 Found 1659 hits with Last Name = 'warmus' and Initial = 'j'
Found 1659 hits with Last Name = 'warmus' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM144227
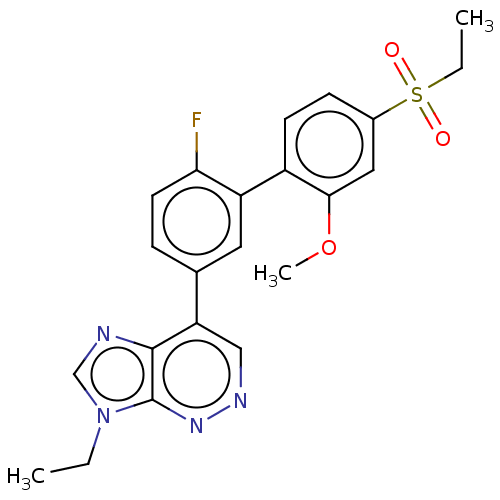
(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha1beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha1beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325241

(CHEMBL1223114)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CON1[C@H]2CCCC[C@H]2CC1=O |r| Show InChI InChI=1S/C29H40N4O9/c1-17(2)26(32-29(40)41-15-19-9-5-4-6-10-19)28(39)30-18(3)27(38)31-21(14-25(36)37)23(34)16-42-33-22-12-8-7-11-20(22)13-24(33)35/h4-6,9-10,17-18,20-22,26H,7-8,11-16H2,1-3H3,(H,30,39)(H,31,38)(H,32,40)(H,36,37)/t18-,20-,21-,22-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325237

((5S,8S,11S)-11-(2-(3,3-diphenylpropanoyloxy)acetyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C36H41N3O9/c1-23(2)33(39-36(46)48-21-25-13-7-4-8-14-25)35(45)37-24(3)34(44)38-29(20-31(41)42)30(40)22-47-32(43)19-28(26-15-9-5-10-16-26)27-17-11-6-12-18-27/h4-18,23-24,28-29,33H,19-22H2,1-3H3,(H,37,45)(H,38,44)(H,39,46)(H,41,42)/t24-,29-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM144227

(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha3beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325240
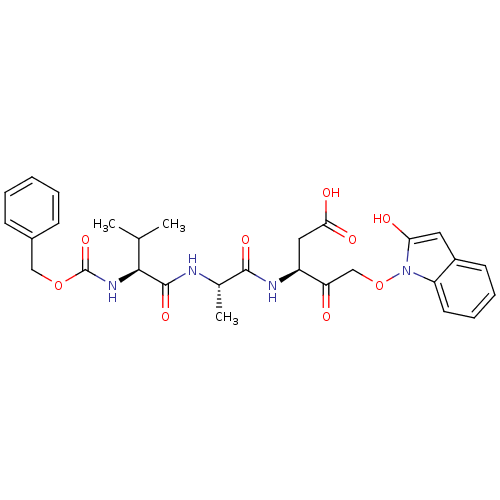
(CHEMBL1223113)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COn1c(O)cc2ccccc12 |r| Show InChI InChI=1S/C29H34N4O9/c1-17(2)26(32-29(40)41-15-19-9-5-4-6-10-19)28(39)30-18(3)27(38)31-21(14-25(36)37)23(34)16-42-33-22-12-8-7-11-20(22)13-24(33)35/h4-13,17-18,21,26,35H,14-16H2,1-3H3,(H,30,39)(H,31,38)(H,32,40)(H,36,37)/t18-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325238

((5S,8S,11S)-11-(2-(2-benzyl-3-phenylpropanoyloxy)a...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)C(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H43N3O9/c1-24(2)33(40-37(47)49-22-28-17-11-6-12-18-28)35(45)38-25(3)34(44)39-30(21-32(42)43)31(41)23-48-36(46)29(19-26-13-7-4-8-14-26)20-27-15-9-5-10-16-27/h4-18,24-25,29-30,33H,19-23H2,1-3H3,(H,38,45)(H,39,44)(H,40,47)(H,42,43)/t25-,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha3beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325236

