(H,74,92)(H,75,97)(H,76,96)(H,77,93)(H,78,99)(H,79,100)(H,80,88)(H,81,98)(H,89,90)/t37-,38+,41?,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,55-,56-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50040253

(3-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...)Show SMILES COc1ccccc1N1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C21H24N4O3/c1-28-19-9-5-4-8-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-6-2-3-7-17(16)22-21(25)27/h2-9H,10-15H2,1H3,(H,22,27) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Kallikrein-14
(Homo sapiens (Human)) | BDBM50125021

(CHEMBL3623779)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C58H92N20O20S2/c1-5-25(2)42-52(93)71-33-23-99-100-24-34(72-53(94)43(26(3)80)73-41(85)21-65-45(86)29(18-38(59)82)67-50(91)35-11-7-15-76(35)55(96)30(19-39(60)83)69-48(33)89)49(90)75-44(27(4)81)54(95)66-28(10-6-14-64-58(62)63)46(87)70-32(22-79)47(88)68-31(20-40(61)84)56(97)78-17-9-13-37(78)57(98)77-16-8-12-36(77)51(92)74-42/h25-37,42-44,79-81H,5-24H2,1-4H3,(H2,59,82)(H2,60,83)(H2,61,84)(H,65,86)(H,66,95)(H,67,91)(H,68,88)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,90)(H4,62,63,64)/t25-,26+,27+,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,42-,43-,44-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK14 (unknown origin) expressed in Sf9 cells using Ac-YANR-pNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50369382
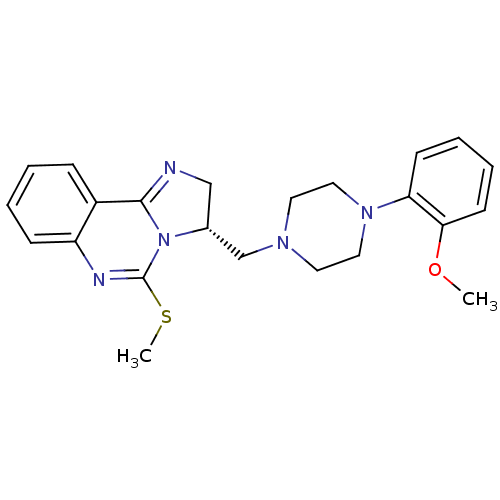
(CHEMBL1788223)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(SC)=Nc2ccccc32)CC1 |r,c:16,22| Show InChI InChI=1S/C23H27N5OS/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)16-17-15-24-22-18-7-3-4-8-19(18)25-23(30-2)28(17)22/h3-10,17H,11-16H2,1-2H3/t17-/m1/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50369382

(CHEMBL1788223)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(SC)=Nc2ccccc32)CC1 |r,c:16,22| Show InChI InChI=1S/C23H27N5OS/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)16-17-15-24-22-18-7-3-4-8-19(18)25-23(30-2)28(17)22/h3-10,17H,11-16H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50369382

(CHEMBL1788223)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(SC)=Nc2ccccc32)CC1 |r,c:16,22| Show InChI InChI=1S/C23H27N5OS/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)16-17-15-24-22-18-7-3-4-8-19(18)25-23(30-2)28(17)22/h3-10,17H,11-16H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1A adrenergic receptorof rat submaxillary gland membranes |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266984
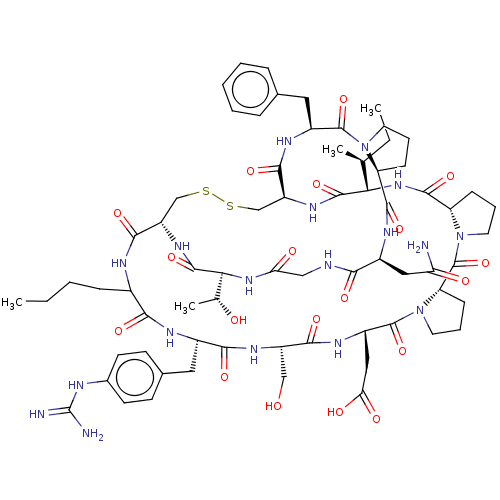
(CHEMBL4092637)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(NC(N)=N)cc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C69H98N18O19S2/c1-5-7-16-40-57(95)76-41(27-38-20-22-39(23-21-38)74-69(71)72)58(96)80-45(32-88)59(97)79-44(30-53(92)93)67(105)87-26-13-19-50(87)68(106)86-25-12-18-49(86)63(101)84-54(35(3)6-2)64(102)81-47-34-108-107-33-46(60(98)75-40)82-65(103)55(36(4)89)83-52(91)31-73-56(94)42(29-51(70)90)77-62(100)48-17-11-24-85(48)66(104)43(78-61(47)99)28-37-14-9-8-10-15-37/h8-10,14-15,20-23,35-36,40-50,54-55,88-89H,5-7,11-13,16-19,24-34H2,1-4H3,(H2,70,90)(H,73,94)(H,75,98)(H,76,95)(H,77,100)(H,78,99)(H,79,97)(H,80,96)(H,81,102)(H,82,103)(H,83,91)(H,84,101)(H,92,93)(H4,71,72,74)/t35-,36+,40?,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266989

(CHEMBL4081142)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C68H95N15O19S2/c1-5-7-21-40-57(91)72-41(28-38-17-10-8-11-18-38)58(92)76-45(33-84)59(93)75-44(31-53(88)89)67(101)83-27-16-24-50(83)68(102)82-26-15-23-49(82)63(97)80-54(36(3)6-2)64(98)77-47-35-104-103-34-46(60(94)71-40)78-65(99)55(37(4)85)79-52(87)32-70-56(90)42(30-51(69)86)73-62(96)48-22-14-25-81(48)66(100)43(74-61(47)95)29-39-19-12-9-13-20-39/h8-13,17-20,36-37,40-50,54-55,84-85H,5-7,14-16,21-35H2,1-4H3,(H2,69,86)(H,70,90)(H,71,94)(H,72,91)(H,73,96)(H,74,95)(H,75,93)(H,76,92)(H,77,98)(H,78,99)(H,79,87)(H,80,97)(H,88,89)/t36-,37+,40?,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Kallikrein-14
(Homo sapiens (Human)) | BDBM50125044

(CHEMBL3623790)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C69H104N20O17S2/c1-7-34(4)53-64(102)78-40(18-12-22-74-69(72)73)57(95)81-44(31-90)59(97)86-55(36(6)9-3)68(106)89-25-15-21-49(89)67(105)88-24-14-20-48(88)63(101)85-54(35(5)8-2)65(103)83-45-32-107-108-33-46(61(99)84-53)82-58(96)41(26-37-29-75-39-17-11-10-16-38(37)39)77-52(93)30-76-56(94)42(27-50(70)91)79-62(100)47-19-13-23-87(47)66(104)43(28-51(71)92)80-60(45)98/h10-11,16-17,29,34-36,40-49,53-55,75,90H,7-9,12-15,18-28,30-33H2,1-6H3,(H2,70,91)(H2,71,92)(H,76,94)(H,77,93)(H,78,102)(H,79,100)(H,80,98)(H,81,95)(H,82,96)(H,83,103)(H,84,99)(H,85,101)(H,86,97)(H4,72,73,74)/t34-,35-,36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,53-,54-,55-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK14 (unknown origin) expressed in Sf9 cells using Ac-YANR-pNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM12623

(2,4-Diamino-5-ketopyrimidine 41 | 2-N-(1-methanesu...)Show SMILES COc1cc(F)c(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H20F3N5O4S/c1-30-12-7-11(19)14(20)15(21)13(12)16(27)10-8-23-18(25-17(10)22)24-9-3-5-26(6-4-9)31(2,28)29/h7-9H,3-6H2,1-2H3,(H3,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Kallikrein-5 [D153N]
(Homo sapiens (Human)) | BDBM50124906