((5S,8S,11S)-5-isopropyl-8-methyl-11-(2-(2-(naphtha...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H37N3O9/c1-20(2)30(36-33(43)45-18-22-10-5-4-6-11-22)32(42)34-21(3)31(41)35-26(17-28(38)39)27(37)19-44-29(40)16-24-14-9-13-23-12-7-8-15-25(23)24/h4-15,20-21,26,30H,16-19H2,1-3H3,(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t21-,26-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325239
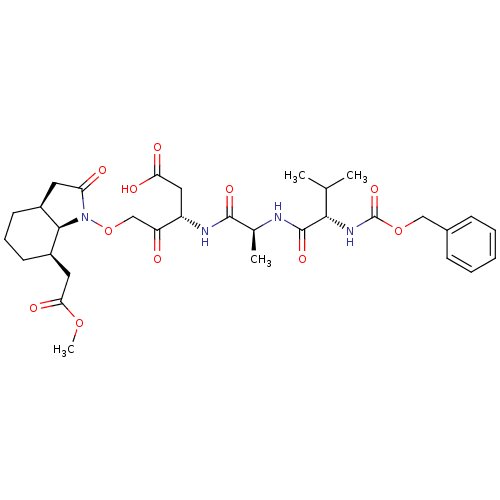
(CHEMBL1223112)Show SMILES COC(=O)C[C@H]1CCC[C@H]2CC(=O)N(OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)OCc3ccccc3)C(C)C)[C@@H]12 |r| Show InChI InChI=1S/C32H44N4O11/c1-18(2)28(35-32(44)46-16-20-9-6-5-7-10-20)31(43)33-19(3)30(42)34-23(15-26(39)40)24(37)17-47-36-25(38)13-21-11-8-12-22(29(21)36)14-27(41)45-4/h5-7,9-10,18-19,21-23,28-29H,8,11-17H2,1-4H3,(H,33,43)(H,34,42)(H,35,44)(H,39,40)/t19-,21-,22+,23-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12197
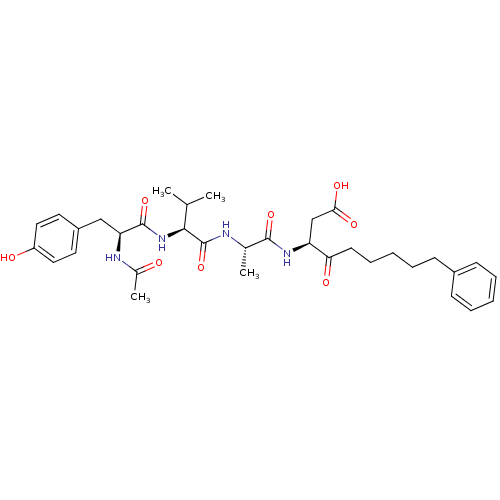
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O8/c1-21(2)31(38-33(45)28(36-23(4)39)19-25-15-17-26(40)18-16-25)34(46)35-22(3)32(44)37-27(20-30(42)43)29(41)14-10-6-9-13-24-11-7-5-8-12-24/h5,7-8,11-12,15-18,21-22,27-28,31,40H,6,9-10,13-14,19-20H2,1-4H3,(H,35,46)(H,36,39)(H,37,44)(H,38,45)(H,42,43)/t22-,27-,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM144227

(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha5beta2gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50255538

((4-(4-(3-chlorobenzyloxy)-3-methylbenzoyl)piperazi...)Show SMILES Cc1cc(ccc1OCc1cccc(Cl)c1)C(=O)N1CCN(CC1)C(=O)C1CCCO1 Show InChI InChI=1S/C24H27ClN2O4/c1-17-14-19(7-8-21(17)31-16-18-4-2-5-20(25)15-18)23(28)26-9-11-27(12-10-26)24(29)22-6-3-13-30-22/h2,4-5,7-8,14-15,22H,3,6,9-13,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG channel |
Bioorg Med Chem Lett 19: 665-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.054
BindingDB Entry DOI: 10.7270/Q28C9W3D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha5beta2gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50255483
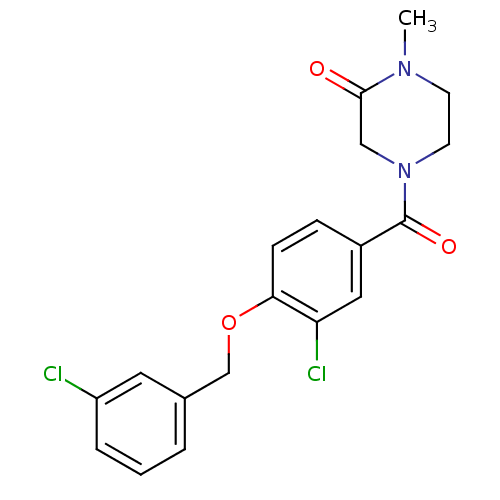
(4-(3-chloro-4-(3-chlorobenzyloxy)benzoyl)-1-methyl...)Show SMILES CN1CCN(CC1=O)C(=O)c1ccc(OCc2cccc(Cl)c2)c(Cl)c1 Show InChI InChI=1S/C19H18Cl2N2O3/c1-22-7-8-23(11-18(22)24)19(25)14-5-6-17(16(21)10-14)26-12-13-3-2-4-15(20)9-13/h2-6,9-10H,7-8,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG channel |
Bioorg Med Chem Lett 19: 665-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.054
BindingDB Entry DOI: 10.7270/Q28C9W3D |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity choline transporter 1
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]hemicholinium-3 from recombinant human choline transporter after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50071542

((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-5-[2-(...)Show SMILES CC(C)C(NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)Cc1c(Cl)cccc1Cl Show InChI InChI=1S/C20H24Cl2N2O7/c1-10(2)19(23-11(3)25)20(30)24-15(8-17(27)28)16(26)9-31-18(29)7-12-13(21)5-4-6-14(12)22/h4-6,10,15,19H,7-9H2,1-3H3,(H,23,25)(H,24,30)(H,27,28)/t15-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (resynthesized compound) |
Bioorg Med Chem Lett 8: 2309-14 (1999)
BindingDB Entry DOI: 10.7270/Q2F47N9K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50255768
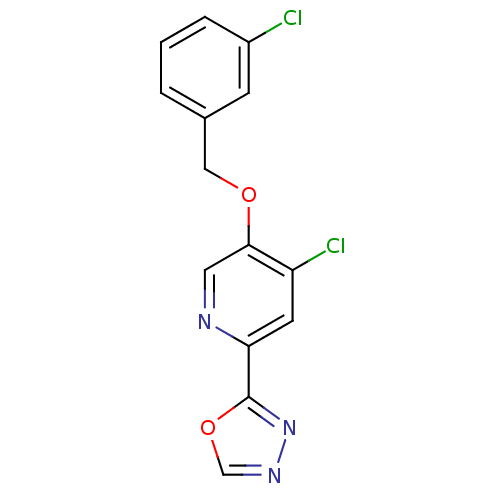
(2-(4-chloro-5-(3-chlorobenzyloxy)pyridin-2-yl)-1,3...)Show InChI InChI=1S/C14H9Cl2N3O2/c15-10-3-1-2-9(4-10)7-20-13-6-17-12(5-11(13)16)14-19-18-8-21-14/h1-6,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG channel |
Bioorg Med Chem Lett 19: 665-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.054
BindingDB Entry DOI: 10.7270/Q28C9W3D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50255662
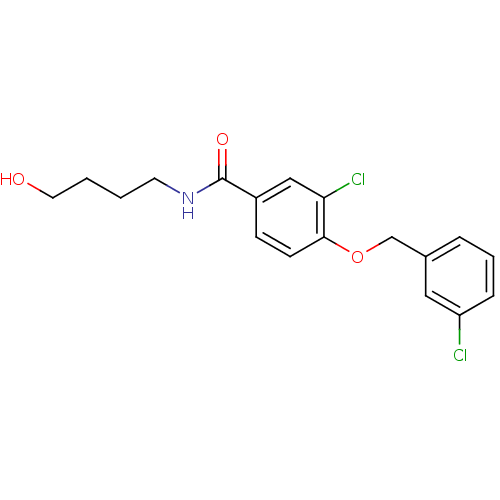
(3-chloro-4-(3-chlorobenzyloxy)-N-(4-hydroxybutyl)b...)Show InChI InChI=1S/C18H19Cl2NO3/c19-15-5-3-4-13(10-15)12-24-17-7-6-14(11-16(17)20)18(23)21-8-1-2-9-22/h3-7,10-11,22H,1-2,8-9,12H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG channel |
Bioorg Med Chem Lett 19: 665-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.054
BindingDB Entry DOI: 10.7270/Q28C9W3D |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50071543