(CHEMBL3623776)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C60H97N19O19S2/c1-7-27(3)43-54(93)71-35-25-99-100-26-36(72-55(94)45(29(5)81)73-42(85)23-66-47(86)32(21-40(61)83)68-52(91)37-14-10-18-77(37)57(96)33(22-41(62)84)69-50(35)89)51(90)76-46(30(6)82)56(95)67-31(13-9-17-65-60(63)64)48(87)70-34(24-80)49(88)75-44(28(4)8-2)59(98)79-20-12-16-39(79)58(97)78-19-11-15-38(78)53(92)74-43/h27-39,43-46,80-82H,7-26H2,1-6H3,(H2,61,83)(H2,62,84)(H,66,86)(H,67,95)(H,68,91)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,88)(H,76,90)(H4,63,64,65)/t27-,28-,29+,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK5 (unknown origin) expressed in Pichia pastoris X33 using Ac-YRSR-pNA by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951
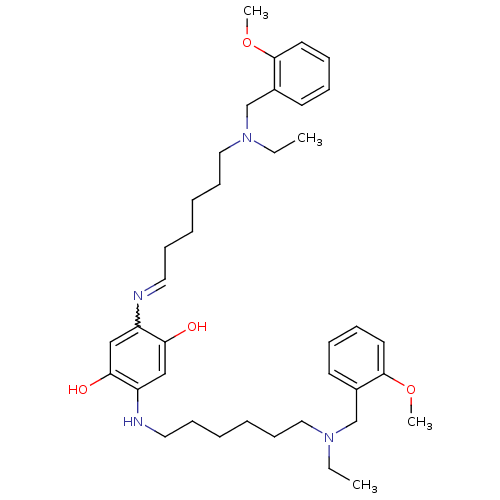
(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266987
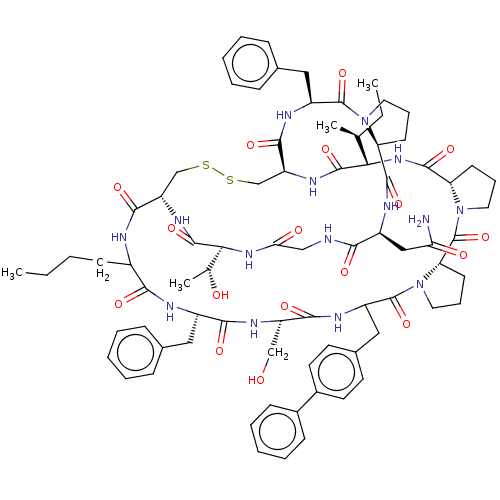
(CHEMBL4097952)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)C(Cc3ccc(cc3)-c3ccccc3)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C79H103N15O17S2/c1-5-7-26-52-68(100)83-53(37-47-20-11-8-12-21-47)69(101)87-57(42-95)70(102)85-56(39-49-30-32-51(33-31-49)50-24-15-10-16-25-50)78(110)94-36-19-29-62(94)79(111)93-35-18-28-61(93)74(106)91-65(45(3)6-2)75(107)88-59-44-113-112-43-58(71(103)82-52)89-76(108)66(46(4)96)90-64(98)41-81-67(99)54(40-63(80)97)84-73(105)60-27-17-34-92(60)77(109)55(86-72(59)104)38-48-22-13-