((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-4-oxo-...)Show SMILES CC(C)C(NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)COC(=O)Cc1c(Cl)ccc(Cl)c1Cl Show InChI InChI=1S/C20H23Cl3N2O7/c1-9(2)19(24-10(3)26)20(31)25-14(7-16(28)29)15(27)8-32-17(30)6-11-12(21)4-5-13(22)18(11)23/h4-5,9,14,19H,6-8H2,1-3H3,(H,24,26)(H,25,31)(H,28,29)/t14-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (resynthesized compound) |
Bioorg Med Chem Lett 8: 2309-14 (1999)
BindingDB Entry DOI: 10.7270/Q2F47N9K |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325235
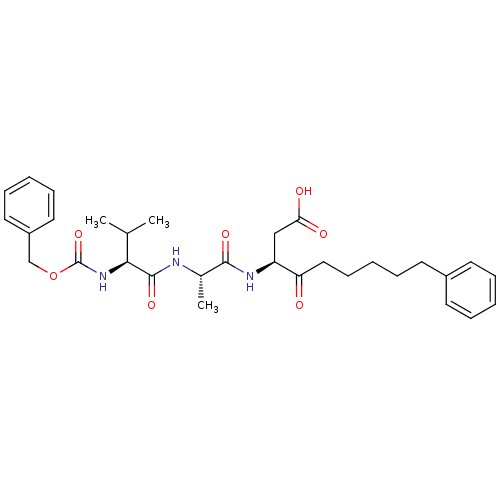
((5S,8S,11S)-5-isopropyl-8-methyl-3,6,9-trioxo-1-ph...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C31H41N3O7/c1-21(2)28(34-31(40)41-20-24-16-10-5-11-17-24)30(39)32-22(3)29(38)33-25(19-27(36)37)26(35)18-12-6-9-15-23-13-7-4-8-14-23/h4-5,7-8,10-11,13-14,16-17,21-22,25,28H,6,9,12,15,18-20H2,1-3H3,(H,32,39)(H,33,38)(H,34,40)(H,36,37)/t22-,25-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133601
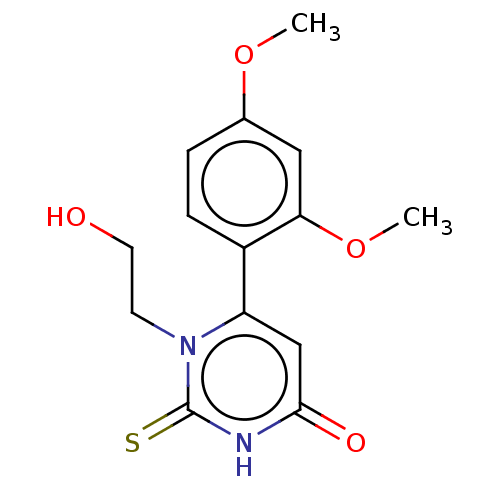
(CHEMBL3633251)Show InChI InChI=1S/C14H16N2O4S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)15-14(21)16(11)5-6-17/h3-4,7-8,17H,5-6H2,1-2H3,(H,15,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133602
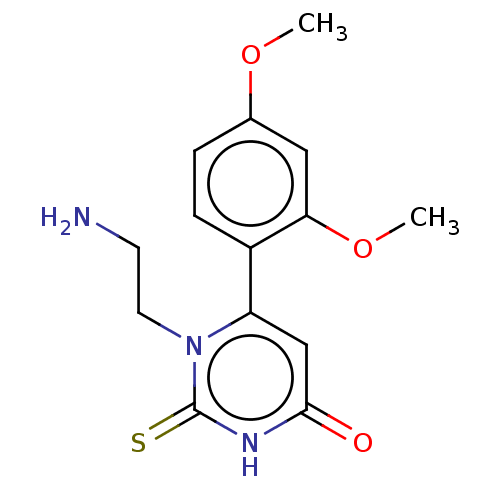
(CHEMBL3633250)Show InChI InChI=1S/C14H17N3O3S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)16-14(21)17(11)6-5-15/h3-4,7-8H,5-6,15H2,1-2H3,(H,16,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human norepinephrine transporter |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133596
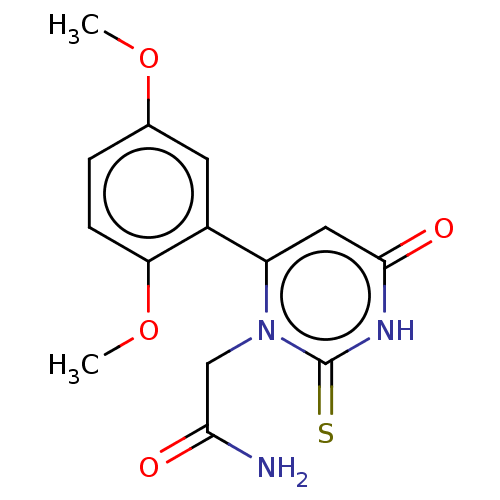
(CHEMBL3633459)Show SMILES COc1ccc(OC)c(c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-11(21-2)9(5-8)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133603
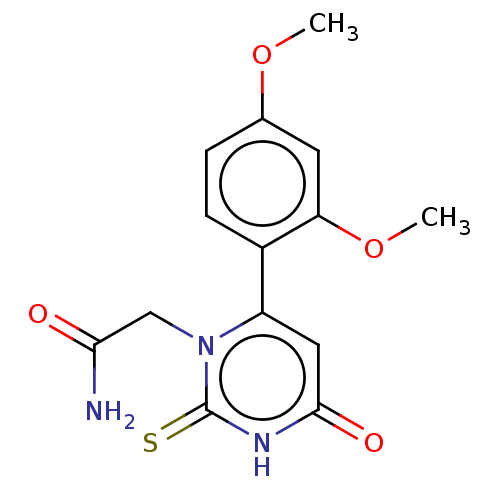
(CHEMBL3633248)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-9(11(5-8)21-2)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325250
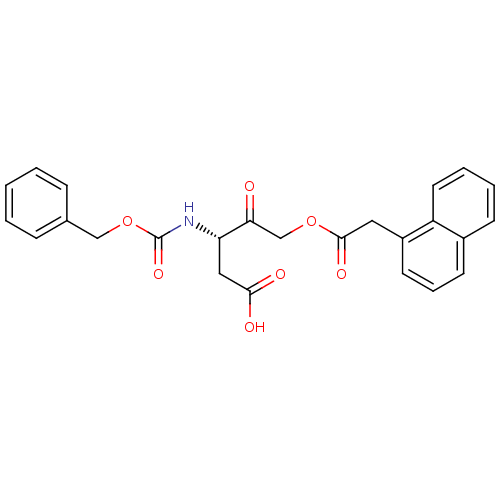
((S)-3-(benzyloxycarbonylamino)-5-(2-(naphthalen-1-...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C25H23NO7/c27-22(16-32-24(30)13-19-11-6-10-18-9-4-5-12-20(18)19)21(14-23(28)29)26-25(31)33-15-17-7-2-1-3-8-17/h1-12,21H,13-16H2,(H,26,31)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133600
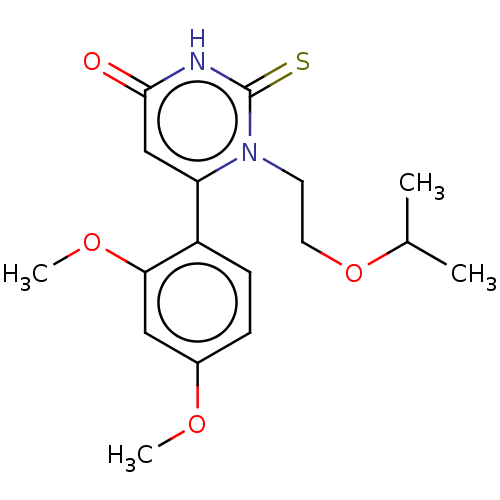
(CHEMBL3633457)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CCOC(C)C Show InChI InChI=1S/C17H22N2O4S/c1-11(2)23-8-7-19-14(10-16(20)18-17(19)24)13-6-5-12(21-3)9-15(13)22-4/h5-6,9-11H,7-8H2,1-4H3,(H,18,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325259
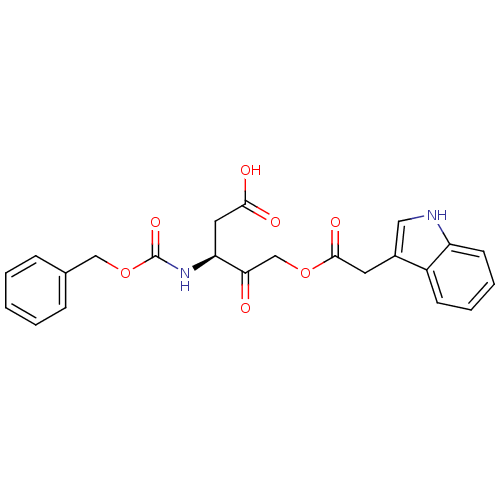
((S)-5-(2-(1H-indol-3-yl)acetoxy)-3-(benzyloxycarbo...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C23H22N2O7/c26-20(14-31-22(29)10-16-12-24-18-9-5-4-8-17(16)18)19(11-21(27)28)25-23(30)32-13-15-6-2-1-3-7-15/h1-9,12,19,24H,10-11,13-14H2,(H,25,30)(H,27,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483375