9-14-23-48/h8-16,20-25,30-33,45-46,52-62,65-66,95-96H,5-7,17-19,26-29,34-44H2,1-4H3,(H2,80,97)(H,81,99)(H,82,103)(H,83,100)(H,84,105)(H,85,102)(H,86,104)(H,87,101)(H,88,107)(H,89,108)(H,90,98)(H,91,106)/t45-,46+,52?,53-,54-,55-,56?,57-,58-,59-,60-,61-,62-,65-,66-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM12619
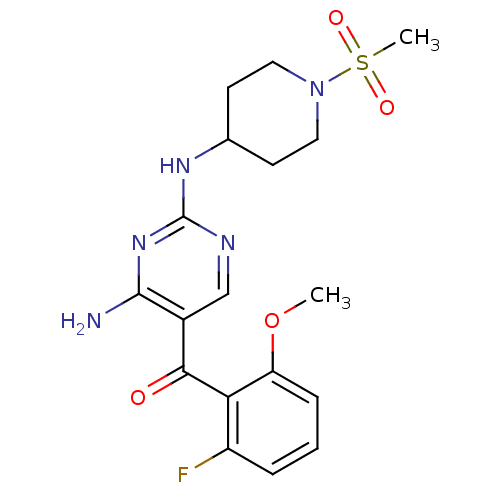
(2,4-Diamino-5-ketopyrimidine 37 | 5-[(2-fluoro-6-m...)Show SMILES COc1cccc(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H22FN5O4S/c1-28-14-5-3-4-13(19)15(14)16(25)12-10-21-18(23-17(12)20)22-11-6-8-24(9-7-11)29(2,26)27/h3-5,10-11H,6-9H2,1-2H3,(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266999
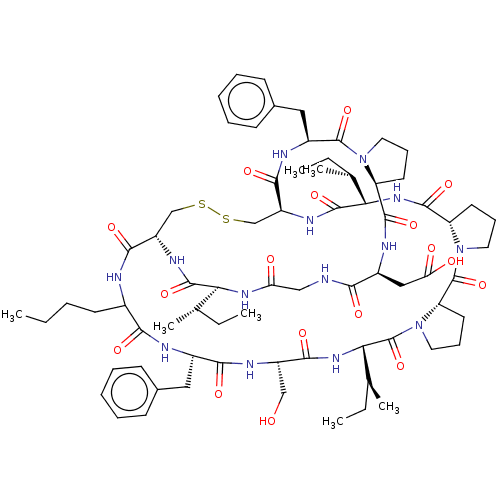
(CHEMBL4069983)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N2 |r| Show InChI InChI=1S/C72H104N14O17S2/c1-8-12-26-45-61(92)75-46(33-43-22-15-13-16-23-43)62(93)78-49(37-87)63(94)83-59(42(7)11-4)72(103)86-32-21-29-54(86)71(102)85-31-20-28-53(85)67(98)82-58(41(6)10-3)69(100)80-51-39-105-104-38-50(64(95)74-45)79-68(99)57(40(5)9-2)81-55(88)36-73-60(91)47(35-56(89)90)76-66(97)52-27-19-30-84(52)70(101)48(77-65(51)96)34-44-24-17-14-18-25-44/h13-18,22-25,40-42,45-54,57-59,87H,8-12,19-21,26-39H2,1-7H3,(H,73,91)(H,74,95)(H,75,92)(H,76,97)(H,77,96)(H,78,93)(H,79,99)(H,80,100)(H,81,88)(H,82,98)(H,83,94)(H,89,90)/t40-,41-,42-,45?,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50369382