(CHEMBL1236446)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](CC(=O)NO)CO[C@H](CO)[C@H]1O |r| Show InChI InChI=1S/C22H41NO7/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(26)30-22-17(14-19(25)23-28)16-29-18(15-24)21(22)27/h17-18,21-22,24,27-28H,2-16H2,1H3,(H,23,25)/t17-,18+,21+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325257
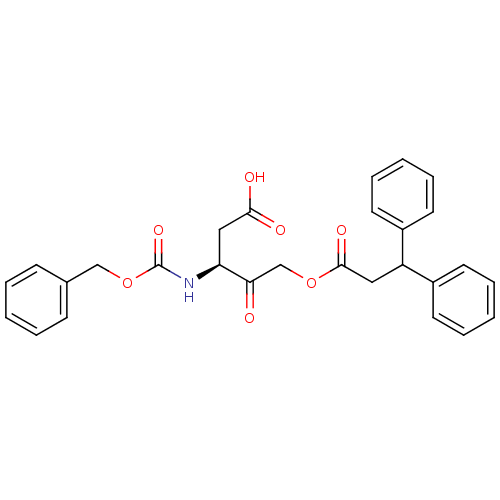
((S)-3-(benzyloxycarbonylamino)-5-(3,3-diphenylprop...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C28H27NO7/c30-25(24(17-26(31)32)29-28(34)36-18-20-10-4-1-5-11-20)19-35-27(33)16-23(21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,34)(H,31,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325260
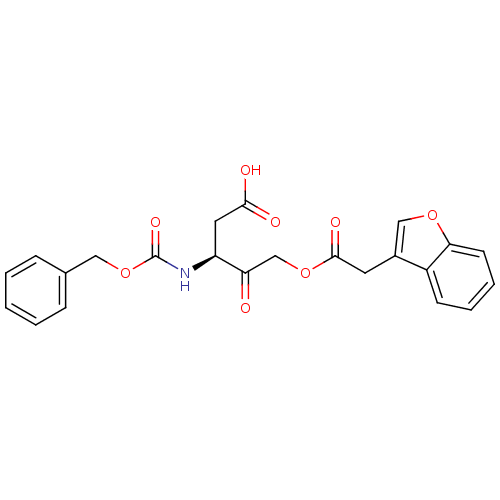
((S)-5-(2-(benzofuran-3-yl)acetoxy)-3-(benzyloxycar...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)Cc1coc2ccccc12 |r| Show InChI InChI=1S/C23H21NO8/c25-19(14-31-22(28)10-16-13-30-20-9-5-4-8-17(16)20)18(11-21(26)27)24-23(29)32-12-15-6-2-1-3-7-15/h1-9,13,18H,10-12,14H2,(H,24,29)(H,26,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325252

((3S)-3-(benzyloxycarbonylamino)-5-(2-(naphthalen-1...)Show SMILES CC(C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)OCc1ccccc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C26H25NO7/c1-17(20-13-7-11-19-10-5-6-12-21(19)20)25(31)33-16-23(28)22(14-24(29)30)27-26(32)34-15-18-8-3-2-4-9-18/h2-13,17,22H,14-16H2,1H3,(H,27,32)(H,29,30)/t17?,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325251

((S)-3-(benzyloxycarbonylamino)-5-(2-(naphthalen-1-...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)COc1cccc2ccccc12 |r| Show InChI InChI=1S/C25H23NO8/c27-21(20(13-23(28)29)26-25(31)34-14-17-7-2-1-3-8-17)15-33-24(30)16-32-22-12-6-10-18-9-4-5-11-19(18)22/h1-12,20H,13-16H2,(H,26,31)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from recombinant human ERG after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325258
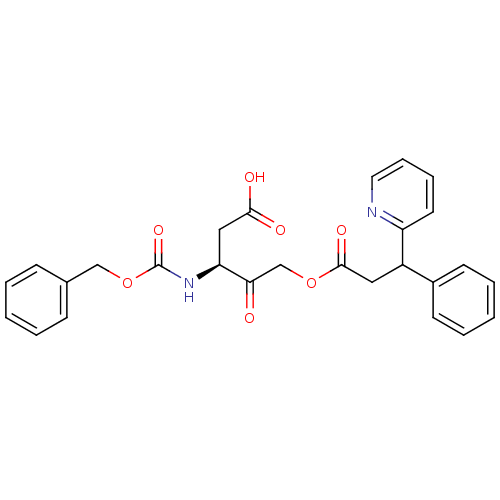
((3S)-3-(benzyloxycarbonylamino)-4-oxo-5-(3-phenyl-...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)CC(c1ccccc1)c1ccccn1 |r| Show InChI InChI=1S/C27H26N2O7/c30-24(23(16-25(31)32)29-27(34)36-17-19-9-3-1-4-10-19)18-35-26(33)15-21(20-11-5-2-6-12-20)22-13-7-8-14-28-22/h1-14,21,23H,15-18H2,(H,29,34)(H,31,32)/t21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325246

((S)-3-(benzyloxycarbonylamino)-4-oxo-5-(2-(phenylt...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)CSc1ccccc1 |r| Show InChI InChI=1S/C21H21NO7S/c23-18(13-28-20(26)14-30-16-9-5-2-6-10-16)17(11-19(24)25)22-21(27)29-12-15-7-3-1-4-8-15/h1-10,17H,11-14H2,(H,22,27)(H,24,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325261
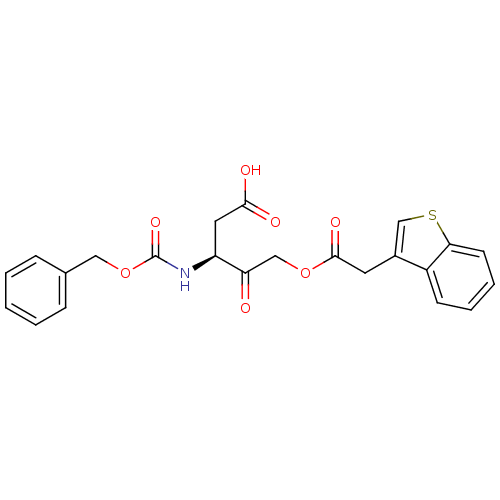
((S)-5-(2-(benzo[b]thiophen-3-yl)acetoxy)-3-(benzyl...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)Cc1csc2ccccc12 |r| Show InChI InChI=1S/C23H21NO7S/c25-19(13-30-22(28)10-16-14-32-20-9-5-4-8-17(16)20)18(11-21(26)27)24-23(29)31-12-15-6-2-1-3-7-15/h1-9,14,18H,10-13H2,(H,24,29)(H,26,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325249