(CHEMBL1788223)Show SMILES COc1ccccc1N1CCN(C[C@H]2CN=C3N2C(SC)=Nc2ccccc32)CC1 |r,c:16,22| Show InChI InChI=1S/C23H27N5OS/c1-29-21-10-6-5-9-20(21)27-13-11-26(12-14-27)16-17-15-24-22-18-7-3-4-8-19(18)25-23(30-2)28(17)22/h3-10,17H,11-16H2,1-2H3/t17-/m1/s1 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266981

(CHEMBL4070520)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)C(CCCC)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2 |r| Show InChI InChI=1S/C73H106N14O17S2/c1-8-12-27-46-62(93)77-48(35-44-23-16-14-17-24-44)64(95)75-47(28-13-9-2)63(94)84-59(42(6)11-4)73(104)87-34-22-31-55(87)72(103)86-33-21-30-54(86)68(99)83-58(41(5)10-3)69(100)80-52-40-106-105-39-51(65(96)76-46)81-70(101)60(43(7)88)82-56(89)38-74-61(92)49(37-57(90)91)78-67(98)53-29-20-32-85(53)71(102)50(79-66(52)97)36-45-25-18-15-19-26-45/h14-19,23-26,41-43,46-55,58-60,88H,8-13,20-22,27-40H2,1-7H3,(H,74,92)(H,75,95)(H,76,96)(H,77,93)(H,78,98)(H,79,97)(H,80,100)(H,81,101)(H,82,89)(H,83,99)(H,84,94)(H,90,91)/t41-,42-,43+,46?,47?,48-,49-,50-,51-,52-,53-,54-,55-,58-,59-,60-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50266980
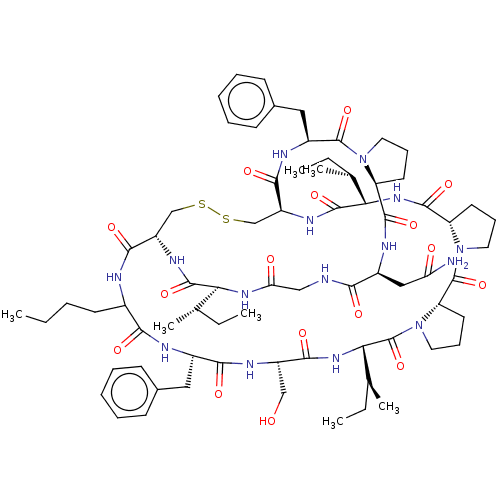
(CHEMBL4097688)Show SMILES CCCCC1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccccc3)NC1=O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N2 |r| Show InChI InChI=1S/C72H105N15O16S2/c1-8-12-26-45-61(92)76-46(33-43-22-15-13-16-23-43)62(93)79-49(37-88)63(94)84-59(42(7)11-4)72(103)87-32-21-29-54(87)71(102)86-31-20-28-53(86)67(98)83-58(41(6)10-3)69(100)81-51-39-105-104-38-50(64(95)75-45)80-68(99)57(40(5)9-2)82-56(90)36-74-60(91)47(35-55(73)89)77-66(97)52-27-19-30-85(52)70(101)48(78-65(51)96)34-44-24-17-14-18-25-44/h13-18,22-25,40-42,45-54,57-59,88H,8-12,19-21,26-39H2,1-7H3,(H2,73,89)(H,74,91)(H,75,95)(H,76,92)(H,77,97)(H,78,96)(H,79,93)(H,80,99)(H,81,100)(H,82,90)(H,83,98)(H,84,94)/t40-,41-,42-,45?,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate after 30 mins |
J Med Chem 60: 658-667 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01509
BindingDB Entry DOI: 10.7270/Q2FB55DF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM12623

(2,4-Diamino-5-ketopyrimidine 41 | 2-N-(1-methanesu...)Show SMILES COc1cc(F)c(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H20F3N5O4S/c1-30-12-7-11(19)14(20)15(21)13(12)16(27)10-8-23-18(25-17(10)22)24-9-3-5-26(6-4-9)31(2,28)29/h7-9H,3-6H2,1-2H3,(H3,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Kallikrein-5 [D153N]
(Homo sapiens (Human)) | BDBM50125021

(CHEMBL3623779)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C58H92N20O20S2/c1-5-25(2)42-52(93)71-33-23-99-100-24-34(72-53(94)43(26(3)80)73-41(85)21-65-45(86)29(18-38(59)82)67-50(91)35-11-7-15-76(35)55(96)30(19-39(60)83)69-48(33)89)49(90)75-44(27(4)81)54(95)66-28(10-6-14-64-58(62)63)46(87)70-32(22-79)47(88)68-31(20-40(61)84)56(97)78-17-9-13-37(78)57(98)77-16-8-12-36(77)51(92)74-42/h25-37,42-44,79-81H,5-24H2,1-4H3,(H2,59,82)(H2,60,83)(H2,61,84)(H,65,86)(H,66,95)(H,67,91)(H,68,88)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,90)(H4,62,63,64)/t25-,26+,27+,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,42-,43-,44-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK5 (unknown origin) expressed in Pichia pastoris X33 using Ac-YRSR-pNA by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50040260