((S)-3-(benzyloxycarbonylamino)-5-(2-(naphthalen-2-...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)Cc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H23NO7/c27-22(16-32-24(30)13-18-10-11-19-8-4-5-9-20(19)12-18)21(14-23(28)29)26-25(31)33-15-17-6-2-1-3-7-17/h1-12,21H,13-16H2,(H,26,31)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325254
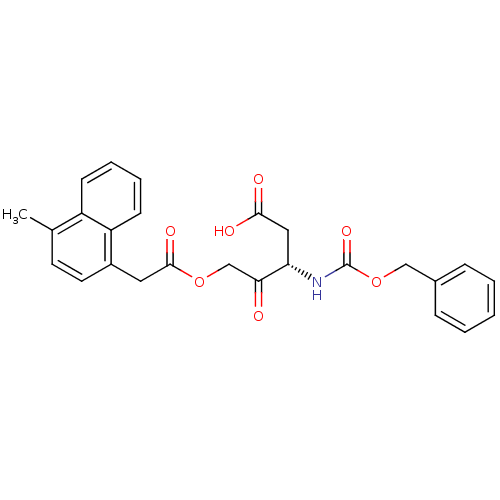
((S)-3-(benzyloxycarbonylamino)-5-(2-(4-methylnapht...)Show SMILES Cc1ccc(CC(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)OCc2ccccc2)c2ccccc12 |r| Show InChI InChI=1S/C26H25NO7/c1-17-11-12-19(21-10-6-5-9-20(17)21)13-25(31)33-16-23(28)22(14-24(29)30)27-26(32)34-15-18-7-3-2-4-8-18/h2-12,22H,13-16H2,1H3,(H,27,32)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325234
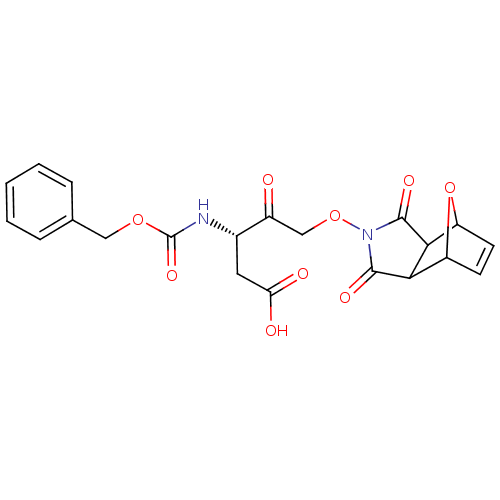
(CHEMBL1223107)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)CON1C(=O)C2C3OC(C=C3)C2C1=O |r,c:28,THB:30:29:27.28:25| Show InChI InChI=1S/C21H20N2O9/c24-13(10-31-23-19(27)17-14-6-7-15(32-14)18(17)20(23)28)12(8-16(25)26)22-21(29)30-9-11-4-2-1-3-5-11/h1-7,12,14-15,17-18H,8-10H2,(H,22,29)(H,25,26)/t12-,14?,15?,17?,18?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325245
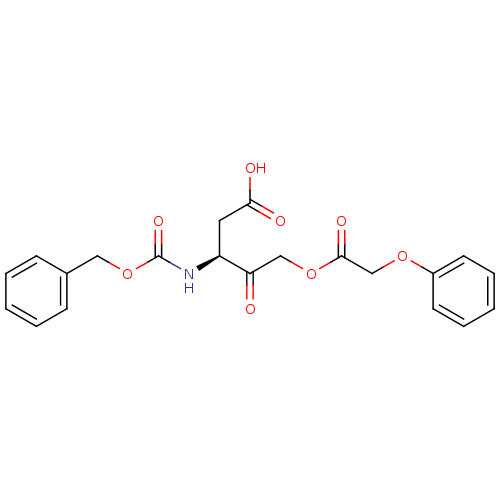
((S)-3-(benzyloxycarbonylamino)-4-oxo-5-(2-phenoxya...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COC(=O)COc1ccccc1 |r| Show InChI InChI=1S/C21H21NO8/c23-18(13-29-20(26)14-28-16-9-5-2-6-10-16)17(11-19(24)25)22-21(27)30-12-15-7-3-1-4-8-15/h1-10,17H,11-14H2,(H,22,27)(H,24,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50325255

((S)-3-(benzyloxycarbonylamino)-5-(2-(5-methylnapht...)Show SMILES Cc1cccc2c(CC(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)OCc3ccccc3)cccc12 |r| Show InChI InChI=1S/C26H25NO7/c1-17-7-5-12-21-19(10-6-11-20(17)21)13-25(31)33-16-23(28)22(14-24(29)30)27-26(32)34-15-18-8-3-2-4-9-18/h2-12,22H,13-16H2,1H3,(H,27,32)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 20: 5089-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.031
BindingDB Entry DOI: 10.7270/Q2SQ90K1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from recombinant human dopamine transporter after 120 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275437
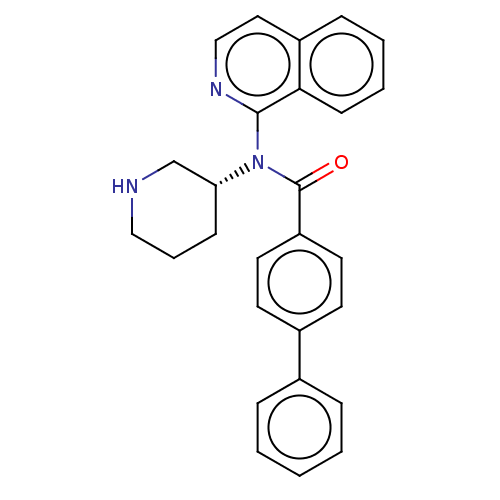
(CHEMBL4129620)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O/c31-27(23-14-12-21(13-15-23)20-7-2-1-3-8-20)30(24-10-6-17-28-19-24)26-25-11-5-4-9-22(25)16-18-29-26/h1-5,7-9,11-16,18,24,28H,6,10,17,19H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data