(2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-...)Show SMILES COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2NC3=O)CC1 |c:17| Show InChI InChI=1S/C22H25N5O2/c1-29-20-9-5-4-8-19(20)26-12-10-25(11-13-26)14-16-15-27-21(23-16)17-6-2-3-7-18(17)24-22(27)28/h2-9,16H,10-15H2,1H3,(H,24,28) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from Alpha-1B adrenergic receptor of rat liver membrane |
J Med Chem 41: 3128-41 (1998)
Article DOI: 10.1021/jm970159v
BindingDB Entry DOI: 10.7270/Q2GM880H |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM12622
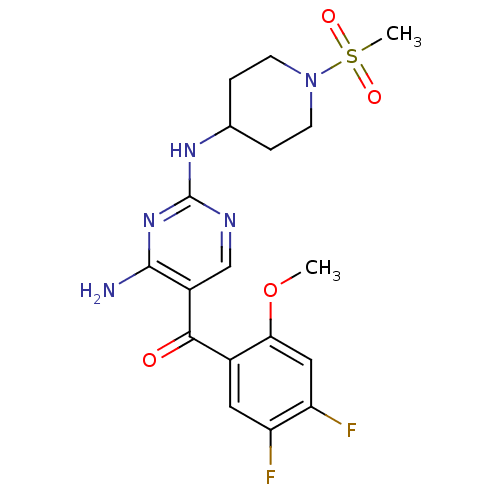
(2,4-Diamino-5-ketopyrimidine 40 | 5-[(4,5-difluoro...)Show SMILES COc1cc(F)c(F)cc1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-15-8-14(20)13(19)7-11(15)16(26)12-9-22-18(24-17(12)21)23-10-3-5-25(6-4-10)30(2,27)28/h7-10H,3-6H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM12622

(2,4-Diamino-5-ketopyrimidine 40 | 5-[(4,5-difluoro...)Show SMILES COc1cc(F)c(F)cc1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-15-8-14(20)13(19)7-11(15)16(26)12-9-22-18(24-17(12)21)23-10-3-5-25(6-4-10)30(2,27)28/h7-10H,3-6H2,1-2H3,(H3,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50251114
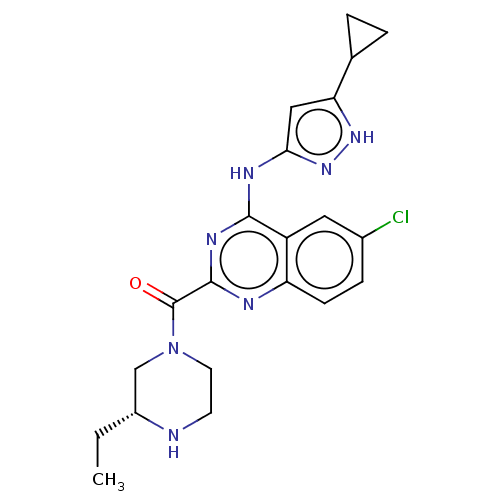
(CHEMBL4076860)Show SMILES CC[C@@H]1CN(CCN1)C(=O)c1nc(Nc2cc([nH]n2)C2CC2)c2cc(Cl)ccc2n1 |r| Show InChI InChI=1S/C21H24ClN7O/c1-2-14-11-29(8-7-23-14)21(30)20-24-16-6-5-13(22)9-15(16)19(26-20)25-18-10-17(27-28-18)12-3-4-12/h5-6,9-10,12,14,23H,2-4,7-8,11H2,1H3,(H2,24,25,26,27,28)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human PAK4 kinase domain using coumarin and fluorescein-labeled ser/thr20 peptide as substrate preincubated for 15 mins followed by ATP... |
J Med Chem 61: 265-285 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01342
BindingDB Entry DOI: 10.7270/Q2BK1FSN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM12617
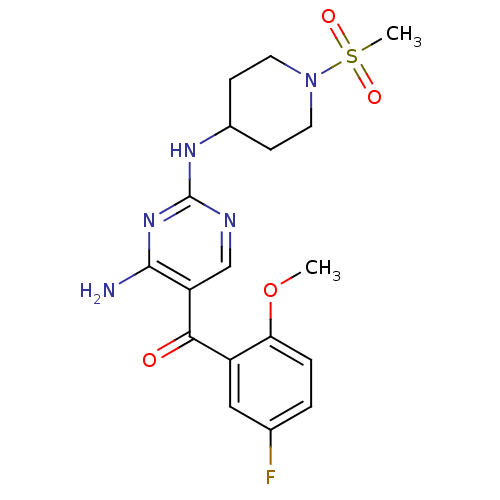
(2,4-Diamino-5-ketopyrimidine 35 | 5-[(5-fluoro-2-m...)Show SMILES COc1ccc(F)cc1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H22FN5O4S/c1-28-15-4-3-11(19)9-13(15)16(25)14-10-21-18(23-17(14)20)22-12-5-7-24(8-6-12)29(2,26)27/h3-4,9-10,12H,5-8H2,1-2H3,(H3,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM12617

(2,4-Diamino-5-ketopyrimidine 35 | 5-[(5-fluoro-2-m...)Show SMILES COc1ccc(F)cc1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H22FN5O4S/c1-28-15-4-3-11(19)9-13(15)16(25)14-10-21-18(23-17(14)20)22-12-5-7-24(8-6-12)29(2,26)27/h3-4,9-10,12H,5-8H2,1-2H3,(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
| Assay Description
Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... |
J Med Chem 49: 6549-60 (2006)
Article DOI: 10.1021/jm0606138
BindingDB Entry DOI: 10.7270/Q2QR4VB2 |
More data for this
Ligand-Target Pair | |
Kallikrein-14
(Homo sapiens (Human)) | BDBM50125028
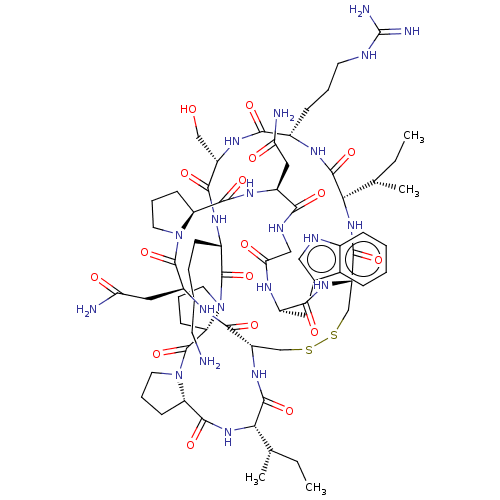
(CHEMBL3623791)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C69H105N21O17S2/c1-5-35(3)54-64(103)79-40(18-11-23-75-69(73)74)57(96)83-45(32-91)59(98)80-41(17-9-10-22-70)66(105)90-26-14-21-50(90)68(107)89-25-13-20-49(89)63(102)87-55(36(4)6-2)65(104)85-46-33-108-109-34-47(61(100)86-54)84-58(97)42(27-37-30-76-39-16-8-7-15-38(37)39)78-53(94)31-77-56(95)43(28-51(71)92)81-62(101)48-19-12-24-88(48)67(106)44(29-52(72)93)82-60(46)99/h7-8,15-16,30,35-36,40-50,54-55,76,91H,5-6,9-14,17-29,31-34,70H2,1-4H3,(H2,71,92)(H2,72,93)(H,77,95)(H,78,94)(H,79,103)(H,80,98)(H,81,101)(H,82,99)(H,83,96)(H,84,97)(H,85,104)(H,86,100)(H,87,102)(H4,73,74,75)/t35-,36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of KLK14 (unknown origin) expressed in Sf9 cells using Ac-YANR-pNA substrate by spectrophotometry method |
J Med Chem 58: 8257-68 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01148
BindingDB Entry DOI: 10.7270/Q2PR7XSJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50251178

(CHEMBL4081464)Show SMILES C[C@@H]1CN(CCN1)C(=O)c1nc(Nc2cc([nH]n2)C2CC2)c2ccc(Cl)cc2n1 |r| Show InChI InChI=1S/C20H22ClN7O/c1-11-10-28(7-6-22-11)20(29)19-23-16-8-13(21)4-5-14(16)18(25-19)24-17-9-15(26-27-17)12-2-3-12/h4-5,8-9,11-12,22H,2-3,6-7,10H2,1H3,(H2,23,24,25,26,27)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human PAK4 kinase domain using coumarin and fluorescein-labeled ser/thr20 peptide as substrate preincubated for 15 mins followed by ATP... |
J Med Chem 61: 265-285 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01342
BindingDB Entry DOI: 10.7270/Q2BK1FSN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data